
Abstract
Aims: The aim of this study was to evaluate predictors and safety of next-day discharge (NDD) after transfemoral transcatheter aortic valve implantation (TF-TAVI) in unselected patients receiving either balloon-expandable or self-expanding devices.
Methods and results: From June 2007 to August 2018, 1,232 consecutive patients undergoing TF-TAVI were discharged alive from our institution. They had a mean age of 80.9±5.4 years and an intermediate estimated surgical mortality risk; they received either balloon-expandable (26.1%) or self-expanding prostheses (73.9%). We compared patients discharged within 24 hours from the procedure (n=160, 13.0%) with those discharged later, and accounted for confounding variables through a propensity matching adjustment. After adjustment, no differences in all-cause mortality (1.2% vs 0.0%, for NDD and no-NDD matched groups, respectively, p=0.16) or permanent pacemaker implantation (PPI) after TAVI (0.6% vs 0.6%, respectively) were encountered at 30 days. At one year, no difference in the composite endpoint of all-cause death and heart failure (HF) rehospitalisation was encountered (Kaplan-Meier [KM] estimates 91.9% vs 90.6% for NDD and no-NDD matched groups, respectively, p=0.69). After excluding patients with post-procedural major complications from the unmatched population, prior PPI (OR 2.06, 95% CI: 1.21-3.51; p<0.01) and availability of preprocedural computed tomography angiography (CTA) (OR 1.71, 95% CI: 1.15-2.54; p<0.01) were found to be predictors of NDD after TAVI.
Conclusions: NDD in unselected patients after TF-TAVI using either balloon-expandable or self-expanding devices was demonstrated to be a safe strategy up to one year in the absence of procedural complications. Patients with prior PPI and undergoing preprocedural CTA had a higher chance of NDD.
Introduction
The PARTNER 3 and Evolut R Low Risk randomised trials established the role of transcatheter aortic valve implantation (TAVI) alongside surgery even for treatment of patients with severe aortic stenosis (AS) at low surgical risk1,2. Apart from clear contraindications for each type of intervention as assessed by local Heart Teams, bioprosthesis durability and patients’ preference remain the main factors in the decision-making process for the treatment of severe AS in elderly patients. In recent years, some groups have been working on local programmes which incorporate specific preprocedural, periprocedural and post-procedural pathways aimed at simplification of the TAVI pathway. The objectives of these pathways are to identify a more efficient system for patient assessment screening, to optimise the TAVI procedure without compromising its safety, to accelerate patient recovery and mobilisation after the procedure and to minimise unnecessary use of medical resources3,4,5,6,7.
The purpose of this study was to evaluate the safety of next-day discharge (NDD) after transfemoral TAVI (TF-TAVI) using either balloon-expandable or self-expanding transcatheter aortic valves (TAVs) in an all-comers population and to determine which patients are more suitable for this strategy.
Methods
This was a single-centre, retrospective analysis obtained from a prospective local TAVI registry. All consecutive patients undergoing TF-TAVI in our institution were included. The TAVI procedures were all performed using a minimalist approach, under local anaesthesia and using only angiographic guidance with back-up transthoracic echocardiography. Transthoracic echocardiographic evaluation was performed immediately after the procedure and before discharge. Patients who died during the index hospitalisation were excluded from the analysis.
First, we reported in-hospital and 30-day outcomes of consecutive patients discharged alive from our institution considering the entire population categorised into two groups (NDD group vs no-NDD group); we accounted for any confounding variables through a propensity matching adjustment and evaluated 30-day outcomes and the one-year composite outcome of all-cause death and rehospitalisation for heart failure (HF) of the NDD and no-NDD matched groups.
Second, we assessed predictors of NDD among patients not experiencing periprocedural and in-hospital major or life-threatening complications, including in-hospital myocardial infarction (MI), stroke or transient ischaemic attack (TIA), major vascular complications, major or life-threatening bleeds, more-than-mild acute kidney injury (AKI), new-onset atrial fibrillation (AF) or permanent pacemaker implantation (PPI). All outcomes were reported according to Valve Academic Research Consortium-2 definitions8. The study participant flow chart is shown in Figure 1.
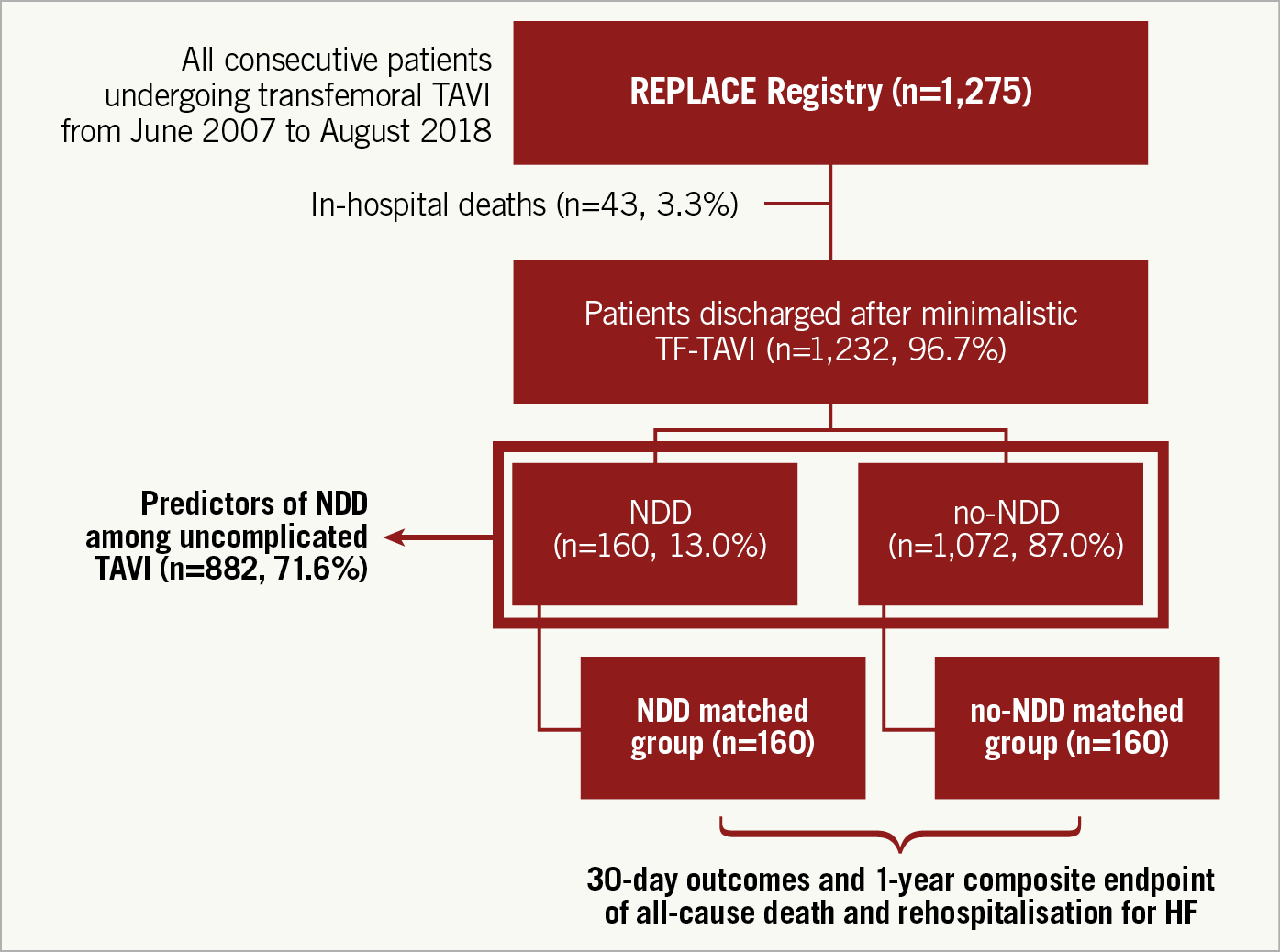
Figure 1. Flow chart of study participants.
STATISTICAL ANALYSIS
Continuous variables are reported as mean±standard deviation (SD), whereas dichotomous parameters are reported as frequencies and percentage. Comparisons were carried out using a Pearson’s chi-square test and t-test for categorical and continuous variables, as appropriate. To adjust for potential bias in treatment assignment, two groups of patients with similar preprocedural characteristics were selected using propensity score (PS) matching with the nearest neighbour method using a non-parsimonious approach. Variables taken into account in the propensity score were: sex, age, body mass index (BMI), hypertension, severe renal impairment, chronic obstructive pulmonary disease (COPD), peripheral artery disease (PAD), prior PPI, Society of Thoracic Surgeons (STS) mortality score, echocardiographic left ventricle ejection fraction (EF) and computed tomography angiography (CTA) assessment. The standardised mean difference plot of PS matching is shown in Supplementary Figure 1. The matching algorithm used in this analysis is implemented in the PS Matching package, version 3.0.4 (IBM Corp., Armonk, NY, USA), whose details have been reported elsewhere. To account for the matched design, the baseline characteristics and the clinical outcomes after matching were analysed using Spearman’s rank correlation and Mann-Whitney U tests between the two groups, as appropriate.
Two-step analysis was used to assess predicting factors. First, a single logistic regression was performed. Variables with a p-value <0.10 were entered into a multiple logistic regression analysis. The results were reported as odds ratio (OR) with a 95% confidence interval (CI).
All statistical tests were performed two-tailed, and a significance level of p<0.05 was considered to indicate statistical significance. The statistical software SPSS Statistics, Version 25.0 (IBM Corp.) was used for all statistical analyses.
CRITERIA FOR NEXT-DAY DISCHARGE
Patients were deemed suitable for an NDD strategy if they had: New York Heart Association (NYHA) Class ≤II; no chest pain attributable to cardiac ischaemia; no untreated major arrhythmias; no fever during the last 24 hours and no signs of an infectious cause; independent mobilisation and self-caring; preserved diuresis (>40 ml/hour during the preceding 24 hours); blood creatinine increase less than 0.3 mg/dL from baseline; stable haemoglobin in two consecutive samples (defined as a decrease of no more than 2 mg/dl); no PVL with aortic regurgitation less than moderate; no stroke/TIA; and no haemodynamic instability.
Results
From June 2007 to August 2018, 1,275 patients underwent TF-TAVI using different devices at our institution. For the purposes of the present analysis, 43 patients (3.3%) who died during the index hospitalisation were excluded from the study.
The study population comprised 1,232 consecutive patients discharged alive after TF-TAVI. Mean age was 80.9±5.4 years and the mean STS mortality score was 4.4±3.4%. TAVI was performed using either balloon-expandable (26.1%) or self-expanding (73.9%) TAVs. Baseline demographic, clinical, electrocardiographic, echocardiographic and preprocedural CTA characteristics of the entire population are summarised in Table 1. Data on the in-hospital length of stay (LoS) of the overall population across the study time period are presented in Figure 2.
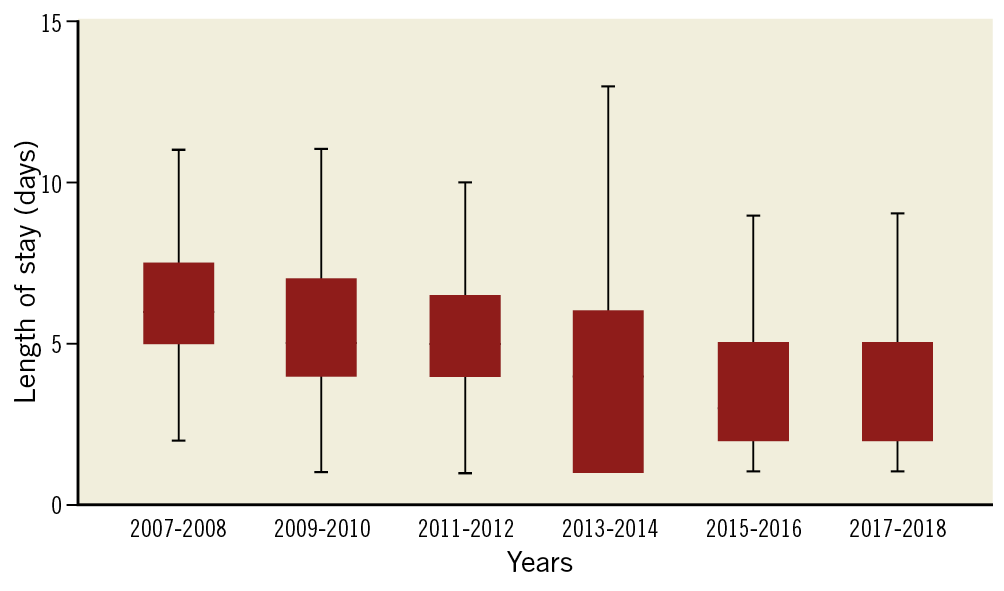
Figure 2. Box plots of post-procedural length of stay (LoS) of the overall population across the study time period.
One hundred and sixty patients (13.0%) were discharged within 24 hours after the procedure. At baseline, NDD patients had a lower incidence of prior stroke (1.3% vs 5.1%, p<0.01), pre-existing atrial fibrillation (AF) (12.6% vs 17.4%, p<0.05), and NYHA Classification III/IV (66.0% vs 74.8%, p<0.05), a higher incidence of prior pacemaker (PM) (16.4% vs 9.3%, p<0.05) and preprocedural computed tomography angiography (CTA) assessment (60.4% vs 44.8%, p<0.01), and a larger native aortic valve area (AVA) (0.7±0.2 vs 0.6±0.2 cm², p<0.01).
All the baseline differences among NDD and no-NDD patients at baseline were resolved after propensity matching analysis (Supplementary Table 1).
Procedural and in-hospital outcomes of the entire population are reported in Table 2 and Supplementary Table 2, respectively. Data on transcatheter aortic valve use and NDD strategy across the study time period are presented in Supplementary Figure 2. Patients discharged at 24 hours after the procedure had a lower contrast volume usage (198±76 vs 217±88 ml, p=0.01). The ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) valve was more frequently used in patients discharged within 24 hours (16.4% vs 8.9%, p=0.01), whereas the CoreValve® (Medtronic, Minneapolis, MN, USA) was used more frequently in those discharged later (26.4% vs 34.9%, p=0.02).
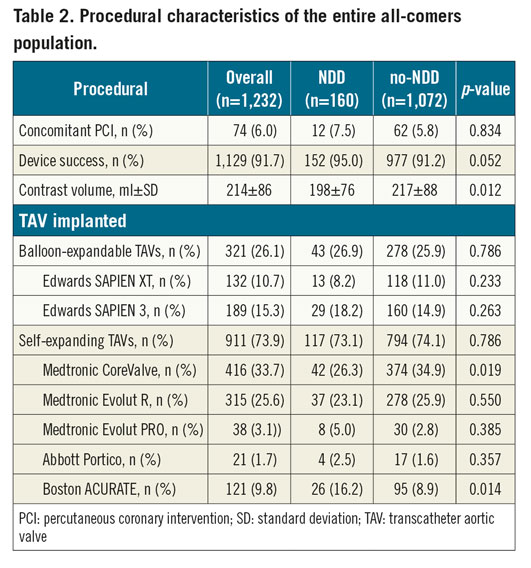
No cases of MI (0.0% vs 0.2%, p=0.51), disabling stroke (0.0% vs 0.7%, p<0.01) or major vascular complication (0.0% vs 10.5%, p<0.01) were reported among NDD patients.
NDD patients showed lower rates of new-onset AF (3.8% vs 7.4%, p<0.01), PPI (2.3% vs 13.5%, p<0.01), AKI (2.5% vs 11.3%, p<0.01) and major or life-threatening bleeding (1.9% vs 20.2%, p<0.01).
The thirty-day outcomes of the entire population are reported in Table 3.
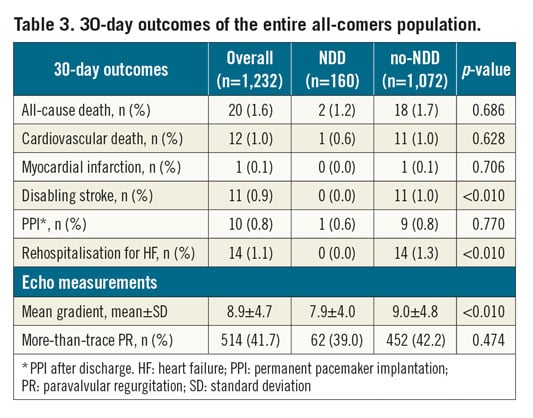
At 30 days, no differences in all-cause (1.2% vs 1.7%, p=0.69) and cardiovascular mortality (0.6% vs 1.0%, p=0.63), or PPI after discharge (0.6% vs 0.8%, p=0.77) were reported between the NDD and no-NDD cohorts. No cases of MI (0.0% vs 0.1%, p=0.71), disabling stroke (0.0% vs 1.0%, p<0.01) or HF rehospitalisation (0.0% vs 1.3%, p<0.01) were reported among NDD patients. Furthermore, patients discharged within 24 hours from TAVI showed a lower mean transvalvular gradient (7.9±4.0 vs 9.0±4.8 mmHg, p<0.01) at 30 days.
After propensity score-matching analysis, no difference regarding all-cause (1.2% vs 0.0% for NDD and no-NDD patients, respectively, p=0.16) and cardiovascular mortality (0.6% vs 0.0% for NDD and no-NDD patients, respectively, p=0.32) was reported between the two groups at 30 days. No cases of MI, disabling stroke or rehospitalisation for HF were reported for either group at 30 days. A trend towards lower rates of PPI after TAVI was observed in the NDD group (2.5% vs 6.9%, for NDD and no-NDD matched groups, respectively, p=0.06) (Table 4).
At one year, no difference in all-cause mortality (6.3% vs 8.1%, p=0.52), stroke (0.6% vs 2.5%, p=0.18) or rehospitalisation for HF (2.5% vs 2.5%, p=1.00) was reported in the NDD and no-NDD matched groups (Table 4). Survival analysis using the Kaplan-Meier (KM) method showed no difference in the composite endpoint of all-cause death and rehospitalisation for heart failure (KM estimates 91.9% vs 90.6% for NDD and no-NDD matched groups, respectively, plog-rank=0.69) (Figure 3).
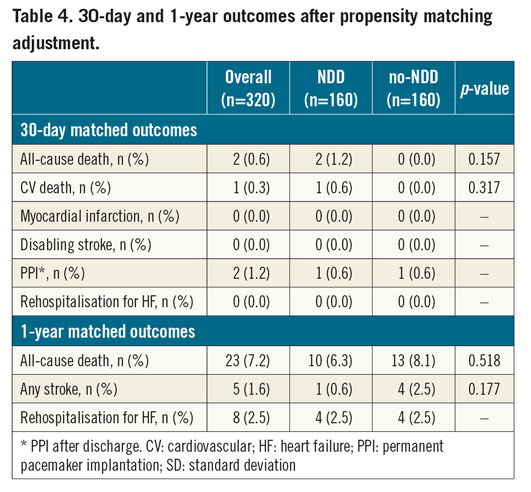
Figure 3. Kaplan-Meier estimates of the composite endpoint of all-cause death and rehospitalisation for heart failure in NDD and no-NDD matched groups at one year.
After excluding patients with procedure-related complications (n=882; 71.6%), NDD patients had a lower incidence of chronic obstructive pulmonary disease (COPD) (20.5% vs 28.4%, p<0.05), a higher incidence of prior PPI (16.4% vs 10.2%, p<0.05) and preprocedural CTA assessment (61.6% vs 40.8%, p<0.01), larger AVA (0.7±0.2 vs 0.6±0.2 cm2, p<0.01) and higher values of haemoglobin at baseline (12.0±1.7 vs 11.6±1.9 mg/dL, p<0.05) (Supplementary Table 3).
Logistic regression of baseline and procedural factors with NDD after TAVI considering patients without procedure-related complications is reported in Supplementary Table 4.
In a multivariate analysis, prior PPI (OR 2.06, 95% CI: 1.21-3.51, p<0.01) and preprocedural CTA assessment (OR 1.71, 95% CI: 1.15-2.54, p<0.01) were associated with discharge within 24 hours from the procedure in patients without procedural complications (Figure 4).
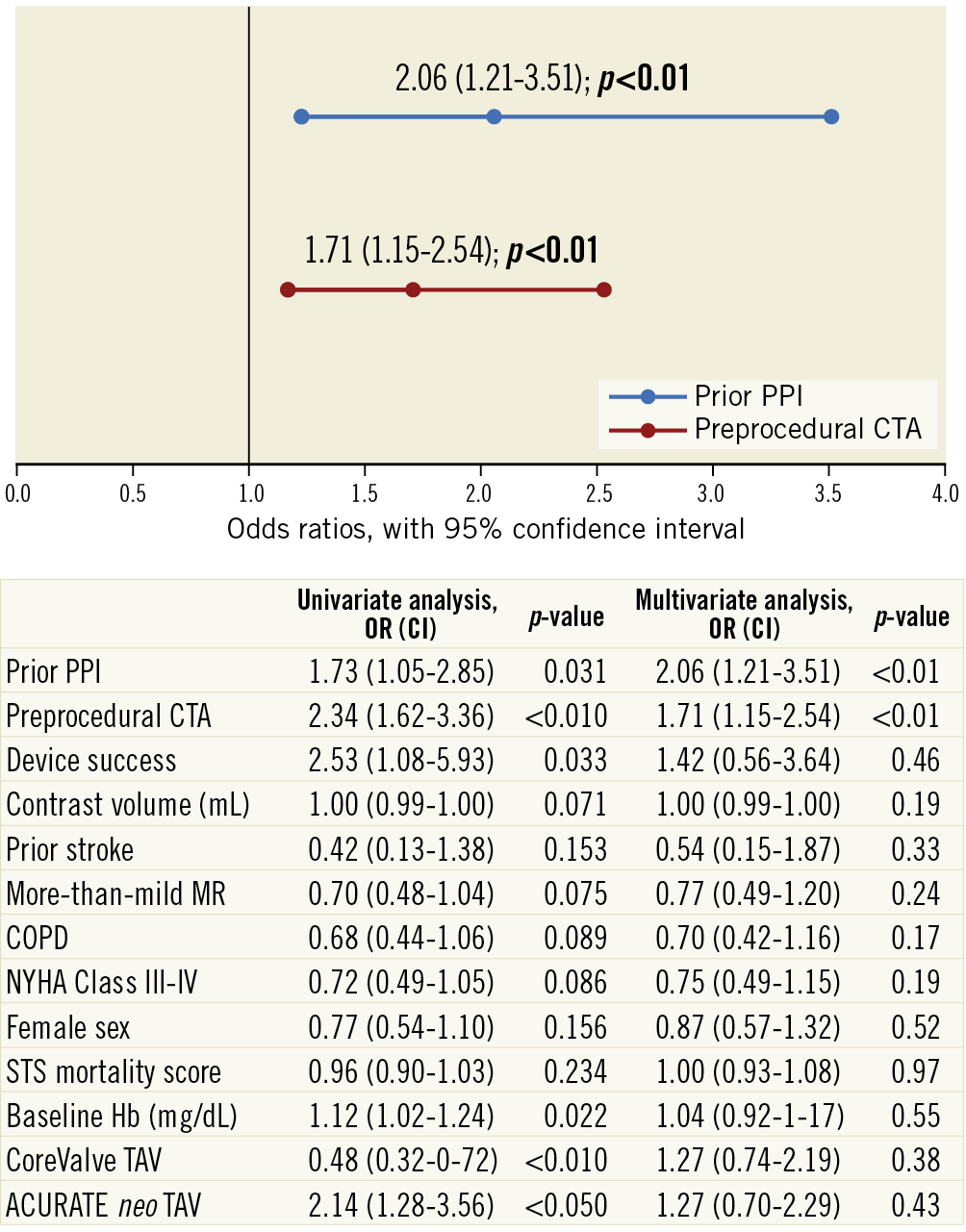
Figure 4. Predictors of next-day discharge after TAVI among patients without procedure-related complications in an all-comers population.
Discussion
To date, the majority of patients undergoing TAVI have been elderly and with many comorbidities; therefore, in most cases, a fast postoperative recovery was challenging to obtain. On the basis of the promising results of the latest trials in younger patients at low surgical risk, the indications for TAVI are expected to increase dramatically1,2. Hence, optimisation of the TAVI procedure and post-procedural pathways is an issue of paramount importance for the near future5. In recent years, the adoption of a minimalist approach (local anaesthesia and conscious sedation under fluoroscopic guidance) has permitted facilitating patients’ mobilisation, shortening postoperative in-hospital stay, and reducing hospitalisation-related complications and costs6,9,10,11,12,13. Recently, two multicentre prospective trials assessed the safety and feasibility of early discharge of patients after TAVI, and an analysis from the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) Transcatheter Valve Therapy (TVT) registry reported a significant increase in mortality for patients with delayed discharge after TAVI1,2,14.
The main findings of our study are as follows. First, early discharge within 24 hours following TF-TAVI using either balloon-expandable or self-expanding devices was feasible in an unselected, “all-comers” population, and was not associated with an increased risk of all-cause mortality or rehospitalisation for HF at one year. Second, among patients who did not experience procedure-related complications, those with prior PPI at baseline and who underwent preprocedural CTA assessment had a higher probability of being discharged within 24 hours.
Hospital stays after TAVI range from 3 to 11 days with an average LoS of at least 6 days in most contemporary trials and registries. The most frequent issues that usually prolong hospitalisations after TAVI are unnecessary prolonged immobilisation, bleeding, conduction disturbances, AKI, and the need for prolonged monitoring due to acute atrioventricular block which is by far the most important one. Early mobilisation promotes a rapid return to baseline status, reduces nosocomial complications, and decreases LoS in frail elderly patients. Several European and North American experiences, including ours, have demonstrated that discharge within 72 hours after TAVI in most of the patients did not compromise the safety of the procedure3,12,13,15,16. This analysis was meant to demonstrate that there is additional room for optimisation of the post-procedural period by discharging patients safely even the day after TAVI. The safety at 30 days of an NDD strategy after TAVI has already been explored in the 3M multicentre prospective trial and in a retrospective study by Kamioka et al. However, both studies included highly selected patients treated with a balloon-expandable TAV with a preprocedural CTA assessment, thus hiding a certain grade of selection in the patient population12,13,15,17,18. The results of the present study are important because the study included different TAVI devices and did not incorporate any selection criteria. Considering the overall population, at 30 days NDD patients showed similar rates of overall (1.2% vs 1.7%, p=0.69) and cardiovascular (0.6% vs 1.0%, p=0.63) mortality, MI (0.0% vs 0.1%, p=0.71) and PPI after discharge (0.6% vs 0.8%, p=0.77), and lower rates of disabling stroke (0.0% vs 1.0%, p<0.01) and HF rehospitalisation (0.0% vs 1.3%, p<0.01) compared to those discharged later. Nevertheless, these differences are probably related to the differences in baseline characteristics between the two cohorts. Indeed, after propensity-matching adjustment, an NDD strategy was not associated with an increased risk of overall (1.2% vs 0.0%, p=0.16) and cardiovascular mortality (0.6% vs 0.0%, p=0.32), stroke (0.0% vs 0.0%), pacemaker implantation (0.6% vs 0.6%) or rehospitalisation (0.0% vs 0.0%) at 30 days. At one year, an early discharge within 24 hours from the index procedure was confirmed to be a safe strategy, as no difference in the composite endpoint of all-cause death and rehospitalisation for HF was encountered (plog-rank=0.69).
In our analysis, we also demonstrated that there was no correlation between using balloon-expandable (26.9% vs 25.9% for NDD and no-NDD patients, respectively) or self-expanding (73.1% vs 74.1% for NDD and no-NDD patients, respectively) devices for TAVI and NDD (p=0.79).
Analysing each TAV, although the ACURATE neo (16.4% vs 8.9%, p<0.05) and the CoreValve (26.4% vs 34.9%, p<0.05) were associated with NDD and no-NDD, respectively, neither of these devices was demonstrated to be an independent predictor of NDD after TAVI at multivariate analysis (p=0.43 and p=0.38, respectively). It might be hypothesised that the observed differences among TAVs simply reflect the tendency to shorten postoperative LoS during recent years (Supplementary Figure 2)14. In fact, the first-generation CoreValve was used at the beginning of our TAVI experience, when the procedure was less standardised and post-procedural management protocols more intensive, whereas the ACURATE neo was the latest device introduced in our practice, when TAVI had already become a streamlined and standardised procedure.
The majority of patients discharged within 24 hours after TAVI did not experience any procedure-related complications (n=146, 91.2%). In an exploratory analysis including only patients free from procedural complications, prior PPI and the presence of preprocedural CTA assessment were found to be predictors of NDD after TAVI.
This finding highlights the importance of pre-procedure planning with high-quality CTA, as it facilitates selecting the most suitable device considering the anatomy of the aortic root and iliofemoral vascular axes, thus permitting in particular a decrease in vascular complication rates18.
The presence of prior PM implantation offers a protection in case of new-onset advanced atrioventricular disease block related to TAVI, avoiding the necessity of close rhythm monitoring after the procedure as well as additional immobilisation for PPI19.
Finally, our study seems to support the feasibility and safety of shortening the LoS up to 24 hours after TAVI even for unselected patients. It also showed that pre-existing PM and preprocedural CTA assessment could help to identify patients who are eligible for this strategy. However, it has to be underlined that discharge timing in some European countries (e.g., Germany) actually clashes with the different reimbursement regimens of each national health system, as in many countries TAVI patients must stay hospitalised for an established minimum number of days after the procedure and therefore cannot benefit from the NDD strategy.
Limitations
The main limitation of our study lies in its single-centre, retrospective design with a relatively small sample size. Furthermore, the influence of unknown confounders cannot be excluded, despite propensity-matching adjustment.
Conclusions
A next-day discharge strategy for unselected patients after transfemoral TAVI was demonstrated to be a safe strategy in the absence of procedural complications. Patients with prior PPI at baseline and undergoing preprocedural CTA assessment had a higher chance of being discharged within 24 hours following the procedure. At one year, no difference in the composite endpoint of all-cause death and HF rehospitalisation was encountered between NDD and no-NDD matched groups.
|
Impact on daily practice On the basis of the promising results of the latest trials on younger patients at low surgical risk, TAVI indications are expected to increase dramatically. Hence, optimisation of the TAVI procedure and post-procedural pathways is an issue of paramount importance for the near future. Recently, the multicentre, prospective 3M and FAST-TAVI trials assessed the safety and feasibility of early discharge (within three days) of selected patients after TAVI using a balloon-expandable device. In our study, we demonstrated that next-day discharge (NDD) of unselected patients undergoing minimalist, transfemoral TAVI is a safe strategy up to one year for those patients not experiencing procedural complications, regardless of the type of prosthesis implanted. Patients with a pre-existing PM at baseline and undergoing preprocedural CTA assessment had a higher chance of being discharged within 24 hours from the procedure. |
Appendix. Study collaborators
Angelo Giuffrida, MD; Division of Cardiac Surgery, Policlinico-Vittorio Emanuele Hospital, C.A.S.T., University of Catania, Catania, Italy. Valeria Garretto, MD; Division of Radiology, Policlinico-Vittorio Emanuele Hospital, C.A.S.T., Catania, Italy. Gianbattista Privitera, MD; Division of Radiology, Policlinico-Vittorio Emanuele Hospital, C.A.S.T., Catania, Italy. Maria Teresa Cannizzaro, MD; Division of Radiology, Policlinico-Vittorio Emanuele Hospital, C.A.S.T., Catania, Italy. Cristina Inserra, MD; Division of Radio logy, Policlinico-Vittorio Emanuele Hospital, C.A.S.T., Catania, Italy. Pierfrancesco Veroux, PhD; Division of Vascular Surgery, Policlinico-Vittorio Emanuele Hospital, C.A.S.T., University of Catania, Catania, Italy.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences and an advisory board member for Biotronik. C Tamburino has received speaker honoraria from Medtronic, Abbott Vascular, Edwards Lifesciences and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

