
Abstract
Aims: Our aim was to evaluate the impact of baseline chronic kidney disease (CKD) on clinical outcomes after percutaneous edge-to-edge mitral valve repair (PMVR).
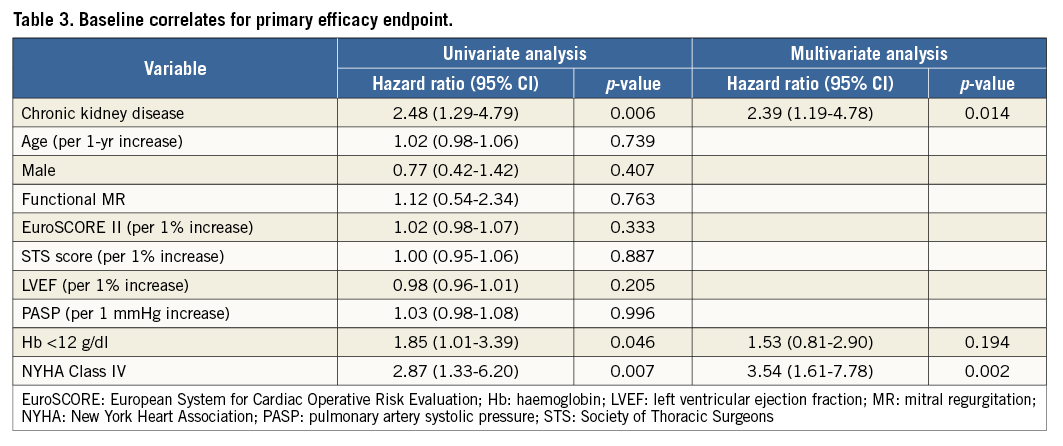
Methods and results: Two hundred and fourteen consecutive patients dichotomised by the presence of baseline CKD (n=113) or no-CKD (n=101) had their clinical outcomes compared up to 12-month follow-up. The primary safety endpoint was the incidence of major adverse events and the primary efficacy endpoint was freedom from death, surgery for MV dysfunction, or grade ≥3+ MR. The primary safety endpoint was demonstrated in 12.4% vs. 2.0% in CKD and no-CKD patients, respectively (p=0.003). The primary efficacy endpoint at 12 months was significantly lower in CKD patients (65.8% vs. 84.2%, respectively, log-rank p=0.005). While MR reduction and NYHA functional class improvement were mostly sustained and equivalent up to 12 months in no-CKD patients, they were impaired in CKD patients. Baseline CKD was an independent predictor of the primary efficacy endpoint (adjusted HR 2.48, 95% CI: 1.29 to 4.79, p=0.006) and calcified leaflet predicted grade ≥3+ MR at 12 months (adjusted HR 6.56, 95% CI: 2.71 to 15.88, p<0.001).
Conclusions: CKD patients had worse clinical outcomes compared with no-CKD patients post PMVR. CKD was an independent predictor of the primary efficacy endpoint, whereas calcified leaflet was an independent predictor of grade ≥3+ MR at 12 months.
Abbreviations
CKD: chronic kidney disease
EF: ejection fraction
eGFR: estimated glomerular filtration rate
FMR: functional mitral regurgitation
LV: left ventricular
MAC: mitral annulus calcification
MR: mitral regurgitation
NYHA: New York Heart Association
PMVR: percutaneous mitral valve repair
Introduction
Severe mitral regurgitation (MR) is associated with progressive left ventricular (LV) dysfunction and congestive heart failure (CHF), ultimately leading to high rates of morbidity and mortality1. Current guidelines recommend surgery for moderate-to-severe (3+) or severe (4+) MR in patients with symptoms or evidence of LV dysfunction2,3; however, when MR is secondary to underlying LV dysfunction (i.e., functional MR [FMR]), the benefit of surgery is controversial4. Therefore, patients with FMR and at high surgical risk are frequently denied surgery and referred to isolated clinical management, carrying a poor long-term prognosis5.
Recently, percutaneous mitral valve repair (PMVR) with the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA) emerged as a safe, less invasive, therapeutic option in patients with 3+ or 4+ MR associated with high surgical risk6. This novel therapy is associated with efficacious MR reduction, improvement in CHF symptoms, as well as LV reverse remodelling7.
Chronic kidney disease (CKD), defined by the presence of reduced estimated glomerular filtration rate (eGFR) or albuminuria, is frequently observed in patients with CHF8. Multiple studies have demonstrated that nearly one third of patients with CHF have concomitant stage 3 or greater CKD (eGFR <60 ml/min/1.73 m2)9. A previous study of mitral valve surgery has shown that preoperative CKD was an independent predictor of late mortality10. The impact of baseline CKD on the outcomes of patients undergoing PMVR with the MitraClip system has not been assessed. In the present study, we sought to evaluate, in consecutive patients undergoing PMVR with MitraClip in a “real-world” setting, the impact of baseline CKD on clinical outcomes up to 12-month follow-up.
Methods
STUDY POPULATION
Patients with symptoms or signs of LV deterioration and 3+ or 4+ MR determined by combined transthoracic and transoesophageal echocardiography11,12, considered to be at high surgical risk by an interdisciplinary team of cardiologists, interventional cardiologists, cardiac surgeons, and anaesthesiologists, underwent PMVR with MitraClip at Ferrarotto Hospital, University of Catania, Catania, Italy, from August 2008 to May 2014 as part of the ongoing Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation (GRASP) registry, the results of which have been partly published elsewhere13,14. After receiving a complete oral and written explanation of the issues surrounding the procedure, all patients included in the study provided written consent. The study was approved by the local ethics committee. Qualifying inclusion and exclusion criteria for MitraClip therapy (clinical and echocardiographic), as well as details of the procedure, have been previously reported15. Patients who had calcification in the grasping area were omitted from the procedure, whereas those with localised calcification not related to the grasping area were included.
DEFINITION OF CKD AND CHANGE IN RENAL FUNCTION
Pre-procedural serum creatinine (SCr) values were used to calculate the baseline eGFR with the Modification of Diet in Renal Disease (MDRD) equation8. CKD was defined as a GFR of <60 ml/min/1.73 m2 (estimated by MDRD), and further classified as moderate (CKD stage 3) if the GFR was 30-59 ml/min/1.73 m2, or severe (CKD stage 4) if the GFR was <30 ml/min/1.73 m2. Baseline eGFR was assessed one day before the MitraClip procedure. Postoperative SCr was measured daily until 72 hours after the procedure, or until peak value, and at discharge. After excluding the patients undergoing chronic dialysis (n=5), we dichotomised our population based on the presence of CKD as follows: patients with CKD and patients with no-CKD. Clinical outcomes up to 12-month follow-up were then compared between the two groups. In addition, we assessed separately the impact of CKD on outcomes in patients with FMR.
MEASUREMENTS
All patients underwent transthoracic two-dimensional (2D) echocardiography at baseline, at hospital discharge, at 30 days, and at 12 months after the procedure. MR severity was graded by the vena contracta (VC) width and the proximal isovelocity surface area (PISA) method to measure effective regurgitant orifice area (EROA) and regurgitant volume (R Vol) according to the current guidelines11-14. Echocardiographic data were separately analysed by a team of two expert echocardiographers and reviewed by a third reader for consensus when there was disagreement.
Calcified mitral valves (i.e., calcified leaflets and mitral annulus calcification [MAC]) were defined using transoesophageal echocardiography and fluoroscopy. Leaflets were considered “calcified” unless the calcification score was 0 based on the Wilkins score16. The extent of MAC was graded into four groups by two observers, from small nodule (grade 1) to extensive calcification (grade 4), according to their extent on fluoroscopic examination (Figure 1)17. The utilisation of fluoroscopy to detect the presence of any calcification was necessary because of the limitations of echocardiography in differentiating nodular fibrosis from calcification18. All echocardiographic and fluoroscopic interpretation was performed without knowledge of the patient’s renal function.
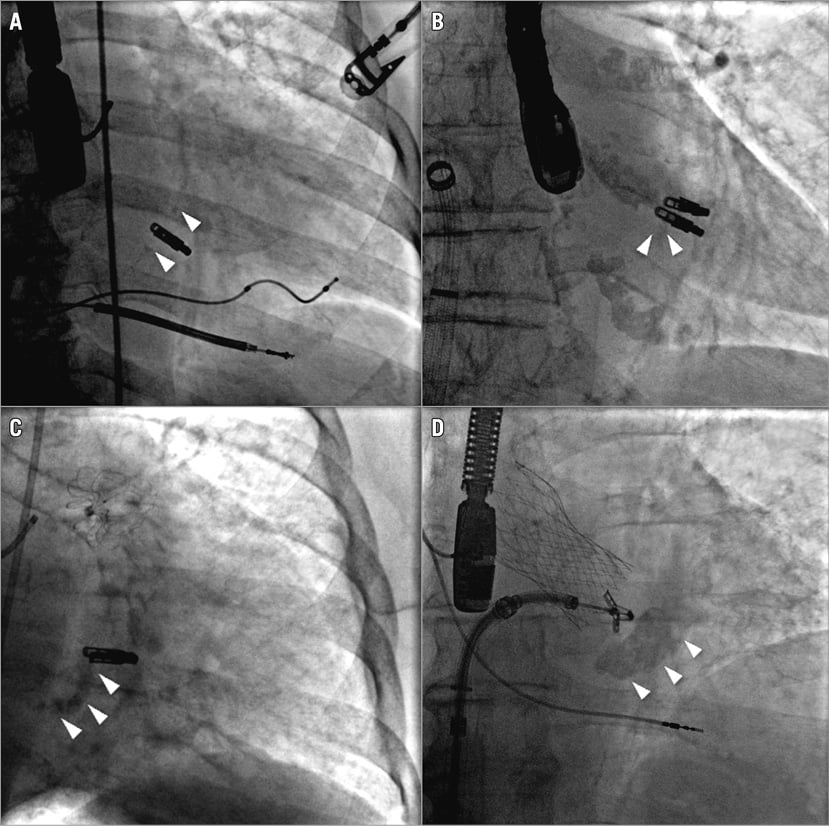
Figure 1. Representative images of the mitral annulus calcification as assessed by fluoroscopy. A) Grade 1. B) Grade 2. C) Grade 3. D) Grade 4. White arrowheads show the calcified lesions.
ENDPOINTS AND FOLLOW-UP
Acute device success was defined as residual MR ≤2+ after the procedure. The primary safety endpoint was the incidence of major adverse events (MAEs) at 30 days, defined as the composite of death, myocardial infarction, reoperation for failed MitraClip implantation, non-elective cardiovascular surgery for adverse events, stroke, renal failure, deep wound infection, mechanical ventilation for >48 hours, gastrointestinal complication requiring surgery, new onset of permanent atrial fibrillation, septicaemia, and transfusion of 2 U of blood. The primary efficacy endpoint was freedom from death, surgery for mitral valve dysfunction, or grade ≥3+ MR at 12-month follow-up after clip implantation. The composite endpoint was defined as death or re-hospitalisation for HF. Re-hospitalisation for HF was defined as new-onset or worsening signs and symptoms of HF that required urgent therapy and resulted in hospitalisation. Clinical follow-up was conducted by clinical visits and/or phone consultation at 30 days, six months, and 12 months, and annually thereafter. The median follow-up was 23 months (interquartile range [IQR]: 11 to 33 months); no patient was lost to follow-up. Any death or re-hospitalisation was recorded during the follow-up period.
Statistical analysis
Continuous variables following a normal distribution are presented as mean±SD and were compared using the Student’s unpaired t-test for comparisons between groups and the Student’s paired t-test for within-group comparisons. Variables that did not follow a normal distribution were compared with a Mann-Whitney test for comparisons between groups and a Wilcoxon signed-rank test for within-group comparisons. Categorical variables are presented as counts and percentages and were compared by the chi-square or the Fisher’s exact test. Survival curves were generated using the Kaplan-Meier method, and log-rank tests were used to evaluate differences between groups. A two-way repeated measures analysis of variance (ANOVA) was used to evaluate the effects of time (baseline vs. 30 days vs. 12 months) and group (CKD vs. no-CKD) on echocardiographic and clinical variables, and post hoc analysis was performed with Bonferroni correction. Prognostic values of baseline CKD compared with no-CKD as the reference were assessed using a Cox hazard regression model. Cox regression analysis was performed to identify independent predictors of the primary efficacy endpoint, expressed as hazard ratio (HR) and 95% confidence interval (95% CI). Candidate variables for the multivariable model were those considered clinically relevant and with a p-value <0.10 at the univariate analysis. Likewise, Cox regression analysis was performed to identify independent predictors of grade ≥3+ MR. All p-values reported are two-sided, and p-values <0.05 were considered significant. All data were processed using SPSS, Version 21 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS
Of the 214 patients included in the present study, more than half (52.8%) presented with baseline CKD (moderate in 91 patients [80.5%] and severe in 22 [19.5%]). Baseline clinical characteristics are shown in Table 1. Patients with CKD were more likely to be female and had a trend to be older compared with no-CKD patients. EuroSCORE II and STS score were higher in the CKD group. Although baseline left ventricular ejection fraction (LVEF) and left chamber sizes were comparable between groups, patients with CKD more frequently demonstrated MAC and calcified leaflets. Patients with CKD were mildly anaemic when compared with no-CKD patients (mean haemoglobin 11.7±2.2 g/dl vs. 12.6±1.9 g/dl, respectively, p=0.003), whereas no differences were revealed in the severity of MR as well as NYHA functional class between groups.
IN-HOSPITAL AND 30-DAY OUTCOMES
All of the procedures were performed without clip-related complications, such as embolisation, cardiac tamponade or periprocedural stroke, except for one case of cardiac tamponade which occurred due to left atrial appendage injury and which was well controlled by pericardial drainage. Marked improvement in MR was demonstrated in both groups (Figure 2A), leading to high rates of device success (97.3% vs. 99.0%, respectively, p=0.354). No significant differences were documented regarding number of clips, device time, procedure time, dose of contrast media, and length of hospital stay between the groups. Data were available for 100% of the patients at 30-day follow-up. A higher rate of renal failure after the procedure was identified in the CKD group compared with the no-CKD group, leading to higher adverse events in the CKD group (primary safety endpoint: 12.4% vs. 2.0%, respectively, p=0.003) (Table 2). Furthermore, post-procedure reduction of MR was sustained while important NYHA functional class improvement was observed up to 30-day follow-up in both groups (Table 2, Figure 2B).
Figure 2. Mitral regurgitation severity and NYHA functional class. A) MR severity at baseline, post-procedure, 30 days, and 12 months. B) NYHA functional class at baseline, 30 days, and 12 months. NYHA: New York Heart Association
TWELVE-MONTH OUTCOMES
Kaplan-Meier freedom from death, surgery for mitral valve dysfunction, or grade ≥3+ MR at 12 months (primary efficacy endpoint) was significantly lower in the CKD group compared with the no-CKD group (65.8% vs. 84.2%, log-rank p=0.005) (Figure 3A). The components of the primary efficacy endpoint when analysed separately were also significantly different between the two groups (Figure 3B, Figure 3C); meanwhile, the estimates for freedom from combined death and re-hospitalisation for CHF were also worse in the CKD group compared with the no-CKD group (Figure 3D). The same results as above were revealed when these outcomes were analysed in patients with only FMR (n=167). While MR reduction and NYHA functional class improvement were mostly sustained and equivalent up to 12 months in the surviving patients of the no-CKD group, they were impaired in the CKD group (Figure 2).
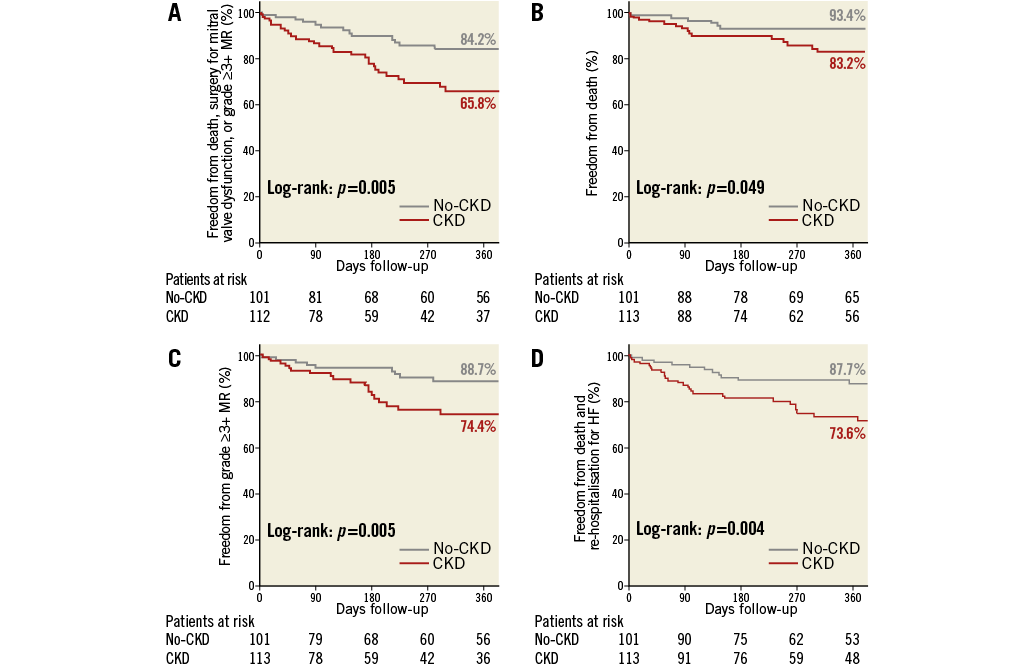
Figure 3. Kaplan-Meier curves at 12-month follow-up. A) Freedom from death, surgery for mitral valve dysfunction, or grade ≥3+ MR. B) Freedom from death. C) Freedom from MR ≥3+. D) Freedom from death and re-hospitalisation for HF. CKD: chronic kidney disease; HF: heart failure; MR: mitral regurgitation
COX REGRESSION ANALYSIS
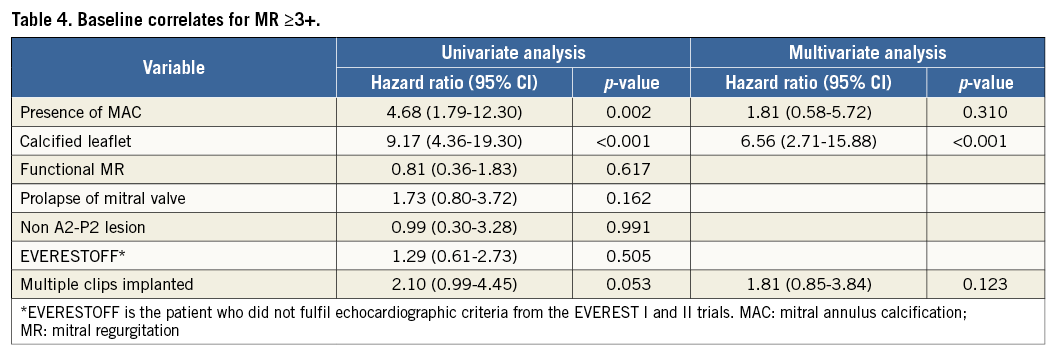
Predictive factors of the primary efficacy endpoint were assessed using a Cox hazard regression model (Table 3), as follows: baseline CKD, anaemia, and NYHA Class IV were identified as predictors in univariate analysis while CKD and NYHA Class IV remained as predictors after multivariate analysis. In addition, predictors of grade ≥3+ MR at 12 months were assessed using the same model (Table 4). The presence of MAC and calcified leaflets were significant predictors in univariate analysis, and calcified leaflets only remained as a significant predictor of grade ≥3+ MR at 12 months after multivariate analysis (adjusted HR 6.56, 95% CI: 2.71 to 15.88, p<0.001).
Discussion
The main findings of the present study were as follows. 1) Baseline CKD is common in patients undergoing MitraClip implantation and is associated with reduced safety and efficacy of the intervention and worse clinical outcomes when compared with no-CKD patients up to 12 months. 2) Despite remarkably high and comparable rates of device success between the groups, CKD patients had higher mortality, more likely had recurrence of MR grade ≥3+, and were more frequently re-hospitalised for heart failure up to 12 months compared with no-CKD patients. 3) Calcified leaflet, which was more frequently observed in patients with CKD, was identified as a significant predictor of grade ≥3+ MR at 12 months. Based on these findings, CKD should be carefully considered when evaluating patients for MitraClip implantation.
PMVR with the MitraClip system has progressively established its role as an alternative treatment for high-risk surgical patients with moderate-to-severe and severe MR19, but the impact of baseline CKD and calcified leaflets on the outcomes after this relatively novel intervention were, until now, unknown. The rationale for understanding this complex clinical setting better is provided by the higher mortality rates demonstrated after MV surgery in patients with preoperative CKD10. Likewise, CKD was identified as an independent predictor of worse MitraClip efficacy up to 12 months in the present study. In fact, all the components of the primary efficacy endpoint, when analysed separately, were also more frequently observed in CKD compared with no-CKD patients; moreover, the rates of re-hospitalisations for CHF were higher in CKD patients. Taken together, these data provide confirmation that patients with CKD who undergo either surgical intervention or the less invasive PMVR with MitraClip are extremely complex and are at higher risk of developing adverse events compared with no-CKD patients. Importantly, renal failure after the procedure, which could be a further concern mostly in CKD patients, was only demonstrated in 4.4% of CKD patients in the present study. Usually no or extremely low amounts of contrast are utilised during PMVR20 (as it is mostly a TEE-guided procedure). In addition, the intervention does not require cardiopulmonary bypass and aorta cross-clamping, which reduces haemodynamic impairment during the intervention, ultimately minimising the likelihood of additional renal function deterioration21. However, considering that patients in the no-CKD group did not develop renal failure after the procedure, one should note that the intervention itself carries a potential risk of worsening renal failure in CKD patients. While clinicians should be cognisant of the potential impact of CKD on the outcomes of MV surgery and PMVR when evaluating patients with 3+ or 4+ MR who are potential candidates for MV repair, a direct randomised comparison between these two therapies will be required to shed more light in this setting.
Previous studies have shown the association between MAC, calcified leaflets and CKD22, which is in line with our results. MAC negatively impacts on the outcomes of surgical MV repair, leading to higher rates of surgical MV repair failure and conversion to MV replacement instead23. Although marked improvement in MR post-procedure was demonstrated regardless of baseline renal function, MAC status, and calcified leaflets, the results were mostly sustained up to 12 months in the surviving no-CKD patients while significantly impaired in CKD patients. A similar trend was observed in patients with or without calcified leaflets. The presence of calcified leaflets was identified as the only and strong independent predictor of MR grade ≥3+ at 12 months. Indeed, among the 29 surviving patients who exhibited this pathological feature, 41% developed MR grade ≥3+ at 12 months. Valvular calcification is considered to be a marker of advanced cardiovascular disease, since cardiovascular risk factors are common in these patients. Moreover, previous histological study observing surgical specimens demonstrated that valve leaflet calcification in patients with CKD is associated with enhanced inflammation24. We therefore speculate that such inflammation could potentially be associated with a “vulnerable” grasping over time, which could lead to late MR recurrence regardless of the effective acute grasping results and MR reduction. Further investigation is required to elucidate these findings completely.
Different outcomes in the MitraClip procedure between degenerative MR and FMR populations have been demonstrated in previous studies25. Since patients with CKD were more likely to have FMR, we have assessed the impact of CKD on outcomes in patients with only FMR (n=167) and revealed the same results, i.e., CKD patients had higher mortality, more likely had recurrence of MR grade ≥3+, and were more frequently re-hospitalised for heart failure up to 12 months compared with no-CKD patients. Moreover, we included “functional MR” as one of the variables in our Cox hazard regression model and it was found to be insignificant.
Study limitations
Our study has the inherent limitations of its retrospective design, although the data were collected prospectively. As a non-randomised study, several confounding factors could have influenced our results, but we performed statistical adjustment for the differences in baseline characteristics in order to minimise this potential caveat; indeed, the results were unchanged after doing so. Although not all of our patients were available for 12-month follow-up due to insufficient time having elapsed since the index procedure, we utilised Kaplan-Meier estimates to assess our 12-month primary endpoint and its components. We cannot completely eliminate the possible contribution of “functional renal insufficiency”, i.e., impaired renal function created by intensive heart failure therapy (i.e., high dose of IV diuretics) for an acute heart failure episode, to the CKD patients, although baseline renal function was assessed one day before the procedure after confirmation of the haemodynamic and renal functional stability.
The interventions performed in this study were undertaken in a high-volume MitraClip implantation centre, hence the results obtained should not be generalised. The echocardiographic data herewith described were not reviewed by an independent core laboratory as it was performed in a clinical setting, reflecting the real-world practice; however, the analyses were conducted by dedicated, highly experienced physicians utilising validated methods and were based on consensus26.
Conclusion
CKD patients had worse clinical outcomes compared with no-CKD patients post PMVR. CKD was an independent predictor of the primary efficacy endpoint, whereas calcified leaflets were an independent predictor of grade ≥3+ MR at 12 months.
| Impact on daily practice In the present study, patients with baseline CKD undergoing MitraClip therapy demonstrated worse safety at 30 days, this being associated with reduced efficacy of the intervention and worse clinical outcomes when compared with no-CKD patients up to 12 months. CKD was an independent predictor of the primary efficacy endpoint, whereas calcified leaflet was an independent predictor of grade ≥3+ MR at 12 months. Therefore, these results can be applied to risk stratification in patients undergoing MitraClip therapy. |
-**
Funding
No financial support was provided for our study. Y. Ohno is supported by a grant from the Japan Heart Foundation and Bayer Yakuhin Research Grant Abroad.
Conflict of interest statement
G. Attizzani is on the speaker’s bureau of Abbott Vascular. C. Grasso serves as a proctor for Abbott Vascular. The other authors have no conflicts of interest to declare.

