
Abstract
Aims: The aims of this study were to determine the incidence and correlates of left ventricular (LV) dysfunction amongst percutaneous patent ductus arteriosus (PDA) device closure patients, and to propose an indexed parameter for predicting LV dysfunction.
Methods and results: In a retrospective cross-sectional analysis of 30 months duration, 447 patients who underwent PDA device closure were studied. The diameter of the PDA at the pulmonary artery end was measured in the angiograms in all patients and was indexed for their body surface area. The indexed PDA size was categorised into group A (1-2.9 mm/m², 35/447), B (3-5.9 mm/m², 254/447), C (6-8.9 mm/m², 66/447) and D (>9 mm/m², 35/447). Systolic LV function was evaluated using echocardiography at frequent intervals. Overall, 62.63% of the patients were female (280/447). At baseline, all 447 patients had normal LV function. LV dysfunction was seen in 102/447 (22.8%) patients with 2.8% in category A (1/35), 10.6% in category B (27/254), 34.1% in category C (42/123) and 91.4% in category D (32/35) after PDA device closure. Correlation of indexed PDA size and LV dysfunction was statistically significant (p<0.05).
Conclusions: Accurate prediction of LV dysfunction is important in risk stratification, ICU management and counselling in PDA device closures. Indexed PDA size correlates well with post-procedural LV dysfunction. The authors propose a new classification of PDA utilising this accurate, reproducible and easy to perform parameter, which does not involve any extra cost, for risk stratification and early management in device closure of PDA.
Abbreviations
ACE: angiotensin-converting enzyme
BSA: body surface area
EF: ejection fraction
ESC: European Society of Cardiology
ICU: intensive care unit
LV: left ventricle
PDA: patent ductus arteriosus
Qp: pulmonary blood flow
Qs: systemic blood flow
SPSS: Statistical Package for Social Sciences
Introduction
Patent ductus arteriosus (PDA) is a post-tricuspid shunt lesion with a left-to-right shunt. A sizeable PDA can cause increased preload of the left heart, resulting in remodelling and an altered Frank-Starling curve1.
Percutaneous transcatheter closure of PDA has evolved over the last four decades and has become the “standard of care” in most patients2,3. Various studies have confirmed the immediate deterioration of systolic4,5 and diastolic6 dysfunction of the left ventricle (LV) which may persist for a few months after the successful device closure of the PDA. However, there is no reliable indicator for predicting which patients are likely to experience LV dysfunction after device closure of the PDA.
The present study was designed to investigate if such a prediction is possible by simplifying the classification of PDA through indexing for body surface area (BSA).
Methods
In a retrospective cross-sectional analysis of 30 months duration from April 2013 to September 2015, patients who underwent PDA device closure at Narayana Institute of Cardiac Sciences, Bangalore, India, were studied. As per protocol, all of these subjects underwent detailed preprocedural and post-procedural transthoracic echocardiography delineating the anatomy of the PDA, and left ventricular dimensions and function, using VIVID™ 7 echocardiography machines (GE Healthcare, Chicago, IL, USA). Along with the echocardiography, all the patients had a standard 12-lead electrocardiogram and chest radiograph at the initial assessment. Just prior to admission for the interventional procedure, all patients were subjected to a complete haemogram, blood and serum biochemistry, coagulation indices such as prothrombin time with international normalised ratio along with partial thromboplastin time, hepatic and renal functions. Each patient was inducted into the procedure only when all of these reports were found to be normal.
Patients with a silent PDA were not offered any therapeutic intervention. PDA patients who had any co-existing congenital cardiac lesions, those who had clinical features of irreversible pulmonary vascular disease, those with a bizarrely shaped PDA which may not have been compatible with device closure, or those with pre-existing LV dysfunction prior to the procedure were excluded from the study. The study was approved by the institutional ethics committee (NHH/AEC-CL-2016-028, dated 23 March 2016), and complied with the Declaration of Helsinki. Informed consent was not applicable as the study was retrospective in design.
The body surface area of every patient was calculated using the DuBois formula with the help of online calculators and rounded off to the second decimal place. The majority of the patients underwent device deployment through the femoral venous approach, and very few through the retrograde route via the arterial end. The PDA was crossed in antegrade fashion. Angiographic evaluation of the PDA was carried out by placing a pigtail catheter in the descending thoracic aorta. The better of the standard lateral view or the right anterior oblique view was chosen to calibrate the size of PDA at the pulmonary arterial end. The best size of the PDA at the pulmonary arterial end was documented in millimetres and rounded off to the first decimal place. The size of the PDA was divided by the patient’s BSA to calculate the “indexed PDA size”. This number was rounded off to the first decimal place and expressed in mm/m2.
An appropriately sized AMPLATZER™ Duct Occluder (St. Jude Medical, St. Paul, MN, USA) or Cera™ PDA Occluder (Lifetech Scientific Co., Ltd., Shenzhen, China) was used for closing the PDA.
Immediately following device deployment but before releasing, echocardiography was performed to assess the location, orientation and stability of the device. Assessment was carried out for residual leaks across the device. Flow and pressure patterns across the left pulmonary artery and descending thoracic aorta were evaluated in at least two transthoracic views. The PDA device was released only when all these parameters were found to be safe and satisfactory. A descending aortogram with the pigtail catheter placed proximal to the location of the device was performed ten minutes after release of the device to ensure the appropriate device location and orientation, and to look for residual shunts.
All patients who underwent the procedure were transferred to the intensive care unit (ICU) for monitoring. Echocardiography was performed on arrival at the ICU to ensure overall post-procedural stability. At the ICU, routine monitoring of vital parameters was carried out as per standard protocol. Six hours after arrival at the ICU, another echocardiogram was performed. The LV ejection fraction (EF) was measured using the modified Simpson’s method in the apical four-chamber view by averaging three cardiac cycles. An ejection fraction of less than 55% was defined as LV systolic dysfunction. The LV systolic dysfunction was categorised as mild (LVEF 40-54%), moderate (LVEF 30-39%) or severe (LVEF <30%). Those with good LV function and successful device closure of the PDA were moved out of the ICU to the ward. Those who had any LV systolic dysfunction were kept in the ICU.
Mild LV dysfunction was treated with diuretics and angiotensin-converting enzyme (ACE) inhibitors (n=69/447; 15.4%), whereas those with moderate or severe LV dysfunction (n=33/447; 7.4%) were given additional inotropic support, as per the latest available European Society of Cardiology (ESC) heart failure guidelines7, in recommended doses adjusted for body weight. Six-hourly echocardiography was continued in this cohort until LV function normalised. Normal LV function was defined as an LVEF of >55% in two successive echocardiography examinations at least six hours apart.
At 24 hours post procedure, detailed echocardiography was performed in all of the patients, irrespective of their LV function recovery. The same VIVID 7 echocardiography machine was used for the assessment. Tabulated data had the following parameters: patient demographics, age, gender, height, weight, BSA, measured size of PDA on angiography, indexing of that PDA size, Qp/Qs, make of PDA occluder used, approach used for device closure, and sequential LV function assessment after PDA device closure.
Statistical Package for Social Sciences, Version 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The primary correlates were indexed PDA size and LV function. Univariate analysis using Pearson’s correlation coefficient was used to correlate the relationship between the variables. The results were tabulated in a master chart using an MS Excel data sheet. Apart from the entire group, univariate analysis using Pearson’s correlation coefficient was extended for each subgroup of indexed PDA sizes. P-value was tabulated using the correlation coefficient. A p-value of <0.05 was considered significant.
Results
A total of 447 patients were inducted into the study. The mean age of this cohort was 68.4 months (range two to 700 months) with a median age of 36 months. Overall, 318 patients were aged under five years. There were 279 female patients and 168 male patients, giving a female predominance of 1.66. The mean weight of the study cohort was 16.04 kg (range 3.7 to 76 kg) with a median weight of 10.8 kg. The under-10 kg category constituted 38% of the patients (n=170). The mean duration of stay in hospital was 4.53 days (range two to 22 days) with a median of four days. There was no mortality in the entire cohort.
The mean size of the PDA in the study cohort was 3.5 mm (range 1.5 to 14 mm) with a median of 3 mm. Correspondingly, the mean indexed PDA size was 5.6 mm/m2 (range 1.7 to 20.3 mm/m2) with a median of 5.1 mm/m2 and a standard deviation of 2.6.
The AMPLATZER Duct Occluder was deployed in 26.6% of patients (n=119/447). A Cera PDA Occluder was used in the remaining 73.4% of the study population (n=328/447). An antegrade approach was used in 439 patients (98.2%) and the remaining eight patients had a retrograde approach for device deployment (1.8%).
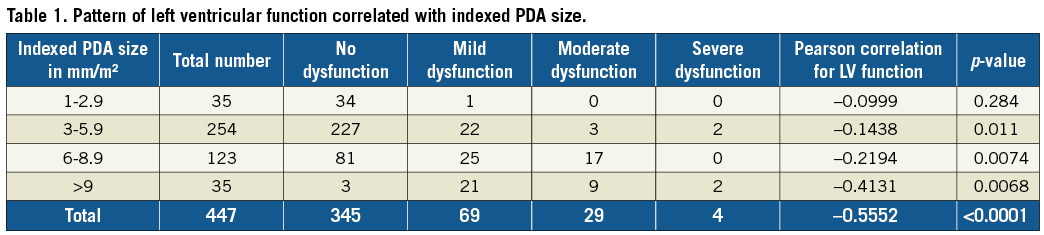
Based on the indexed PDA size, the cohort of 447 patients was divided into four categories: group A (1 to 2.9 mm/m2), group B (3 to 5.9 mm/m2), group C (6 to 8.9 mm/m2) and group D (more than 9 mm/m2). The LV dysfunction patterns were studied in each of these groups and statistically correlated with indexed PDA size (Figure 1).
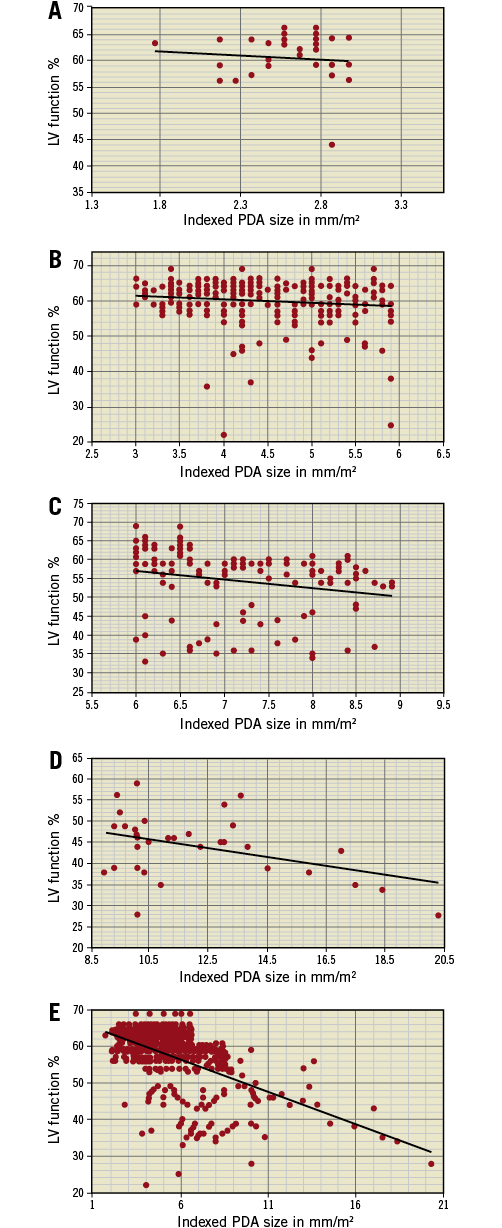
Figure 1. These five graphs represent indexed PDA size on the X-axis plotted against LV function after device closure of PDA on the Y-axis. Orientation represents relative decline in LV function as the indexed PDA size increases. Data for group A (n=35/447) (panel A), group B (n=254/447) (panel B), group C (n=123/447) (panel C), group D (n=35/447) (panel D) and for the entire cohort of 447 patients (panel E) are plotted.
Group A with an indexed PDA size of 1 to 2.9 mm/m2 had 35 patients. Of these, 34 patients had normal LV function after the procedure. One patient had mild LV dysfunction which recovered in five days post procedure. The Pearson correlation between indexed PDA size and LV function in this group was –0.099 with a p-value of 0.284 (not significant for p<0.05) (Figure 1A).
Group B with an indexed PDA size of 3 to 5.9 mm/m2 had 254 patients. Among these, 227 had normal LV function after the procedure. Twenty-two patients had mild LV dysfunction, with an average stay of 3.4 days (range two to 10 days) after the procedure for normalisation of LV systolic function. Three patients had moderate LV dysfunction which recovered in an average of four days (range two to six days). Two patients had severe dysfunction which took six days to normalise. The Pearson correlation between indexed PDA size and LV function in this group was –0.144 with a p-value of 0.011 (significant for p<0.05) (Figure 1B).
Group C with an indexed PDA size of 6 to 8.9 mm/m2 had 123 patients. Among these, 81 had normal LV function following the procedure. Mild dysfunction was seen in 25 patients and it took an average of 2.8 days (range two to six days) before the LV function normalised following the procedure. The remaining 17 patients had moderate LV dysfunction and recovered in an average of 5.5 days (range two to 18 days) after the procedure. No patient in this group had severe LV dysfunction. The Pearson correlation between indexed PDA size and LV function in this group was –0.219 with a p-value of 0.0073 (significant for p<0.05) (Figure 1C).
Group D consisted of 35 patients with an indexed PDA size of 9 mm/m2 and above. The average indexed PDA size in this cohort was 12.2 mm/m2. Only three patients in this group had normal LV function after the procedure. Twenty-one patients had mild dysfunction and recovered in an average of 6.5 days (range two to 16 days) after the procedure. Moderate LV dysfunction was observed in nine patients, which took an average of 10 days (range three to 22 days) to normalise following the procedure. Two patients had severe LV dysfunction; they each recovered in seven days after the procedure. The Pearson correlation between indexed PDA size and LV function in this group was –0.413 with a p-value of 0.0068 (significant for p<0.05) (Figure 1D).
Among the 447 patients in the study cohort, 77.2% (n=345) did not have LV dysfunction, 15.4% had mild LV dysfunction (n=69), moderate LV dysfunction was found in 6.5% (n=29), and only 0.9% had severe LV dysfunction (n=4). The Pearson correlation between indexed PDA size and LV function for the entire cohort was -0.555 with a p-value of <0.0001 (significant for p<0.05) (Figure 1E).
Patient data based on indexed PDA size in all four groups are detailed in Table 1.
Discussion
Patent ductus arteriosus is a common congenital heart defect with a prevalence rate of 0.25/1,000 of those diagnosed with a congenital heart defect8 and a relative frequency of 6.5% amongst all variants of paediatric cardiac defects9.
In 1967, Porstmann and colleagues described the transcatheter closure of PDA for the first time10. Closure of PDA with a device has now become a well-established “standard of care” procedure2. The heart gets accustomed to the altered Frank-Starling curve induced by high LV preload due to the PDA1. This causes LV remodelling, which needs to be reversed after PDA closure11. When a haemodynamically significant PDA is closed, it reduces preload to the LV by abolishing the left-to-right shunt, but increases the afterload by eliminating the low-resistance pulmonary circulation from LV outflow circulation. This simultaneous reduction in the LV preload and increase in the afterload may lead to LV systolic dysfunction, due to a phenomenon called “afterload mismatch”6. The incidence and predictors of LV systolic dysfunction after PDA closure have been well studied12. Studies based on 2D and 3D echocardiography have shown that LV volume and function normalisation may take about six months5. It is speculated that the larger the size of the PDA, the longer it takes for the LV to recover4.
However, considering the age group that can be affected with PDA, the “size of the PDA” is a relative term with no standardisation attached to it. The present study tried to address this ambiguity by standardising PDA size. The study considered the most reliable sizing of the PDA obtained through angiography for indexing with body surface area in a relatively large cohort of 447 patients. Although an attempt was made earlier to follow the indexed PDA sizing in a small cohort of 32 pre-term babies to determine the need for closure of ductus arteriosus either medically or surgically13, to the best of the authors’ knowledge, any study using an indexed PDA size has not been documented in the literature.
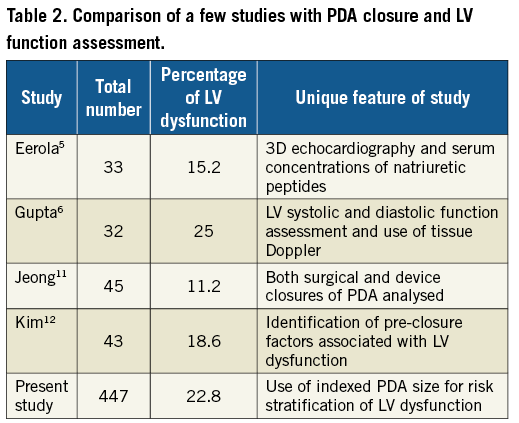
The current study documented the incidence of LV systolic dysfunction in 22.8% of the cohort (n=66/447). This is comparable with another Indian study by Gupta6 which reported LV dysfunction in 25% of patients, but was higher than in the reports from Jeong11 (11.1%), Kim12 (18.6%) and Eerola5 (15.2%). However, all of these studies had a relatively small number of patients in their respective cohorts (Table 2). It is of interest that both Indian studies have shown a higher percentage of LV dysfunction than studies from developed countries. Comparing data from other developing countries might be worthwhile, but such data are not currently available.
The present study used a novel parameter called “indexed PDA size”, which was not used to study LV dysfunction in any of the earlier reports. Prediction of LV dysfunction was more accurate and statistically significant when measured using the indexed PDA size in the entire cohort of 447 patients. On low values of indexed PDA size up to 2.9 mm/m2, there was an insignificant statistical correlation with LV dysfunction with a p-value of >0.05. Possibly, in this cohort of patients, stretch of the LV induced by high Qp of the PDA may still be within the Frank-Starling curves. However, as the indexed PDA size increased, the statistical correlation for LV dysfunction became more significant. Thus, higher statistical correlations with LV dysfunction were seen as the indexed PDA size increased. This is enumerated in Table 1, which shows that the Pearson correlation progressively increased with increasing indexed PDA size.
The results of the present study can be interpreted for their usefulness to triage the patients undergoing PDA device closure to understand who is likely to have LV dysfunction after PDA closure. These data would prove useful in the allocation of resources and frequency of monitoring in the immediate and short-term follow-up of patients undergoing PDA device closure. The authors thereby propose a new classification of PDA into small (indexed PDA size of less than 3 mm/m2), moderate (indexed PDA size from 3 mm/m2 to less than 6 mm/m2), large (indexed PDA size from 6 mm/m2 to less than 9 mm/ m2) and very large (indexed PDA size of 9 mm/m2 or more). In addition to the prevailing structural classification of PDA by Krichenko14, the authors believe that the proposed functional classification of indexed PDA size would be helpful in managing the practical problem of LV dysfunction in PDA patients after device closure.
Limitations
The major limitation of the present study was its retrospective design. The study was conducted in a high-volume centre over a reasonably long duration. Multiple trained echocardiography consultants were involved in the assessment. The possibility of interobserver bias cannot be ruled out even though the entire patient population was screened in the same set of echocardiography machines. Also, the study involved assessment of only systolic function of the LV. Since diastolic function was assessed only in a few patients, the authors could not use the data for lack of distribution over the entire study cohort.
A number of patients underwent surgical closure during the same study period. However, this group of patients was not considered because accurate assessment of PDA size was not possible within the prerequisites set for the study. Also, the surgical stress and difference in anaesthetic agents used for the surgical approach may lead to confounding of the results.
Although the pulmonary blood flow and systemic blood flow (Qp/Qs) were measured in each patient, the values were not considered due to multiple brands of kits used in blood gas analysis during the long study period. Also, the assessment of consumed oxygen was assumed and not actually measured. It was thought that these factors might affect the accuracy of numbers in the cohort.
The treatment for LV dysfunction in the entire study cohort was along the same therapeutic lines using dobutamine infusion as an intravenous inotropic agent, furosemide as diuretic and enalapril as ACE inhibitor. However, the dosage variations were not accounted for, although the accepted upper limit for the medications was never crossed in any patient.
In 2007, Dalsgaard showed the value of isovolumic acceleration measured by tissue Doppler echocardiography as an effective preload independent parameter in the assessment of global LV contractility15. Incorporating such parameters is likely to enhance the value of prospective studies.
Despite these shortcomings, the present study shows a statistically significant correlation between the indexed PDA size and systolic function. The authors strongly propose the utility of PDA classification by indexed PDA size as a parameter for further prospective studies incorporating both systolic and diastolic functions of LV, along with the use of tissue Doppler and myocardial strain pattern to verify the hypothesis.
Conclusions
Reversible LV dysfunction is a known effect following PDA closure. Indexed PDA size is an accurate, reproducible, easy to perform and useful parameter in predicting LV dysfunction in patients undergoing PDA device closure. It is a very simple parameter to calculate and can be carried out as soon as the PDA size is accurately measured in the cardiac catheterisation laboratory. This gives ample time to triage the patient into risk stratification for the development of LV dysfunction, thereby planning allocation of resources and the need for closer monitoring. The authors strongly recommend using the classification of PDA based on indexed PDA size to plan the functional recovery of patients undergoing PDA device closure.
| Impact on daily practice LV dysfunction is well known after device closure of PDA. Indexed PDA size correlates well with post-procedural LV dysfunction. Indexed PDA size is an accurate, reproducible, easy to perform parameter, which does not involve any extra cost, for risk stratification and early management in device closure of PDA. |
Acknowledgements
The authors would like to acknowledge the contribution of the Paediatric Interventional Cardiology team of Narayana Institute of Cardiac Sciences comprising Dr P.V. Suresh, Dr Satheesh Siddaiah, Dr Srisha Shankar Maiya, Dr Rahul Saraf and Dr Prakash Ramachandra. Contributions of Dr Sejal Shah and Ms Varsha Walavalkar from the Paediatric Echocardiography Lab of NICS have been invaluable for the present study.
Conflict of interest statement
The authors have no conflicts of interest to declare.

