
Abstract
Aims: This study sought to determine the incidence and identify predictors of acute kidney injury (AKI) following percutaneous edge-to-edge mitral valve repair (PMVR) and compare the risk of AKI between PMVR and surgical mitral valve repair (SMVR).
Methods and results: We performed a single-centre analysis of 378 patients receiving treatment for mitral regurgitation (196 consecutive patients undergoing PMVR and 182 patients undergoing SMVR). The incidence of AKI (any stage according to KDIGO) following PMVR was 17.9%. Intervention duration (OR 1.01, 95% CI: 1.00-1.02) and peripheral vascular disease (OR 7.69, 95% CI: 3.25-18.17) predicted AKI after PMVR. Patients suffering from AKI after PMVR demonstrated poorer survival (median follow-up 428 days). SMVR patients were significantly younger, had fewer comorbidities and better renal function at baseline. Nevertheless, AKI occurred numerically more often after SMVR than PMVR (25.8% vs. 17.9%, p=0.060), and a multivariable regression model adjusting for differences between both groups confirmed a significantly lower risk for AKI following PMVR (OR 0.22, 95% CI: 0.11-0.44, p<0.001).
Conclusions: These data show a significant incidence of AKI after PMVR that must be taken into account in periprocedural care. Nevertheless, our data suggest that SMVR carries an even higher risk of AKI, which should be considered when a decision has to be made between the two therapies.
Abbreviations
AKI: acute kidney injury
KDIGO: kidney disease: improving global outcomes
MAP: mean arterial pressure
MR: mitral regurgitation
PMVR: percutaneous edge-to-edge mitral valve repair
Sd: standard deviation
SMVR: surgical mitral valve repair
TAVR: transcatheter aortic valve replacement
Introduction
Acute kidney injury (AKI) is a frequent complication following cardiac surgery, with incidences as high as 42%, albeit with a considerable degree of variation depending on the definition and baseline characteristics of the study population1,2. Importantly, AKI is independently associated with mortality3-6. It has been shown that even slight decreases of renal function after cardiac surgery are associated with a significant increase in mortality5,7.
Percutaneous edge-to-edge mitral valve repair (PMVR) has emerged as an alternative to surgery for high-risk patients with mitral valve regurgitation (MR)7,8. Thus, patients being treated with PMVR are typically older and present with a higher number of comorbidities, in particular a higher prevalence of renal dysfunction9. Only very limited data exist on the occurrence and impact of AKI after PMVR, a procedure which usually does not require contrast media. The objectives of this study were to assess the incidence, predictors and prognosis of AKI following PMVR and to compare these data with SMVR.
Methods
STUDY DESIGN AND PATIENT POPULATION
Three hundred and seventy-eight patients undergoing treatment for MR at the Heart Centre of the University Hospital of Cologne were analysed retrospectively: 196 consecutive patients undergoing PMVR between November 2012 and April 2015, and 182 consecutive patients undergoing SMVR between July 2007 and August 2015. The differing, but overlapping, time periods for both procedures are explained by the fact that PMVR was not performed in our institution before November 2012. We manually screened all patients initially indexed as having AKI and relabelled 12 patients as “AKI NO” afterwards. These patients had a significant decrease of serum creatinine on postoperative day 1 or 2 compared to baseline, which returned to baseline level at day 3 or 4, an effect most likely explained by haemodilution caused by intravenous fluids administered during ICU stay or anaesthesia as also suggested in the current KDIGO practice guidelines for acute kidney injury by Kellum et al10. A study flow chart is presented in Figure 1.
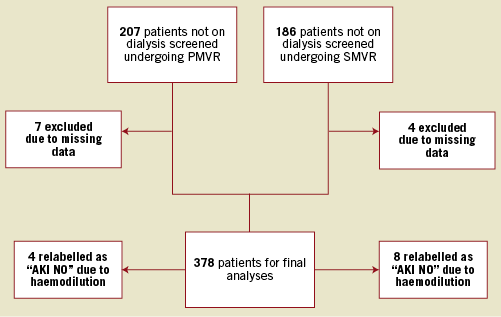
Figure 1. Study flow chart.
All patients treated for MR were discussed by an interdisciplinary Heart Team. In the surgical cohort, patients received singular minimally invasive mitral valve repair. All operations were performed via a video-assisted right minithoracotomy by senior surgeons.
All data were retrieved from either digitalised patient files or (mainly) an automated information system (ORBIS; Agfa Healthcare, Bonn, Germany). Follow-up and mortality data were retrieved during routine visits in our outpatient clinic. Where this was not possible, patients or their general practitioner were contacted by phone. Data collection was approved by the local ethics committee of the Faculty of Medicine of the University of Cologne (13-019).
DEFINITION OF ACUTE KIDNEY INJURY
AKI was defined according to recent KDIGO guidelines10,11. Here, AKI is defined as an increase in serum creatinine ≥0.3 mg/dl within 48 hours or increase in serum creatinine to ≥1.5 times baseline within seven days. Baseline renal function was determined by using the most recent serum creatinine, mostly taken on admission to hospital. Due to the retrospective character of the study, creatinine levels were not evaluated in a standardised manner. In general, laboratory evaluation was conducted at least once daily during intensive care unit stay, and at the physician’s discretion thereafter.
STATISTICAL ANALYSIS
Covariates were described using mean values±standard deviation (Sd), median (interquartile range [IQR]), or frequencies and percentages. Differences between two groups were evaluated using Fisher’s exact tests for categorical variables and unpaired t-tests or Mann-Whitney U tests for continuous variables, depending on normality. Survival curves were estimated using the Kaplan-Meier method and compared by log-rank test. The primary endpoint of our analysis was the incidence of AKI defined by KDIGO. Baseline characteristics (age, STS score, EuroSCORE II, coronary artery disease, prior myocardial infarction, chronic obstructive pulmonary disease, diabetes, atrial fibrillation, arterial hypertension, prior stroke, baseline creatinine, baseline urea, baseline haemoglobin, baseline left ventricular ejection fraction and type of MR [functional/degenerative]) showing a p-value <0.05 in pairwise comparisons by procedure and/or by AKI were included in multivariable logistic regression models. Age and, in case of comparison between PMVR and SMVR, type of procedure were forced into the models, respectively. The potential impact of periprocedural mean arterial pressure on AKI was analysed using a generalised estimating equation (GEE) model with binary logistic outcome (first-order autoregression, taking into account repeated measurements).
Statistical analyses were performed using SPSS Statistics, Version 23 (IBM Corp., Armonk, NY, USA), and R Version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
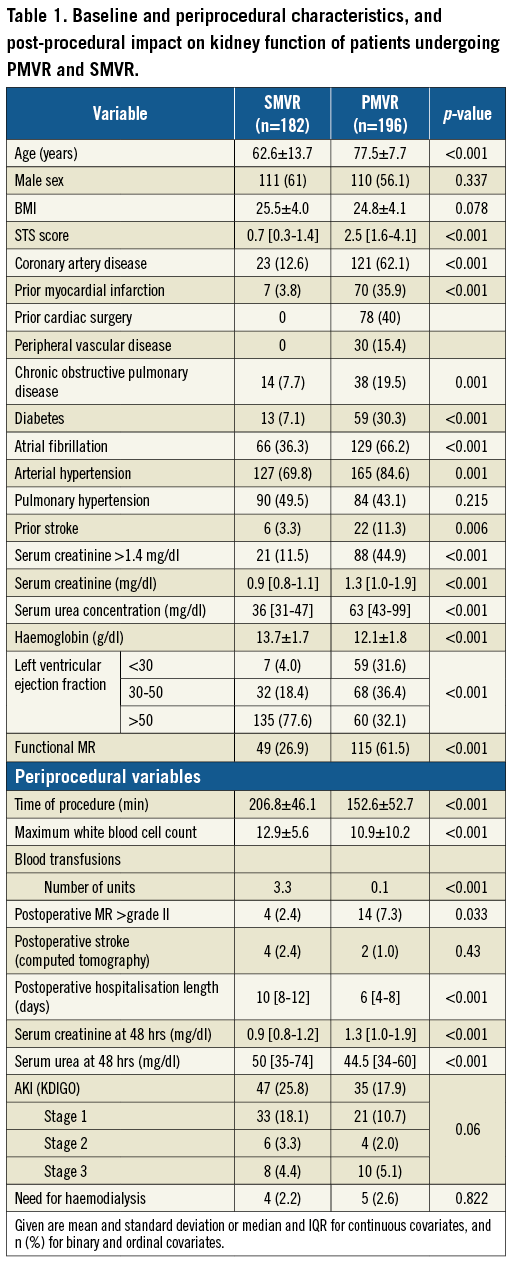
Baseline characteristics and the main procedural characteristics of patients undergoing PMVR are shown in Table 1. AKI occurred in 17.9% (n=35) of PMVR patients, with a majority of patients suffering from stage 1 AKI. In most instances, AKI occurred within 72 hours (77.4% of AKI cases). We detected AKI after more than five days in only four patients.
PREDICTIVE FACTORS OF ACUTE KIDNEY INJURY AFTER PMVR
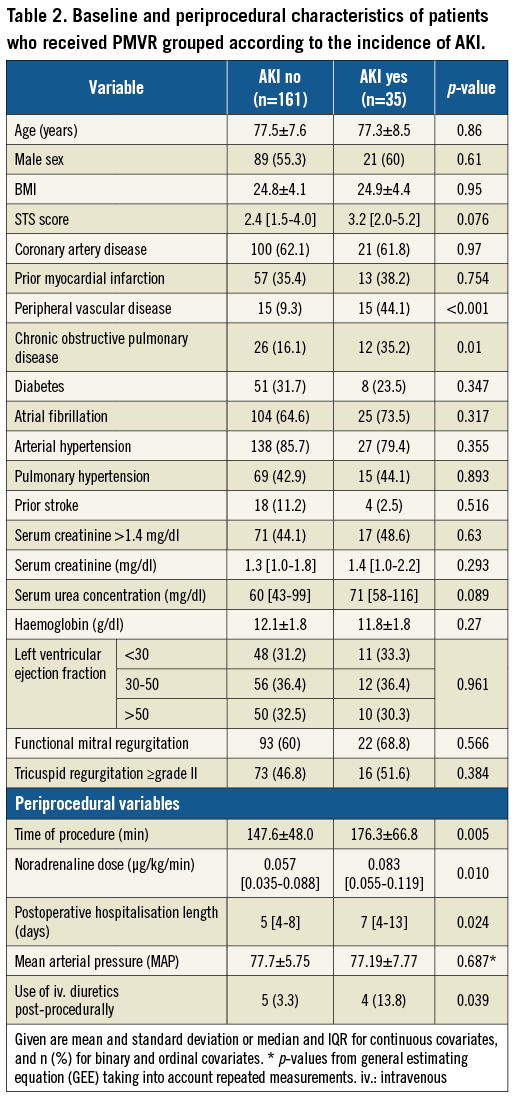
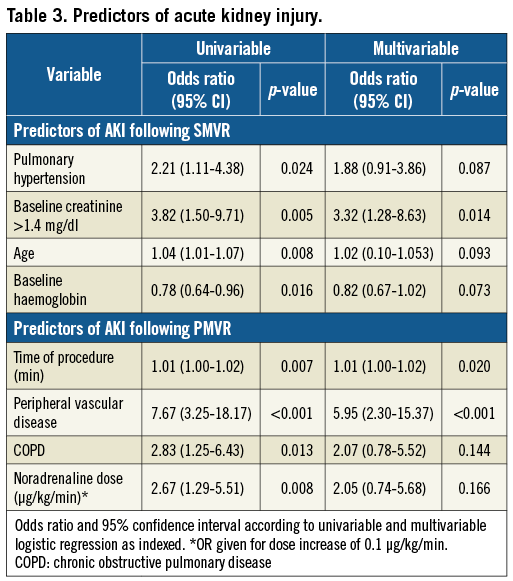
Baseline and procedural characteristics of PMVR patients according to the incidence of AKI are shown in Table 2. Patients with AKI significantly more often suffered from peripheral vascular disease (p<0.001) and chronic obstructive pulmonary disease (p=0.01). Furthermore, intervention duration was significantly longer in patients with AKI (p=0.005) and the procedural dose of noradrenaline was statistically higher (p<0.010). Patients with AKI had a significantly longer postoperative hospital stay (p=0.024) and a significantly higher likelihood of receiving intravenous diuretics (p=0.039). The predictors of AKI according to logistic regression are shown in Table 3. Peripheral vascular disease and intervention duration were significant predictors of AKI in univariable and multivariable models.
ACUTE KIDNEY INJURY FOLLOWING PMVR AND MORTALITY
In-hospital mortality was 1.9% (n=4). Of these patients, two died in cardiogenic shock and two in septic shock. Three of the deceased patients suffered from post-interventional AKI. One-year survival was 72.9% (55.5%-90.3%) for patients with AKI and 90.5% (88.1%-95.4%) for patients without AKI. Median follow-up was 428 days (range 2-1,092 days), and overall survival differed significantly depending on AKI (p<0.001, log-rank test). Kaplan-Meier curves are shown in Figure 2.

Figure 2. The impact of AKI on survival after PMVR. Kaplan-Meier overall survival by AKI versus non-AKI, unadjusted log-rank p<0.001.
PERI-INTERVENTIONAL BLOOD PRESSURE AND THE RISK FOR AKI AFTER PMVR
We derived the peri-interventional mean arterial pressure (MAP) of 191 patients (mean number of measurements 42.8, range 26-63, per patient) (Table 2). Using a GEE, we did not find an association between MAP (p=0.687) and AKI. Patients with AKI received higher doses of noradrenaline which, however, was not independently predictive for AKI (Table 2, Table 3).
ACUTE KIDNEY INJURY FOLLOWING PMVR VERSUS SMVR
Baseline characteristics of SMVR patients are shown in Table 1 along with those of PMVR patients. In comparison to PMVR, SMVR patients presented with a lower rate of comorbidities, better left ventricular ejection fraction and lower incidence of preoperative renal impairment. Moreover, only 26.9% of SMVR patients presented with functional MR, whereas 61.5% of PMVR patients exhibited functional MR (Table 1). Nevertheless, AKI incidence was numerically higher after SMVR compared to PMVR (25.8% vs. 17.9%, p=0.060) with a similar distribution of AKI stages (Table 1) and similar rates of patients requiring haemodialysis (2.2% vs. 2.6%, p=0.822). To compare the impact of PMVR and SMVR on acute kidney injury further, we established a multivariable regression model. After the selection procedure described in the Methods section, variables remaining in the final model were type of procedure, chronic obstructive pulmonary disease, baseline urea and haemoglobin. Age and type of procedure were forced into the model. The risk of AKI was significantly lower following PMVR compared to SMVR (OR 0.22, 95% CI: 0.11-0.44, p<0.001, sensitivity analysis). To account for discrepancies in missing laboratory values at late time points (days 4-7) between both groups, a sensitivity analysis comparing the incidence of AKI at day 3 was performed, which included only patients with completely available creatinine levels. Similar to the original analysis, this analysis showed a statistically non-significant, higher incidence of AKI in surgical compared to interventional patients despite the described unbalanced baseline criteria between the groups (unadjusted OR: 0.84, 95% CI: 0.49-1.46, p=0.539).
Discussion
Despite PMVR being performed without contrast agent under stable haemodynamic conditions, almost one fifth of patients exhibited AKI following PMVR. This is in accordance with Taramasso et al, who reported (in a letter to the editor) the occurrence of AKI after PMVR in 126 patients to be 23.8%12. In contrast, the incidence of renal failure after PMVR in the EVEREST II trial was less than 1%7. This difference may be related to the underlying definition of AKI, which is not further specified in EVEREST II, whereas Taramasso et al defined AKI according to KDIGO criteria, which has a high sensitivity for detecting AKI due to its prolonged observation interval10. However, the fact that EVEREST II included younger patients with fewer comorbidities than our study and the study of Taramasso is another likely explanation contributing to the observed discrepancy.
A considerable number of our patients were elderly, and most had long-standing arterial hypertension, chronic kidney disease, diabetes mellitus and severe systolic left ventricular function impairment as predisposing factors of AKI. Interestingly, we identified only peripheral vascular disease as an independent patient-related predictor of AKI in logistic regression analysis. This is in line with previous data from a high-risk population of transcatheter aortic valve replacement (TAVR) patients, which identified peripheral vascular disease as an independent predictor of AKI, whereas comorbidities such as baseline renal function, heart failure or hypertension did not seem to have an impact13. The pathophysiological explanation for this association between peripheral artery disease and AKI is most likely the close interrelation between advanced atherosclerosis and kidney injury. However, it also has to be considered that the high prevalence of other factors which probably predispose to AKI (e.g., chronic kidney disease) in these populations might blur their identifiability as predictors of AKI. Therefore, AKI may also contribute to mortality independently due to activation of the humoral, vascular or renin-angiotensin-aldosterone cascade14. With regard to the absent predictive effect of baseline renal function on procedural AKI in PMVR, it is furthermore intriguing to speculate that the untoward procedural effects of PMVR are partly counterbalanced by its favourable haemodynamic effects after mitral valve repair, particularly in patients with advanced renal and cardiac impairment, as already suggested by data showing that in the long term renal function improvement is particularly observed in those with severe renal impairment at baseline15. Obviously, future studies are required to investigate this hypothesis. It has to be pointed out that an impact of baseline renal function on AKI might be identified when analysing larger patient numbers.
Since PMVR does not require the use of contrast media and is still frequently followed by AKI, our data support the hypothesis that AKI following catheter-based procedures is only partially explained by contrast media-induced nephropathy13,16,17. On the other hand, intervention duration was a significant predictor of AKI following PMVR in our study, which was also found in a previous study by Schnabel et al, evaluating AKI following TAVR18. A possible explanation seems to be intraprocedural hypotension, which has been linked to postoperative AKI in SMVR19-21, whereas data remain controversial with regard to the protective impact of increasing the mean arterial pressure intraoperatively22,23. We did not find an association between peri-interventional blood pressure and the incidence of AKI but found an association between the incidence of AKI and higher doses of noradrenaline, which are probably a better reflection of haemodynamic stability than blood pressure. Furthermore, patients with AKI had a significantly higher likelihood of receiving intravenous diuretics, which we, however, interpreted as a manifestation of a more unstable status. Thus, the exact pathophysiology of postinterventional AKI and with it its causal predictors still remain unclear.
Importantly, our data demonstrate that AKI is relevant to patient outcome due to its association with longer duration of hospital stay and poorer survival. This is corroborated by previous studies focusing on AKI after cardiac surgery and TAVR, which also established a relationship between acute and chronic renal dysfunction and higher rates of complications2,13,24-26. Regarding renal function, for PMVR so far only baseline renal function has been shown to be a predictor for procedural outcomes, prolonged hospital stay and mortality27,28.
Despite the higher risk profile of PMVR compared to SMVR patients, the incidence of AKI was lower in PMVR. Strikingly, after accounting for baseline confounders in a multivariable regression model, the risk for AKI was about 80% lower after PMVR compared to SMVR, suggesting a favourable risk profile of PMVR with regard to AKI. Of importance, median sternotomy is associated with a higher rate of postoperative AKI. We therefore included only patients treated with minimally invasive mitral valve surgery29-31.
Clearly, these data have to be interpreted with caution. Viewed in a clinical context, however, it appears fair to assume that, based on these data, a risk profile for periprocedural AKI supports interventional treatment in otherwise suitable patients. This is furthermore underlined by the fact that predictors of AKI in SMVR (age, baseline renal function and lower haemoglobin levels) occurred more frequently in PMVR patients at baseline while the incidence of AKI was still lower in these patients. Moreover, as outlined above, recent data suggest that successful PMVR even improves renal function, particularly in patients with severe renal impairment15,32.
For both PMVR and SMVR, a strong association between AKI and poorer survival has been established, underlining the prognostic importance of AKI1,2,5. In agreement, SMVR patients with AKI had a significantly longer hospital stay than patients without AKI. We did not survey mortality data in our surgical collective since it was not the main focus and its relation to AKI is already well described1,2,4.
Interestingly, STS scores for both PMVR and SMVR were rather low. One explanation for this observation is that many factors, which are well established as predictors of surgical risk, are not incorporated in current scoring systems. Furthermore, even factors included in the STS score only incompletely reflect current risk-benefit considerations, particularly in the context of secondary MR. Thus, a 70-year-old patient with three-vessel disease and previous bypass surgery and a left ventricular ejection fraction of 30% and no current option for revascularisation has an STS score of 1.289%. However, given that damaging a bypass graft in such a patient would have rather severe consequences and given the absence of prognostic data and strong guideline recommendations for isolated mitral valve surgery, surgeons are often (correctly) reluctant to perform surgery in such patients. This is potentially also reflected by the fact that patients in our SMVR group indeed had even lower STS scores overall, representing a very low risk cohort (mean 0.7).
Limitations
Due to the retrospective, non-randomised character of our study, unmeasured confounders might have an impact on AKI not visible in our analysis. Therefore, preferably randomised data are needed to verify our hypothesis-generating findings. Also, due to the retrospective character of this study, serum creatinine levels were not obtained systematically. Nevertheless, as most AKI incidences occurred in the first three days, it is unlikely that we missed an important number of patients who suffered AKI. In addition, a sensitivity analysis comparing risk of AKI at day three confirmed the robustness of our findings. Clearly, the comparison of surgically versus interventionally treated patients has to be interpreted with caution regarding the obvious baseline differences, particularly the difference in the underlying aetiology of MR (26.9% vs. 61.5% functional). A comparison of technical aspects (e.g., procedural success rate) would therefore clearly be inadmissible, despite statistical adjustments. In contrast, comparing both procedures with regard to their effect on renal function, though clearly still limited, provides relevant insight, as renal function is largely unrelated to technical aspects but rather is a systemic adverse consequence of both procedures. This holds true particularly in the present constellation, where AKI was numerically more common in the procedure that was performed in patients with a much lower risk profile.
Conclusions
Our data show a significant rate of AKI after PMVR, which was associated with a prolonged hospital stay and poorer survival. Accordingly, kidney function should be closely monitored in patients undergoing PMVR. Nevertheless, our data suggest that SMVR carries an even higher risk for AKI, which should be considered when a decision has to be made between the two therapies.
| Impact on daily practice This study shows a surprisingly high incidence of acute kidney injury following PMVR, especially considering that usually no contrast media is used. The occurrence of acute kidney injury was associated with worse patient survival after PMVR. On the other hand, we could see that compared to SMVR the risk of acute kidney injury was significantly lower following PMVR, despite the clinically higher risk profile of this cohort. This could support the clinical decision to perform PMVR over SMVR in otherwise suitable patients. Furthermore, kidney function should be closely monitored postinterventionally. |
Conflict of interest statement
S. Baldus and V. Rudolph have received an unrestricted research grant from Abbott Vascular. The other authors have no conflicts of interest to declare.

