
Abstract
Aims: The aim of this study was to present our experience with the AMPLATZER Duct Occluder II Additional Sizes (ADOIIAS) for the closure of different types of patent ductus arteriosus (PDA) in patients of various age groups.
Methods and results: A group of 103 patients, in whom the PDA (diameter below 3.5 mm) was closed using the ADOIIAS, was analysed. The median age of treated patients was 3.0 years (from 0.1 to 24 years), and 55 patients (53.4%) were older than three years. Ductal anatomy defined by angiography showed type A in 42 patients (40.8%), type C in six patients (5.8%), type D in 21 patients (20.5%), and type E in 34 patients (33.0%). In two cases, embolisation of the device occurred shortly after implantation. Both occluders were retrieved percutaneously. One death occurred in a neonate four days after ADOIIAS implantation (not related to the procedure: multi-organ failure). Total occlusion of PDA was confirmed in all patients the day after the procedure. No protrusion of the device into the aorta or pulmonary artery was observed in any patient during follow-up.
Conclusions: The use of the ADOIIAS is a good therapeutic option for the treatment of selected PDA. The implant may be successfully substituted for coil implantation in all age groups.
Introduction
Transcatheter closure of the patent arterial duct (PDA) is an established treatment method using coils and the AMPLATZER™ Duct Occluder I or II (ADO) (St. Jude Medical, St. Paul, MN, USA).
Recently, the AMPLATZER Duct Occluder II Additional Sizes (ADOIIAS) was introduced into clinical practice. This device is available in various sizes and was initially designed for PDA closure in smaller children because it is softer and shorter. It consists of two retention discs joined with a tubular central part.
Published experience related to the application of this device in larger series is limited primarily to infants and small children1-4. Here, we will describe our preliminary experience in transcatheter PDA closure using the ADOIIAS, not only in young children but also in older children. To our knowledge, this is the largest published series of PDAs closed using the ADOIIAS.
Methods
A retrospective analysis of the interventional closures of PDAs performed in two tertiary cardiology centres from May 2013 until September 2015 was performed. Angiography records before, during and immediately after release of the device were analysed. PDA anatomy was classified according to Krichenko’s classification5. Duct length and diameter in the medium part (in types C, D, and E) and narrowest point (in type A) were noted. Whether the device was inserted using an arterial or venous approach was recorded.
Additionally, the presence of any residual shunt after device release was recorded. A residual shunt was regarded as significant if a jet of contrast was observed projecting into the pulmonary artery. If only a small puff of contrast was observed in the pulmonary artery, the shunt was not considered significant. Echo examinations 24 hours after the procedure were analysed. The presence of any residual shunt or device protrusion was noted. Device protrusion was defined as the device visibly protruding into the lumen of the descending aorta or pulmonary artery in either angiography or echocardiography.
PATIENTS
All patients with a diagnosis of PDA who underwent transcatheter closure were included in the study. Special attention was paid to those who were treated with the ADOIIAS device (Table 1). A total of 170 patients had the PDA closed percutaneously during the study period. There were 103 patients (68 females) in whom ADOIIAS was used (details analysed later), 27 patients with ADO I devices, one patient in whom an AMPLATZER™ Muscular Ventricular Septal Defect (VSD) Occluder (St. Jude Medical) was used, four patients with ADO II devices, and 35 patients with detachable coils (used only in 2014). Patients with PDA and other significant cardiac anomalies requiring cardiac surgery were excluded from the study.
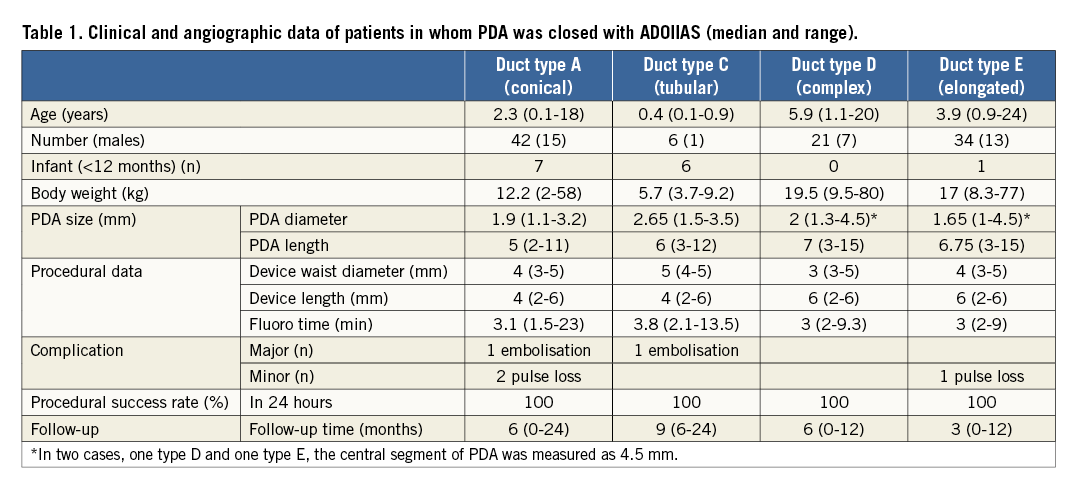
The decision on ADOIIAS size was based on the anatomy and diameter of the PDA. There were two patients with small ventricular septal defects and two others with atrial septal defects type II (ASD). In one child, non-compaction cardiomyopathy was present. Another child (3.7 years) had a significant residual shunt after PDA closure using a detachable coil (procedure performed in another hospital one year earlier). Other comorbidities included diaphragmatic hernia with heart and renal failure in a premature two-week-old infant (weight 2 kg) and chronic respiratory insufficiency (respiratory support-dependent; with coexisting ASD II) in a six-month-old infant (weight 6 kg). In both patients, the procedure of PDA closure was considered to be urgent. Informed parental (patient) consent was obtained for each patient. Follow-up has been planned for one, three, six and 12 months and then yearly after the procedure in the outpatient clinic and includes transthoracic echocardiography (TTE).
DEVICE
The design and characteristics of the new ADOIIAS have been described in detail previously6. Briefly, the ADOIIAS, similar to its previous version, the ADOII, is a self-expanding nitinol wire mesh occluding device, with a central part designed to lie within the ductal lumen, and two retention discs, 1 mm greater than the central waist, deployed at both ends of the PDA. These discs additionally increase the occlusive properties of the device and anchor it at both the pulmonary and aortic ends. There are nine available sizes of the ADOIIAS with waist sizes of 3, 4, and 5 mm and lengths of 2, 4 and 6 mm for each. Accurate measurement of the PDA is crucial for optimal occluder selection. For PDA type B (window type with a length <2 mm), this type of occluder should not be used. The device structure is soft, and all sizes can be easily implanted using a 4 Fr delivery catheter (the ADOIIAS comes pre-attached) from either the arterial or venous side. The delivery cable distal tip is extremely flexible and has a fluoro-visible marker.
DEPLOYMENT TECHNIQUE
The technique of transcatheter PDA closure using the ADOIIAS has been described previously1,6. All procedures were performed in children under general anaesthesia or in adults and teenagers under local anaesthesia. The decision to use the ADOIIAS was based on the narrowest diameter of PDA (less than or equal to 3.5 mm). Following heparin 50 U/kg and a dose of an intravenous antibiotic, a haemodynamic and angiographic evaluation was performed. The ductal anatomy was accessed with contrast medium injection using a 4-5 Fr pigtail catheter placed in the descending aorta. Angiography was recorded generally in the lateral 90° position. In some patients, an additional right oblique projection was performed for precise duct measurements. The device diameter usually selected was 2-3 mm larger than the duct (in types C, D, and E) or the narrowest ductal diameter (in type A). The device length was 2-4 mm less than the duct, such that the aortic disc was placed in the duct or ampulla without protruding into the aortic lumen. Generally, the aortic disc of the device was mostly pulled into the arterial duct in patients with long ducts (Figure 1A, Figure 1B). Device stability was assessed using mild pulling and pushing on the delivery cable. The correct device position was confirmed by hand contrast medium injection through the delivery catheter prior to device release. Repeat angiography was performed after the device was released to assess the residual shunt and evaluate the final position of the device. Colour Doppler 2D TTE was performed the following day to assess ductal closure and to evaluate the left pulmonary artery and aortic isthmus blood flow.
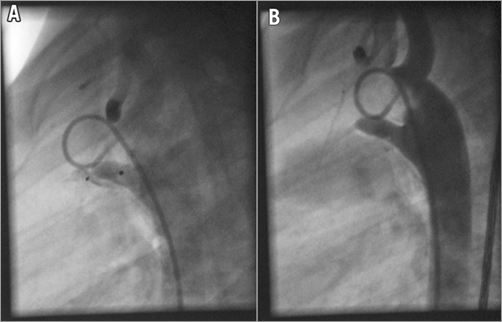
Figure 1. A 2.5-year-old female patient. A) Aortography LAO 90. PDA type E. B) Aortography after implantation of the device. Distal disc in the pulmonary artery and the rest of the device with the proximal disc placed in the duct.
Results
PDA closure using the ADOIIAS was performed in 103 patients with 104 devices (in one patient two devices were used). Clinical and angiographic data of patients in whom a PDA was closed with the ADOIIAS are presented in Table 1. The mean age of the treated patients was 4.7±4.7 years, and the median age was 3.0 (ranging from 0.1 to 24) years. The mean weight of the treated patients was 20.2±16.3 kg, with a median of 14.6 (2-80 kg). There were 48 children aged younger than three years (46.6%). Of these, there were nine infants, age <6 months (all with body weight below 6 kg; 8.7%), and 55 patients were older than three years (53.4%). Of these, 10 children and teenagers were 10 to 18 years old, and there were three adult patients (two 20-year-old and one 24-year-old males and one woman who was 18 years old). Ductal anatomy defined by angiography showed type A in 42 patients (40.8%), type C in six patients (5.8%), type D in 21 patients (20.5%), and type E in 34 patients (33.0%). The mean ductal diameter measured by angiography was 2.0±0.6 mm, with a median of 2.0 mm (1.1-3.5 mm), and the mean length was 6.3±2.7 mm, with a median of 6.0 mm (2-15 mm). Arterial access for PDA closure was selected for all except three patients, in whom the venous route was used (according to operator preference).
All procedures were successful, except for two, in which embolisation occurred. The first case was a one-month-old infant (3.7 kg) with a heart failure caused by isolated PDA. In this case, a 2.8 mm PDA type C was closed using a 5×2 mm ADOIIAS occluder, but three hours later embolisation occurred to the right pulmonary artery (a continuous murmur was noted again). The device was removed percutaneously, and the PDA was ligated surgically. In the other case, a 2.5-year-old girl with a 1.6 mm PDA type A experienced embolisation with the 3×2 mm ADOIIAS, which occurred in the right pulmonary artery (RPA). The device was snared and a larger device was used successfully. The reason for embolisation in both of these cases was probably similar and related to a duct spasm during aortography and the underestimation of its diameter.
One death occurred in a premature neonate who was four days old (not related to the occlusion of PDA) after ADOIIAS implantation (PDA was properly closed in autopsy examination) because of other paediatric reasons (multi-organ failure). For another infant with chronic respiratory failure during the same session, the PDA was closed using a 4×2 mm ADOIIAS and an ASD with an AMPLATZER Atrial Septal Occluder. In another child with inappropriate morphology of the ASD, first PDA and then eventually surgical ASD closure were performed. In one patient after a previous coil implantation and a significant residual shunt, the duct was completely closed using a 4×6 mm ADOIIAS. In three patients, there was transient pedal pulse loss, and they required heparin infusion. The median fluoro time was 3.0 (1.5-23) minutes.
All patients showed complete ductal closure the day after the procedure. The median follow-up period was six months (1-25 months). No evidence of obstruction or Doppler flow acceleration in the aortic arch or left pulmonary artery was observed during the procedure or follow-up. There were no other complications related to the procedures in the follow-up.
Discussion
It has been well documented that ADO I and II are very effective devices for the closure of large diameter PDAs2. The disadvantage of their use in smaller patients is the possibility of protrusion of the device into the aorta or the pulmonary artery. Other limitations include a larger introducer sheath and stiff delivery system. These disadvantages have been overcome with the new ADOIIAS. Bass and Wilson demonstrated the first successful PDA closure with this device in eight small pigs6. The ADOIIAS with its special construction characteristics was designed for smaller children and to overcome these disadvantages. The ADOIIAS has a range of several diameters and lengths and is dedicated for the closure of PDAs with a narrowest diameter less than or equal to 3.5 mm, even in association with a small or shallow ductal ampulla. In clinical practice for smaller PDAs, our daily practice was the application of detachable coils7,8. These are inexpensive and easy to use, and the complication rate is low in experienced hands. However, there are disadvantages to their use, such as a relatively high percentage of residual shunt and the lack of implantation control. Notably, we have not observed any residual shunt for the ADOIIAS. Although the latter device is not recommended for routine use by the manufacturer for children less than 6 kg, we found, similarly to Sungur et al3, that the use of the ADOIIAS was safe and effective in this group of children. Furthermore, we documented that the ADOIIAS was also a useful and effective treatment for children older than 10 years and even in three adult patients. Additionally, we found that almost 60% of our patients had a tubular-like form of PDA (types C, D and E). The diversity of the sizes of the ADOIIAS allowed us to choose an appropriate device for the particular anatomy of each PDA. Our experience with embolisation in two cases with PDA types A and C warrants special attention in these types of PDA, similar to the experience of Liddy et al3. This may occur due to the underestimation of the diameter of the PDA (duct constriction). In both patients, percutaneous retrieval was successfully performed. In one patient, subsequent closure of the PDA was performed with a larger implant, and in the second case (a neonate) the PDA was surgically ligated. Another important advantage of this device is its relatively reasonable price. From a practical point of view, the use of the ADOIIAS has replaced the use of coils in our patients, with excellent results and a relatively low complication rate.
Conclusions
The use of the AMPLATZER Duct Occluder II Additional Sizes is a good therapeutic option to treat PDA. This implant may be successfully substituted for coil implantation in all age groups.
| Impact on daily practice The AMPLATZER Duct Occluder II Additional Sizes is a good therapeutic option for the closure of different types of PDA (particularly types C, D, and E), as was observed in our group of 103 treated patients. All patients had PDA diameters at their narrowest point of less than 3.5 mm. The crucial step is the accurate measurement of the PDA for optimal occluder selection. This device is soft and can be delivered using a 4 Fr delivery catheter. The use of different sizes of the ADOIIAS can prevent the protrusion of the device into the aorta or pulmonary artery. This makes the device highly effective (total PDA occlusion occurred in all patients the day after the procedure), and complications are rare (two embolisations in our study). Therefore, this implant may be successfully substituted for coil implantation. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

