Abstract
Aims: Treatment pathway optimisation in TAVI should include timely patient discharge with a minimised risk for out-of-hospital adverse events. The aim of this study was to define a standardised set of risk criteria that allows a safe and timely discharge, to validate their appropriateness prospectively in different centres and multiple European countries, and to assess post-discharge outcomes.
Methods and results: We defined and validated the adequacy of a set of discharge criteria and its ability to predict timely and safe discharge properly after the intervention in a prospective, European, multicentre registry. A total of 502 unselected patients were enrolled at 10 sites in three countries. The primary endpoint, defined as a composite of all-cause mortality, vascular access-related complications, permanent pacemaker implantation, stroke, re-hospitalisation due to cardiac reasons, kidney failure and major bleeding at 30 days, was reached in 12.9% of patients (95% CI: 11.3-16.5). The overall 30-day mortality was 1.1% (95% CI: 0.2-2.0), and the rates of stroke/TIA 1.7% (95% CI: -0.6 to 4.0), PPI 7.3% (95% CI: 5.8-8.9), major vascular complications 1.9% (95% CI: 0.7-3.1), major/life-threatening bleeding 2.4% (95% CI: 1.0-3.8) and cardiac re-hospitalisation 3.7% (95% CI: 1.4-6.0). Patients appropriately discharged early had a significantly lower risk of the primary endpoint (7.0 vs. 26.4%; p<0.001) which was reflected in some of its relevant components: stroke (0.0 vs. 2.8%; p=0.015), PPI (4.3 vs. 15.9%; p<0.001), major vascular complications (0.3 vs. 4.7%; p=0.004) and major/life-threatening bleeding (0.3 vs. 6.5%; p<0.001).
Conclusions: We validated the appropriateness of a pre-specified set of risk criteria that allows a safe and timely discharge. The rate of 30-day complications did not reveal any risk increase with this strategy compared with the reported outcomes in major TAVI trials and registries. ClinicalTrials.gov Identifier: NCT02404467
Introduction
TAVI is an established treatment for severe symptomatic aortic stenosis1. Current treatment pathways result in a mean duration of hospitalisation of between six and seven days in France to 16 days in Belgium2,3.
The adoption of well-standardised TAVI-specific clinical care pathways can help to identify suitable candidates for early discharge without complications3,4,5. In selected patients deemed as being at low risk for complications, there is no increase in 30-day mortality, disabling stroke, bleeding, permanent pacemaker implantation (PPI) or readmission when discharged early3,5. Furthermore, appropriate early discharge has been demonstrated to improve patient outcomes and quality of life after surgical and interventional procedures. In fact, over the last decade, the duration of hospitalisation after TAVI has steadily decreased3.
It is, however, obvious that neither a generally minimised length of stay (LoS) nor a standard prolongation of hospitalisation appropriately considers individual patient characteristics and medical needs. Optimising the LoS needs to result in a balance of the potential benefits of early discharge (reduced hospital-borne complications, accelerated patient recovery and mobilisation, lower costs) and the potential benefits of late discharge which are related to the timely detection and appropriate treatment of infrequent complications such as arrhythmia as well as vascular and bleeding complications in the hospital setting6,7.
The rationale for further investigating the optimisation of LoS post-TAVI was born from the desire to define a standardised set of risk criteria that allows a safe and timely discharge, to validate their appropriateness prospectively in different centres and multiple European countries, and to assess post-discharge outcomes.
Methods
The FAST-TAVI registry is an observational, prospective, multicentre project. It has been conducted at five sites in Italy, two sites in the Netherlands and three sites in the UK. All centres involved had no administrative restrictions and reimbursement issues potentially affecting post-procedural LoS8. The registry was approved by the independent ethics review board at each of the participating institutions and all patients provided written informed consent.
PATIENT SELECTION
In brief, patients undergoing transfemoral TAVI with the SAPIEN 3 transcatheter heart valve (Edwards Lifesciences, Irvine, CA, USA) were enrolled. Valve selection was restricted according to the potential bias introduced by different valves on the rate of PPI or other relevant complications. Catania was the first centre to enrol patients starting in May 2015 while Cambridge started enrolment in May 2017. Patients were enrolled until the recruitment goal of a mean of 50 patients per centre was reached in November 2017. The decision to perform the procedure, as well as the date of patient discharge, was made by the local team at each institution adhering to medical judgement and standard local practice.
DISCHARGE CRITERIA
According to the protocol, a list of pre-specified criteria was considered after TAVI to define a low risk of complications after early discharge, including NYHA Class ≤II, no chest pain attributable to cardiac ischaemia, no untreated major arrhythmias, patients having complications on day 0 to 1, but free of signs or symptoms on day 3, no fever during the last 24 hours and no signs of an infectious cause, independent mobilisation and self-caring, preserved diuresis (>40 ml/hour during the preceding 24 hours), no unresolved acute kidney injury type 3 (according to VARC-2 criteria), no red blood cell (RBC) transfusion during the preceding 72 hours, stable haemoglobin in two consecutive samples (defined as a decrease of no more than 2 mg/dl), no paravalvular leak (PVL) with aortic regurgitation less than moderate, no stroke/transient ischaemic attack (TIA), and no haemodynamic instability.
OBJECTIVES
The primary objective of the study was to determine the incidence of a composite of VARC-2 defined all-cause mortality, vascular access-related complications, PPI, stroke, re-hospitalisation due to cardiac reasons, kidney failure and major bleeding, occurring during the first 30 days after hospital discharge. The secondary objective prospectively tested a set of pre-specified risk criteria for out-of-hospital complications after early discharge (≤72 hours) post-procedure.
STATISTICAL ANALYSIS
The data are presented descriptively, using means with standard deviation (SD), medians with interquartile range (IQR) or absolute values with percentages. Comparisons were made using a Pearson’s chi-square or Fisher’s exact test for categorical variables. For continuous variables, a t-test or Mann-Whitney-Wilcoxon rank-sum test, and ANOVA for comparison of more than three groups was used. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
PATIENT CHARACTERISTICS AND PROCEDURAL DETAILS
Between May 2015 and October 2017, a total of 502 patients were enrolled, of whom 499 underwent a procedure (Figure 1).
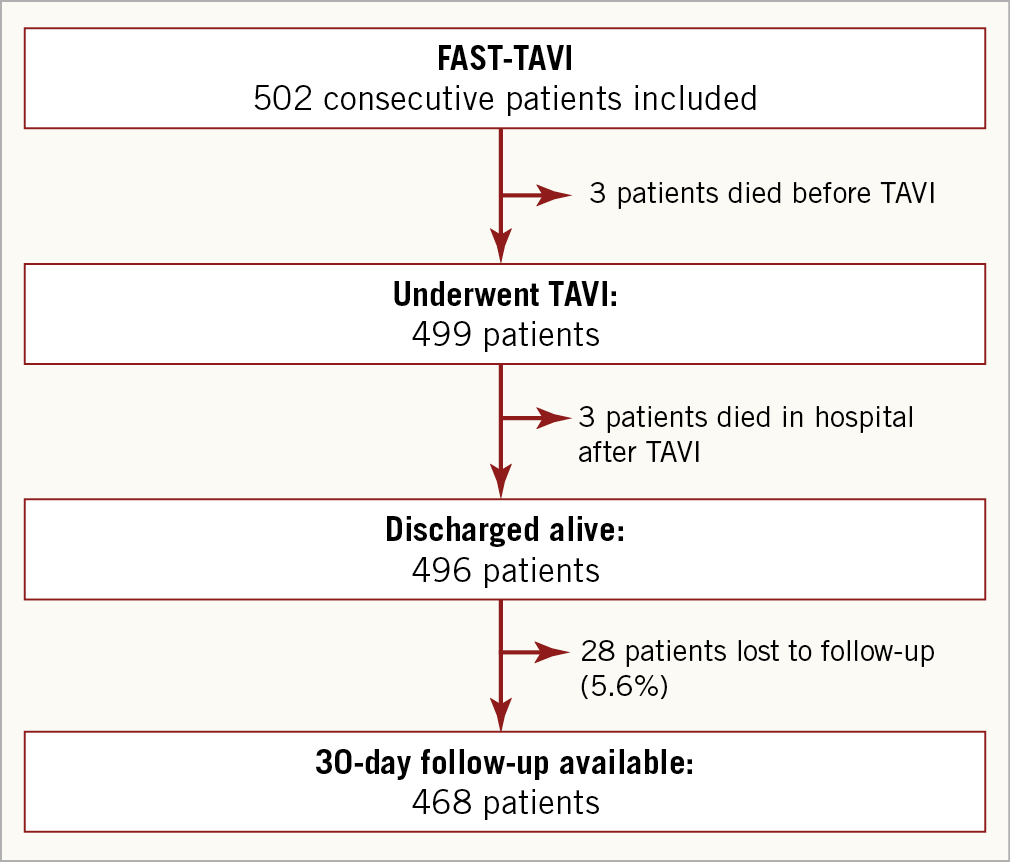
Figure 1. Patient flow chart.
Patients undergoing TAVI (n=499) had a mean age of 81.4±6.0 years, 48.2% were female and the mean EuroSCORE II was 5.0±6.0% . A minority of patients had undergone prior cardiovascular intervention. More than half of the study population were in NYHA Class III/IV (53.3%) and 5.0% had angina CCS III/IV.
Overall, 82.8% of patients underwent transfemoral TAVI with conscious sedation or local analgesia. Three patients (0.6%) required the use of a second valve during the TAVI procedure. Five patients required conversion to open heart surgery (1.0%).
ACTUAL DISCHARGE MANAGEMENT BASED ON RISK
After the TAVI procedure, 68.6% of patients were admitted to the coronary intensive care unit (CICU), 18.8% to an intermediate care unit and 12.6% directly to the general ward (Supplementary Table 2). Overall, three patients died in-hospital post-TAVI (0.6%) and 99.4% (496/499) were discharged (Table 1). The median LoS was 2 (IQR 1-4) days. Of the patients who were discharged, 26.8% (n=133) were discharged within one day, 51.0% (n=253) within two days and 72.6% (n=360) within three days. The majority of patients were discharged home (79.0%) with the other patients mostly being transferred to the referring hospital.
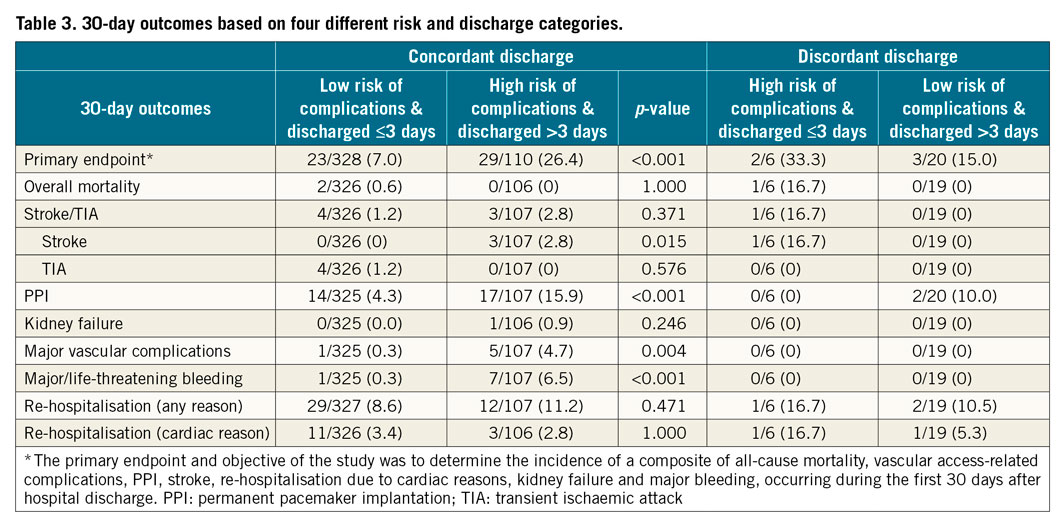
A total of 71.0% of patients with low-risk criteria for complications after discharge were actually discharged early; 23.5% were assessed as being high-risk for complications and were discharged late (resulting in an appropriate discharge rate of 94.5%). Discordant discharge (time to discharge inconsistent with the specified risk of discharge) was only seen in 5.5% of patients, comprising high-risk for out-of-hospital complications but still discharged early in 1.2% of patients and discharged late despite being at low risk in 4.3% of patients (Figure 2). The principal reasons for a prolonged hospitalisation (>72 hours) included logistical reasons (34.8%), conduction disturbances (25.9%) and bleeding complications (16.3%). Other predefined reasons also cited as a reason for prolonged hospitalisation included delayed mobilisation as well as renal and vascular complications (Table 1). The principal reason for late discharge in the 20 patients considered to be at low risk of complications was logistics (17/20; 85.0%). Two further patients were kept in hospital due to “mobilisation problems” (2/20; 10.0%) and one patient because of “vascular problems” (1/20; 5.0%).
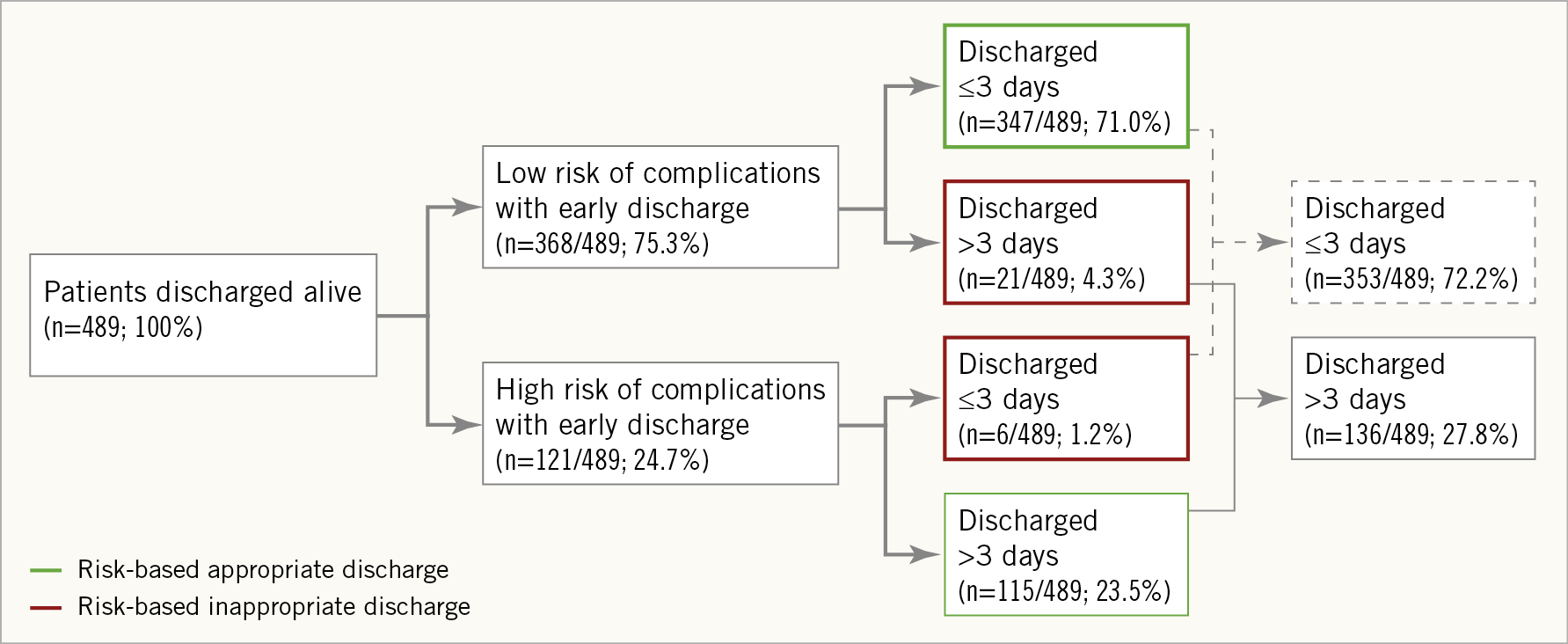
Figure 2. Patient discharge by risk. Includes all patients alive at discharge; excludes seven patients for whom risk status at discharge was unknown. Definitions for high and low risk complications: see Methods section. Green, desired discharge based on risk; red, undesirable discharge based on risk.
THIRTY-DAY OUTCOMES AS A RESULT OF PREDEFINED DISCHARGE CRITERIA
Of the 496 patients discharged alive (Figure 1), 468 were available for the 30-day follow-up (94.4%). The primary endpoint was reached in 12.9% of patients (95% CI: 11.3-16.5). The overall 30-day mortality was 1.1%, and the rates of stroke/TIA 1.7%, PPI 7.3%, major vascular complications 1.9%, major/life-threatening bleeding 2.4% and cardiac re-hospitalisation 3.7% (Table 2). Of those re-hospitalised, 4.8% had valve-related reasons, 9.5% were admitted because of worsening heart failure, 40.5% had other cardiac reasons and 54.8% were not considered cardiac (two patients with TIA, one patient with bleeding complications, the others being unrelated).
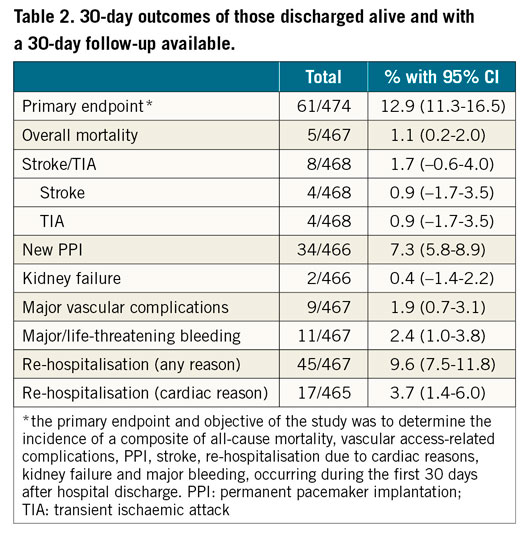
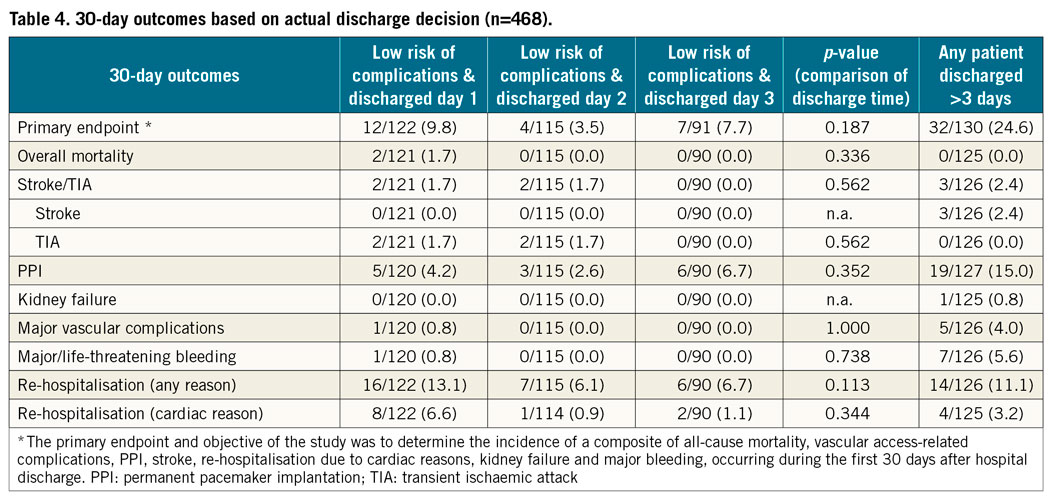
Patients discharged early because of a perceived low risk of complications (Table 3) actually had a significantly lower risk of the primary endpoint (7.0 vs. 26.4%; p<0.001), which was reflected in some of its relevant components: stroke (0 vs. 2.8%), PPI (4.3 vs. 15.9%), major vascular complications (0.3 vs. 4.7%) and major/life-threatening bleeding (0.3 vs. 6.5%). A comparison of out-of-hospital events is provided in Supplementary Table 3. No significant increase in the rate of out-of-hospital adverse events was noted with early discharge in patients at low risk of complications. A separation of patients with low risk and early discharge into those discharged within one, two or three days revealed a nominal risk increase for the primary endpoint and for the rate of re-hospitalisation in those discharged very early (Table 4).
COUNTRY-SPECIFIC DIFFERENCES
More patients were enrolled in Italy (n=264) than in the Netherlands (n=127) and UK (n=111). In the Italian group, three patients died prior to the TAVI procedure. Overall, three patients died in hospital (one patient in the Italian group and two patients in the UK group). In the Dutch and UK groups, 51.2% and 49.5% of patients were discharged post TF-TAVI within 24 hours, compared with only 5.4% of patients in the Italian group (Supplementary Table 4). The majority of all patients had early discharge (ED) (≤72 hours) – 61.9% of patients in Italy, 80.3% of Dutch patients and 89.0% of patients in the UK.
Most patients were discharged home in Italy (92.3%) and the UK (93.6%), while many patients were transferred back to the referring hospital in the Netherlands (58.3%) (both p<0.001 across groups).
There were no statistically significant differences in the primary outcome at 30 days among the three countries. The rate of re-hospitalisation was, however, higher in the Netherlands (14.9%) and UK (13.3%) compared to Italy (5.7%) (p=0.006). Re-hospitalisation for cardiac reasons was substantially higher in the Netherlands (12.3%) than in Italy (0%) and the UK (2.9%) (p<0.001).
Clinical events at 30 days of those actually discharged were low in general, but there were between-country differences in pacemaker rates (lowest 1.9% in the UK) and re-hospitalisation (lowest 5.7% in Italy).
Discussion
A predefined set of risk criteria was able to guide discharge decisions appropriately for patients undergoing TF-TAVI with a balloon-expandable valve in multiple centres across Europe. The criteria allowed the appropriate selection of patients eligible for early discharge because of a low risk of out-of-hospital complications as well as the identification of those patients most probably benefiting from a longer hospitalisation. An optimised discharge strategy appeared to be associated with a low rate of adverse events and a low re-hospitalisation rate.
DISCHARGE CRITERIA
The potential for the optimisation of hospital discharge after TAVI has previously been investigated in a single centre3,4,9 and one multicentre study10. The first3 was a retrospective analysis from Italy where factors indicating low risk for early discharge (≤3 days) were identified from multivariable analysis. The second4 reported on the feasibility of safe early discharge (≤3 days) of patients who had undergone TAVI. The primary endpoint (death and re-hospitalisation at 30 days) was reached in a comparable number of patients in the early and late discharge groups. The investigators analysed predictive factors of early discharge using multivariable analysis of the baseline criteria. The third publication9 prospectively assessed the eligibility for discharge after TF-TAVI within 72 hours. Fifty-nine percent (59%) were discharged early. This was considered safe as the mortality was one in 130 patients at 30 days and three (4%) were re-hospitalised.
Based on the evidence described above, a prospective assessment of predefined early discharge criteria was warranted to optimise post-TAVI outcomes. However, an approach different from the recently presented 3M TAVR study was chosen10. The 3M study excluded a significant number of patients not meeting predefined criteria with regard to life expectancy, computed tomography (CT) quality, vascular access, iliofemoral artery size, clinical stability and mobilisation, language skills, level of social support, cognitive function, and airway anatomy. In contrast, FAST TAVI included unselected patients undergoing transfemoral TAVI with SAPIEN 3, where individual patient risk and the actual risk of complications with early or late discharge was assessed after the intervention. The post-interventional assessment was then compared to the actual discharge pattern and 30-day outcomes analysed. Our two groups (low risk and early discharge, and low risk and late discharge) would be comparable with those included in the 3M study, while our high-risk patients would have been excluded from 3M.
OUTCOMES
Clinical outcomes after TF-TAVI have steadily improved over time. Major studies and registries11,12 published in recent years (2014 to 2016) report 30-day rates of mortality as low as 1.1 to 5.1%, stroke rates of 1.7 to 6.4%, cardiac readmission rates of 4.3 to 6.5% and new pacemaker rates of 8.5 to 27.0%. Reported rates for 3M were at the lower end of this range (1.5%, 1.5%, 5.7%, and 5.7%, respectively)10 but were the result of the aforementioned selection of lower-risk patients in the trial. Our event rates in an unselected patient population (including those with high risk of complications when discharged early) confirmed the adequacy of our predefined discharge criteria in that rates of mortality at 30 days (1.1%), stroke (1.7%), re-hospitalisation for cardiac reasons (3.7%), and new pacemaker (7.3%) were all lower than in the unselected large registries referred to above. A word of caution is warranted, however, as strategies to recognise the need for pacemaker implantation early before may decrease PPI after TAVI.
We further explored the pre-specified discharge criteria and showed that the majority (>70%) of patients undergoing TF-TAVI can be safely discharged within three days post intervention if this set of pre-specified criteria is applied. Furthermore, our study indicates that patients at low risk of post-discharge complications are actually at a very low risk of death, re-hospitalisation, PPI, major vascular complications and life-threatening bleeding, even below the above-mentioned rates. While delayed discharge is medically mandated for some patients, for approximately one third of patients delayed discharge was due to logistical (non-medical) reasons.
COUNTRY ANALYSIS
Our study also confirms that timely discharge of patients who have undergone TF-TAVI can be performed safely in different European geographies (80.3% of patients in the Netherlands and 89.0% of patients in the UK). A lower percentage of patients (61.9%) was discharged early in the Italian cohort, which is probably due to the fact that those patients were sicker at study enrolment (including a higher percentage of patients in NYHA Class III/IV and patients with higher aortic valve [AV] peak and mean gradients) and were more frequently discharged home rather than to a referring hospital as documented for the Netherlands.
While the multinational nature of this study is a key advantage as it increases the applicability of our findings to other countries, it has to be noted that differences in the healthcare systems might have an impact on LoS after TAVI. It is also possible that there could be variations in patient management and treatment between institutions within the same country. There is a further project underway in France (ClinicalTrials.gov Identifier: NCT02956915) to determine average LoS post TF-TAVI. The results of the French study may add further support to the development of guidance for specific discharge criteria post TF-TAVI.
Limitations
While patients were unselected with respect to their potential risk after discharge, inclusion was restricted to patients undergoing TF-TAVI using the Edwards SAPIEN 3 valve. A specific valve and access route were mandated in the protocol as different valves are associated with, for example, differing pacemaker rates and this is one of the key early discharge criteria. Centres participating started recruiting patients at different time points, resulting in a prolonged recruitment period spanning three years. This may have resulted in a loss of consecutiveness but will not interfere with the objective of the study. Finally, as in every registry, data completeness may not be achievable for every variable, but in this study the key variables for the current manuscript were available in >90% of patients.
Conclusions
The FAST-TAVI registry validated the appropriateness of a pre-specified set of risk criteria that allow a safe and timely discharge. The rate of 30-day complications did not reveal any risk increase with this strategy compared with the reported outcomes in major TAVI trials and registries.
|
Impact on daily practice The data support previous findings that, in selected patients, optimising discharge post TAVI is feasible, safe and beneficial to the patient13. As LoS appears to be an important contributor to the overall cost of the procedure2, a decrease would be associated with a significant decrease in treatment costs6,7. As well as being medically advantageous for patients, optimising LoS would reduce procedure-related and resource utilisation costs as well as other risks associated with prolonged hospitalisation14. In order to facilitate optimised timely discharge, the logistics of patient discharge should be planned in advance of the patient admission. |
Acknowledgements
Data were captured using the s4trials software provided by Software for Trials Europe GmbH, Berlin, Germany. F. Iacovelli is currently attending the Cardiopath PhD programme. The authors wish to thank Rodolfo Estay, Sandra Lauck, David Wood, Michelle McEvoy, and Lenka Sykorova for their tremendous help with the study.
Funding
This work was funded by a research grant provided by Edwards Lifesciences (Nyon, Switzerland) to the Institute for Pharmacology and Preventive Medicine (IPPMed).
Appendix. Study collaborators
Jan Baan, MD; Cardiology Department, Amsterdam UMC, University of Amsterdam, the Netherlands. Jana Kurucova, MD; Edwards Lifesciences, Nyon, Switzerland. Martin Thoenes, MD; Edwards Lifesciences, Nyon, Switzerland. Claudia M. Lüske, PhD; Institute for Pharmacology and Preventive Medicine, Cloppenburg, Germany. Cornelia Deutsch, MD; Institute for Pharmacology and Preventive Medicine, Cloppenburg, Germany. Giuliano Costa, MD; Cardiology Department, Policlinico-Vittorio Emanuele Hospital, University of Catania, Italy. Colum G. Owens, MD; Cardiology Department, Royal Victoria Hospital, Belfast, United Kingdom.
Conflict of interest statement
P. Bramlage represents IPPMed, Cloppenburg. M. Barbanti is a consultant for Edwards and advisory board member for Medtronic. M. Spence is a proctor and consultant for Edwards. D. Muir is a proctor for Edwards. The other authors have no conflicts of interest to declare.

