
Abstract
Aims: It is unclear whether detection of prediabetes (pre-DM) by routine assessment of glycated haemoglobin A1c (HbA1c) and fasting plasma glucose (FPG) among patients undergoing percutaneous coronary intervention (PCI) with contemporary drug-eluting stents (DES) may help identify subjects with increased event risk. We assessed the relation between glycaemia status and one-year outcome after PCI.
Methods and results: Glycaemia status was determined in 2,362 non-diabetic BIO-RESORT participants, treated at all four study sites, to identify pre-DM (HbA1c 42-47 mmol/mol; FPG 6.1-6.9 mmol/L) and unknown diabetes mellitus (DM) (HbA1c ≥48 mmol/mol; FPG ≥7.0 mmol/L). Another 624 patients had medically treated DM. The main composite endpoint consisted of death, myocardial infarction, or revascularisation. Glycaemic state was known in 2,986 participants: 324 (11%) patients had pre-DM, 793 (27%) had DM (known or new), and 1,869 (63%) patients had normoglycaemia. Pre-DM and DM patients differed from normoglycaemic patients in cardiovascular risk factors. The composite endpoint occurred in 11.1% in pre-DM, 10.5% in DM, and 5.7% in normoglycaemia (p<0.001). Pre-DM was associated with a twofold higher event risk compared to normoglycaemia (adj. HR 2.0, 95% CI: 1.4-3.0).
Conclusions: Following PCI with contemporary DES, all-comers with pre-DM had significantly higher event risks than normoglycaemic patients. In non-DM patients requiring PCI, routine assessment of HbA1c and FPG appears to be of value to identify subjects with increased event risk.
Abbreviations
DES: drug-eluting stents
DM: diabetes mellitus
FPG: fasting plasma glucose
HbA1c: haemoglobin A1c
IEC: international expert committee
MACE: major adverse cardiac events
NG: normoglycaemia
NICE: National Institute for Health and Care Excellence
pre-DM: prediabetes
Introduction
Before the onset of type 2 diabetes mellitus (DM), some patients can, for many years, experience an abnormal glucose metabolism which is a risk factor for coronary artery disease1,2. A metabolic state with borderline high glucose levels that do not meet the diabetes mellitus (DM) criteria is referred to as prediabetes (pre-DM), although some prefer the term risk of diabetes3. Pre-DM can be detected by measuring glycated haemoglobin A1c (HbA1c), which gives an indication of long-term blood glucose concentration, and fasting plasma glucose (FPG)4,5. Because HbA1c measurements do not require fasting or a glucose load and are not affected by acute changes in glucose levels due to stress or acute illness, they are easy to use in emergency settings and show only limited within-day or day-to-day variation4-7.
General population studies have investigated the association between cardiovascular risk and glucose abnormalities to a great extent, showing in subjects without known DM that elevated HbA1c is a risk factor for cardiovascular events2,8. In addition, in patients with or without known DM, who had an ST-elevation MI, elevated HbA1c levels were associated with increased mortality4,9,10. Moreover, DM increases the event risk after coronary stenting11-15. Nevertheless, there is a lack of data on the potential relevance of pre-DM for clinical outcome of all-comer patients with proven obstructive coronary disease, who require percutaneous treatment with current drug-eluting stents (DES).
The BIO-RESORT randomised trial recently examined the outcome of all-comer patients who underwent percutaneous coronary intervention (PCI) with implantation of contemporary DES, showing similar and very low clinical event rates for all stents used16. In this pre-specified analysis of the BIO-RESORT trial, we assessed the relation between pre-DM and one-year clinical outcome after PCI.
Methods
STUDY DESIGN AND PATIENTS
In the investigator-initiated, multicentre, randomised BIO-RESORT trial, all-comer patients were treated with PCI and contemporary biodegradable polymer or durable polymer DES. Both the design and the primary outcome of BIO-RESORT, which is registered at ClinicalTrials.gov (NCT01674803), have been described previously16. All four clinical study sites, located in the Netherlands, were encouraged to determine HbA1c levels at baseline in non-DM patients in order to identify patients with pre-DM or newly diagnosed DM; if no HbA1c levels were available, the FPG levels were used. In addition, the presence of known DM was recorded in the study files. HbA1c was measured using Tina-quant® 3rd generation assay on a Cobas 6000 analyser (Roche Diagnostics, Almere, the Netherlands) or CAPILLARYS 2 (Sebia, Lisses, France) calibrated using IFCC standards. The trial complied with the CONSORT 2010 Statement and the Declaration of Helsinki and was approved by the Medical Ethics Committee Twente and the institutional review boards of all participating centres. All patients provided written informed consent.
GLYCAEMIC CATEGORIES
Based on HbA1c and FPG levels measured at the index hospitalisation, and medical history, patients were stratified into three groups. Glycaemic categories were based on the National Institute for Health and Care Excellence (NICE)17 and International Expert Committee (IEC) 2009 criteria18. DM was defined as either known DM for which patients received medical treatment (either insulin or oral antidiabetics) or newly diagnosed DM, defined by an HbA1c ≥48 mmol/mol (≥6.5%), or FPG ≥7.0 mmol/L. Pre-DM was defined by HbA1c 42-47 mmol/mol (6.0-6.4%) and FPG 6.1-6.9 mmol/L and normoglycaemia by HbA1c levels ≤41 mmol/mol (≤5.9%) or FPG <6.1 mmol/L. In addition, we studied glycaemia according to the American Diabetes Association (ADA) recommendations, which advocate for prediabetes an HbA1c ≥39 mmol/mol (5.7%) but <48 mmol/mol (6.5%), and for new diabetes an HbA1c ≥48 mmol/mol (≥6.5%) (consistent with the IEC criteria). Sites were encouraged to assess the levels twice during hospitalisation, in case of any discordance between the diagnostic tests. Patients were classified into their “worst” category based on their lab results.
STUDY ENDPOINTS, PROCEDURES, AND MONITORING
Clinical endpoints were pre-specified, using the Academic Research Consortium definitions16. Myocardial infarction was defined by any creatine kinase concentration of more than double the upper limit of normal with elevated confirmatory cardiac biomarkers19. Revascularisation procedures were considered clinically indicated if the angiographic percent diameter stenosis of the then treated lesion was ≥50% in the presence of ischaemic signs or symptoms, or if the diameter stenosis was ≥70% irrespective of ischaemic signs or symptoms16,19. The main composite clinical endpoint of the present study comprised (components in hierarchical order): all-cause death, myocardial infarction, or revascularisation16.
Coronary interventions and concomitant medication did not differ from standard treatment and were performed according to current medical guidelines16. In general, dual antiplatelet therapy was prescribed for six to 12 months. Staged procedures were permitted within six weeks and did not count as events. Electrocardiograms were systematically assessed and recommended at routine clinical follow-up. Laboratory tests included systematic assessment of cardiac markers after the intervention and subsequent serial measurements in case of suspected ischaemia. Clinical follow-up data were obtained at visits to outpatient clinics or, if not feasible, by telephone follow-up or a medical questionnaire. There was no routine angiographic follow-up. A formal data safety monitoring committee reviewed the outcome data periodically. Data monitoring, processing of clinical outcome data, and independent clinical event adjudication were performed by an independent clinical research organisation (Diagram, Zwolle, the Netherlands).
STATISTICAL ANALYSIS
Data were reported as frequencies and percentages for dichotomous and categorical variables. Continuous variables were expressed as mean±standard deviation (SD). The time to primary endpoint and components thereof were assessed according to Kaplan-Meier methods; the log-rank test was applied for between-group comparisons. Pearson’s chi-square test or Fisher’s exact test was used to compare categorical variables, and the t-test to compare continuous variables. The relationship between pre-DM and the composite clinical endpoint of death, myocardial infarction and revascularisation was assessed with Cox proportional hazards analyses. A multivariate Cox proportional hazards analysis was used to adjust for potential confounders, accounting for differences in clinical outcome between the groups. Age, gender, body mass index, haemoglobin level at admission, hypertension, positive family history, hypercholesterolaemia, smoking, previous MI, previous revascularisation, multivessel disease and clinical syndrome at admission were included in the multivariate analysis. All statistical tests were two-tailed and p-values <0.05 were considered significant. Statistical analyses were performed with SPSS, Version 22 (IBM Corp., Armonk, NY, USA).
Results
From December 2012 to August 2015, a total of 3,514 participants in the BIO-RESORT trial were treated for obstructive coronary disease with contemporary DES. A total of 624 (18%) trial participants had known DM for which they already received medical treatment with insulin or oral antidiabetics at the time of their PCI. In 2,362 trial participants without known DM, HbA1c levels and FPG were determined at the index procedure: 324 (14%) had pre-DM, 169 (7%) had newly diagnosed DM, and 1,869 (79%) were normoglycaemic.
As a result, the glycaemic state was known in 2,986/3,514 (85%) trial participants. They represent the study population of the present analysis, in which pre-DM was present in 324 (11%), DM (known or new) in 793 (27%), and normoglycaemia in 1,869 (63%) patients. A total of 2,630 (99.4%) patients completed 12-month follow-up or had died. Only two (0.07%) patients were actually lost to follow-up; 12 (0.4%) patients withdrew consent during the trial and were censored (clinical outcome was used until time of withdrawal).
The baseline characteristics of the study population are presented in Table 1. The majority of participants were Caucasian (95%). Patients with pre-DM and DM were significantly older and had a higher body mass index than normoglycaemic patients. Furthermore, they had similar cardiovascular risk profiles with higher rates of hypertension, hypercholesterolaemia, and previous myocardial infarction and coronary revascularisation than normoglycaemic patients (Table 1). Information on medication prior to hospitalisation and at discharge is provided in Supplementary Table 1.
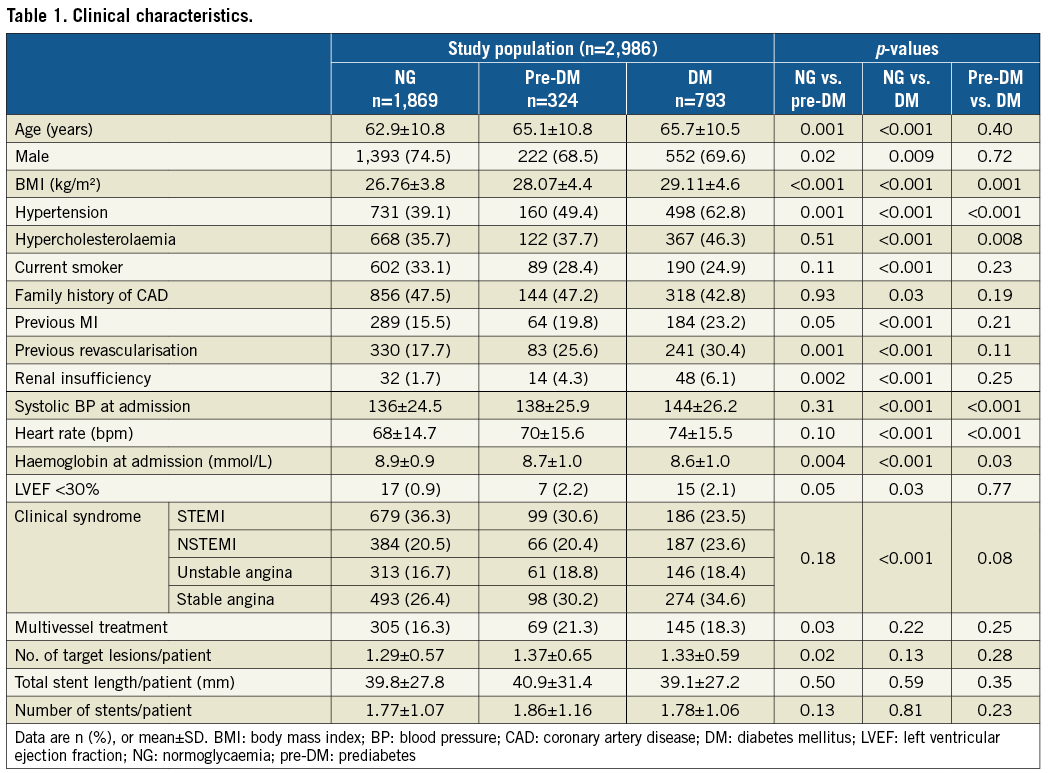
Clinical outcome data are presented in Table 2. The composite endpoint death, myocardial infarction or revascularisation at one year was met by 36 (11.1%) pre-DM patients, 83 (10.5%) diabetic patients, and 106 (5.7%) normoglycaemic patients (p<0.001) (Figure 1). The individual components are presented in Figure 2. Mortality rates were higher in pre-DM (2.8%) and DM (2.8%) than in normoglycaemic patients (1.2%, p=0.006) (Figure 2A). Similar revascularisation rates were found during one-year follow-up in patients with pre-DM and patients with DM (5.2% and 5.9%, respectively), which were significantly higher than revascularisation rates in normoglycaemic patients (3.0%, p<0.001) (Figure 2C).
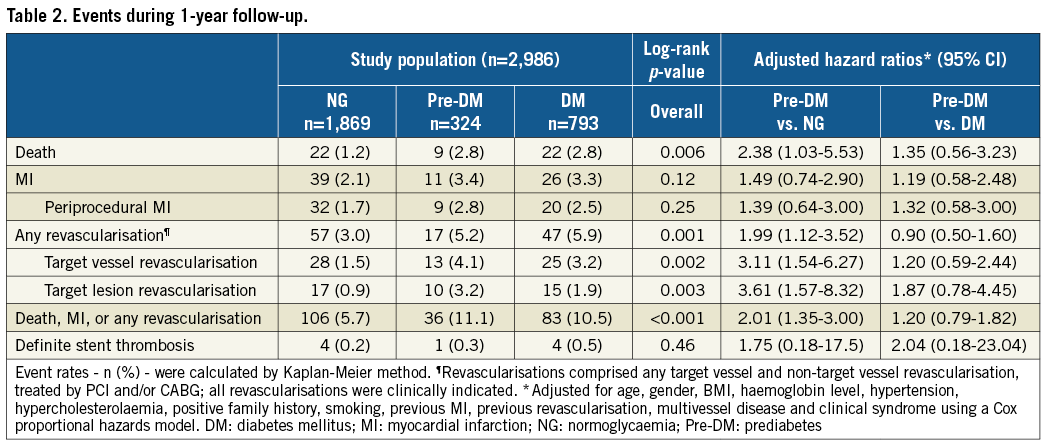
Figure 1. Kaplan-Meier curves of the composite endpoint. Composite clinical endpoint consisting of death, myocardial infarction, or revascularisation at one year.
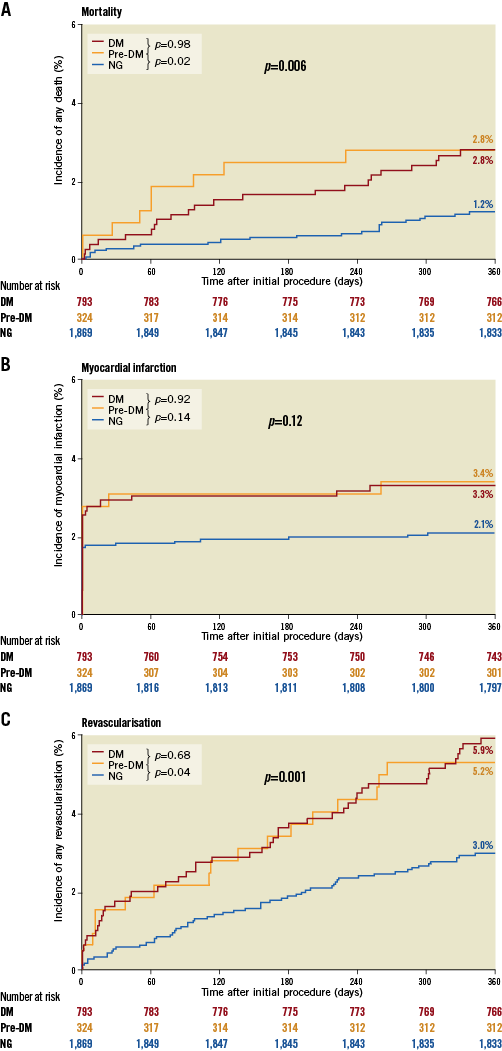
Figure 2. Kaplan-Meier curves of mortality, MI, and revascularisation. A) Event curves for mortality. B) Event curves for myocardial infarction. C) Event curves for revascularisation.
Multivariate analysis demonstrated that pre-DM was independently associated with the composite endpoint at one-year follow-up: pre-DM patients had a twofold higher event risk than patients with normoglycaemia (adjusted HR 2.0, 95% CI: 1.4-3.0). Adjusted hazard ratios are presented in Table 2 (unadjusted hazard ratios in Supplementary Table 2). There were no significant differences in one-year event risk between patients with pre-DM and those with DM. Revascularisation data on stent level can be found in Supplementary Table 3.
When the ADA definitions were applied, 906 (30%) patients were classified as prediabetic with a composite endpoint rate of 8.4% (vs. 5.1% in normoglycaemics and 10.6% in diabetics, p<0.001). Further details are presented in Supplementary Table 4.
Discussion
MAIN FINDINGS
In the present prospective study in all-comers treated with contemporary DES, pre-DM at baseline was present in 14% of all non-DM patients. Patients with pre-DM had higher death and revascularisation rates than normoglycaemic patients, and the composite clinical endpoint rates were higher in patients with pre-DM (11.1% versus 5.7%). Multivariate analysis demonstrated a twofold higher event risk of death, MI and revascularisation in pre-DM than normoglycaemia.
Patients with pre-DM and DM showed similar higher risk profiles versus patients with normoglycaemia, including older age, higher BMI, and higher rates of hypertension, hypercholesterolaemia, prior MI, and prior revascularisation. Most of these variables are components of the metabolic syndrome or linked to insulin resistance, suggesting that individuals with pre-DM have a “diabetic phenotype”20. Despite the use of contemporary DES, the rates of most clinical endpoints were at least as high in pre-DM as in DM, which underlines that PCI patients with pre-DM are prone to experience adverse clinical events. Our data suggest that, in non-diabetic all-comer patients scheduled for PCI, routine assessment of HbA1c and FPG may be of great value.
Further studies are needed to corroborate our findings, but the ease of HbA1c and FPG testing, relatively cheap cost and the pick-up of somewhere between 11% (using more conservative prediabetes criteria used in Europe) and 30% using the ADA criteria mean that many patients at risk of diabetes and a small percentage (4%) with new diabetes are identified with relevant clinical implications for both groups.
PREDIABETES AND CARDIOVASCULAR RISK
Although oral glucose tolerance testing (OGTT) has been suggested as the gold standard in the diagnosis of DM21, there has been an increased interest in HbA1c and cardiovascular risk. In 2008 the IEC, appointed by the ADA, the European Association for the Study of Diabetes, and the International Diabetes Federation, modified previous recommendations and suggested that HbA1c levels would represent a better diagnostic tool for DM than single measures of glucose concentration18. The IEC also recommended HbA1c of 6.0-6.4% (42-47 mmol/mol) for the identification of an intermediate-risk group, i.e., prediabetes, since identification of these individuals provides an opportunity for intervention through lifestyle modification and pharmacological interventions to prevent progression to diabetes18. Pragmatic considerations which support the use of HbA1c are that no fasting is required, and that it can be assessed at any time, even shortly after an acute event. This flexibility is different from FPG assessment, which (ideally) should be performed at least four days after an MI to account for the acute phase response4-7. As for the comparison of glucose measures for the prediction of first-onset cardiovascular disease, results from a meta-analysis of patient-level data from 73 prospective studies suggest that the assessment of HbA1c is equal to or modestly better than the assessment of fasting, random, or post-load plasma glucose22.
Several general population studies have been performed to evaluate associations between pre-DM (according to different definitions) and cardiovascular events, finding an increased risk of composite cardiovascular events, coronary heart disease, stroke, and all-cause mortality7,8,23.
PREVIOUS STUDIES ON PREDIABETES AND PCI OUTCOMES
Studies on HbA1c level and clinical outcome after PCI have shown conflicting results. A recent meta-analysis of 20 observational studies involving 22,428 patients assessed the association between HbA1c level and clinical outcomes in non-diabetic patients with coronary artery disease, showing that elevated HbA1c levels (prediabetes) were associated with long-term mortality24. Only nine of the studies included in this meta-analysis concerned studies in which patients were treated with PCI, of which the majority was treated with bare metal stents or plain old balloon angioplasty (POBA). We will highlight the largest of these studies.
A retrospective, observational study in 4,176 non-diabetic STEMI patients showed that HbA1c levels were independently associated with adverse outcome at one-year follow-up4. In contrast, a Chinese observational registry of STEMI patients with and without DM found that baseline HbA1c levels did not independently predict 30-day clinical outcome (90% had primary PCI with bare metal stents or DES)25. Nevertheless, follow-up duration was very short and event rates were low25. A single-centre study evaluated the relation between HbA1c and major adverse cardiac events (MACE) in non-diabetic patients who underwent elective PCI with balloon angioplasty or bare metal stent implantation26. The study showed that abnormal HbA1c was associated with significantly higher risk of MACE and target vessel revascularisation, although it should be noted that event rates were high, which can be explained by the use of bare metal stents. Another recently published retrospective cohort study assessed non-diabetic patients with ischaemic heart disease treated with PCI in 2010, with a mean follow-up of 42 months, showing that prediabetes as determined by HbA1c was not associated with long-term adverse cardiovascular outcomes27. Again, the majority of these patients (>60%) were treated with bare metal stents and groups were relatively small.
A secondary analysis of the EARLY ACS trial showed similar rates of pre-DM as compared to our trial, in patients who were admitted to the hospital with non-ST-elevation acute coronary syndromes, treated either with PCI or CABG. Their primary outcome was a composite of all-cause death or myocardial infarction at 30 days, showing no significant differences in event rates in prediabetic patients as compared to diabetic patients28.
ADDED VALUE AND CLINICAL IMPLICATIONS
The previously discussed studies assessed only specific subsets of PCI patients (i.e., patients with DM, elective patients, or STEMI patients), and generally used techniques or devices that have been largely replaced (i.e., balloon angioplasty, bare metal stents, or first-generation DES)4,24-28. Our current study, on the other hand, reports findings of a recent prospective multicentre PCI study in all-comers who were all treated with contemporary newer-generation DES16. The findings obtained in patients with pre-DM, DM, and normoglycaemia provide present-day evidence of the importance of pre-DM for the clinical outcome of all-comer patients after PCI with contemporary DES.
Patients with coronary disease share risk factors with the metabolic syndrome and are therefore at an increased risk of developing DM5,29. It is important to identify patients with an abnormal glycaemic state as early as possible in order to prevent (or delay) the onset of DM. Routine assessment of glycaemic state in PCI patients may help to identify patients with pre-DM who are at an increased risk of adverse events. Close follow-up of these patients appears to be warranted. Particular attention should be paid to lifestyle counselling and/or pharmacological therapy to reduce the risk of developing DM, and intensified secondary prevention measures to prevent clinically apparent coronary artery disease (e.g., more aggressive lipid-lowering and hypertension treatment). Furthermore, identification of patients with new diabetes can lead to their immediately gaining the benefit of newer diabetes therapies that decrease MACE and mortality in patients with diabetes and cardiovascular disease30.
Limitations
The glycaemic state was known in 85% of the trial participants. While this percentage is reasonable, data from an even greater number of subjects would have been welcome. OGTT is a well-known method to assess glucose metabolism and has detected more patients with DM in the general population as well as in patients with coronary disease, which is why ideally it would also have been assessed in order to complement the findings of the HbA1c and FPG measurements. However, OGTT is more labour-intensive and onerous on patients, and thus more difficult to integrate into routine clinical practice. To ensure that results could be generalised as much as possible to common clinical settings, we based the diagnosis of pre-DM and newly diagnosed DM on HbA1c or FPG values determined at admission. Only in subjects who had a single HbA1c or FPG value determined at the time of discharge did we use that value. For prediabetes, there are currently no formal recommendations regarding repeating the test for confirmation; however, in the present analysis, in 85% of the patients, glycaemic testing was performed twice to confirm the diagnosis. Iron deficiency anaemia may increase HbA1c levels; however, pre-DM is still independently associated with the composite endpoint, despite including haemoglobin levels in the multivariate analysis. Finally, no data were available on the duration of DM before study enrolment or the occurrence of contrast-induced acute kidney injury, which count as predictors of worse prognosis.
Conclusions
Following PCI with contemporary drug-eluting stents, all-comer patients with pre-DM had a significantly higher risk of adverse events than normoglycaemic patients. In a non-diabetic patient population with obstructive coronary artery disease, routine assessment of HbA1c prior to PCI may be of clinical value.
| Impact on daily practice Routine assessment of HbA1c and fasting plasma glucose in patients requiring PCI with contemporary DES may help to identify a specific subpopulation who are at increased risk of experiencing adverse events. Close follow-up of these patients appears to be warranted. Particular attention should be paid to lifestyle counselling and/or pharmacological therapy to reduce the risk of developing DM, and to prevent clinically apparent coronary artery disease. |
Funding
The BIO-RESORT study was funded equally by Biotronik, Boston Scientific, and Medtronic.
Conflict of interest statement
C. von Birgelen was a consultant to Biotronik, Boston Scientific and Medtronic. N. Sattar reports personal fees from Boehringer Ingelheim, and Janssen, and grants/personal fees from AstraZeneca. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Medication at hospitalisation and discharge.
Supplementary Table 2. Clinical outcome including hazard ratios.
Supplementary Table 3. Revascularisations at 1-year follow-up; data on stent level.
Supplementary Table 4. Clinical outcome based on ADA definitions.
To read the full content of this article, please download the PDF.

