Abstract
The number of individuals with diabetes and pre-diabetes is constantly increasing. These conditions are overrepresented in patients undergoing percutaneous coronary intervention and are associated with adverse prognosis. Optimal glycaemic control during an acute coronary syndrome is a relevant factor for the improvement of longer-term outcomes. In addition, the implementation of newer glucose-lowering drugs with proven cardiovascular benefits has a remarkable impact on recurrence of events, hospitalisations for heart failure and mortality. In this narrative review, we outline the current state-of-the art recommendations for glucose-lowering therapy in patients with diabetes undergoing coronary intervention. In addition, we discuss the most recent evidence-based indications for revascularisation in patients with diabetes as well as the targets for glycaemic control post revascularisation. Current treatment goals for concomitant risk factor control are also addressed. Lastly, we acknowledge the presence of knowledge gaps in need of future research.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has been growing steadily in most countries1. Glucose abnormalities are well established risk factors for coronary artery disease (CAD)2, and they are considerably more common in patients with acute and chronic coronary syndromes (ACS, CCS) than in the general population. Approximately 20-30% of patients with CAD have known diabetes, mainly T2DM, and many more (up to 70%) have newly detected diabetes or pre-diabetes when investigated with an oral glucose tolerance test (OGTT)3,4,5,6,7,8,9. Moreover, hyperglycaemia in patients undergoing percutaneous coronary intervention (PCI) is associated with adverse outcomes. A recent meta-analysis of randomised controlled trials (RCTs) and observational studies comparing post-PCI outcomes showed a twofold increase of in-hospital and short-term (<1 year post PCI) mortality in patients with diabetes10. In addition, newly diagnosed diabetes as well as pre-diabetes at hospital admission for ACS is associated with a similarly adverse long-term prognosis to that of patients with previously known diabetes5,11,12,13,14. Lastly, stress hyperglycaemia is associated with a poorer outcome of ACS6,15.
Different scientific societies have published guidelines with specific recommendations for diabetes management in patients with CAD16,17,18,19. In 2019, the European Society of Cardiology (ESC) in collaboration with the European Association for the Study of Diabetes (EASD) published the third set of guidelines providing guidance on the management of patients with cardiovascular disease (CVD) and diabetes or pre-diabetes18. The guidelines came about following a period that saw an unprecedented increase in the available evidence regarding the CV safety of novel glucose-lowering agents with –for the first time in the history of diabetes– evidence for the improvement in CV outcomes. This paper provides a focused overview of the current state-of-the-art management for patients with diabetes and pre-diabetes undergoing PCI (Central illustration).
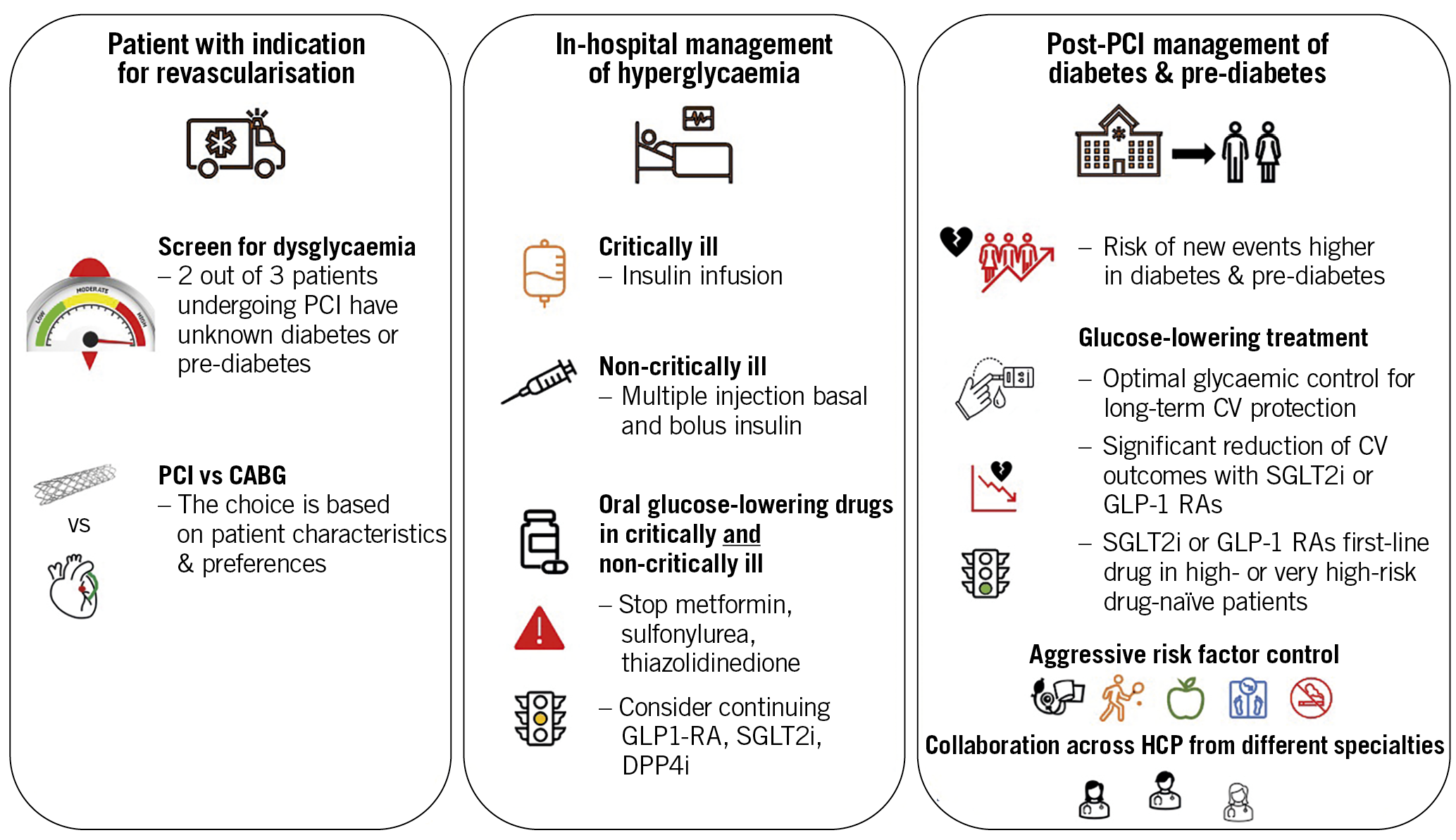
Central illustration. Considerations relating to dysglycaemia in patients undergoing percutaneous coronary intervention. The left panel highlights that diabetes and pre-diabetes are overrepresented in patients undergoing percutaneous coronary intervention (PCI). Patients with an indication for revascularisation therefore should be screened for dysglycaemia at presentation and attention should be given to the most appropriate revascularisation method. The middle panel highlights important considerations for glucose-lowering therapy in these patients during and after PCI. The right panel highlights that patients with dysglycaemia have a higher risk of new events after PCI and points to the importance of optimal long-term control of dysglycaemia as well as other risk factors post PCI.
Pre-PCI hyperglycaemia
DEFINITION OF DIABETES
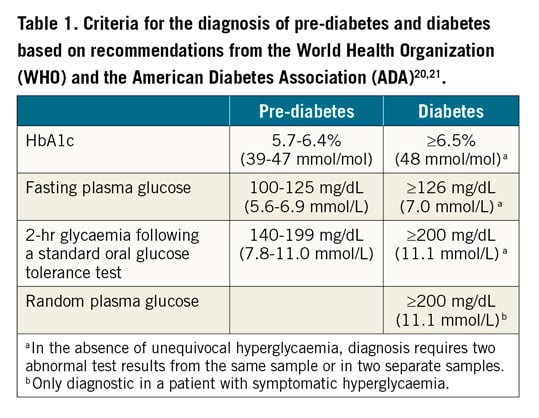
The diagnosis of T2DM is based on blood glucose levels in the fasting state and/or after an oral glucose load, and glycated haemoglobin (HbA1c)20,21,22. Intermediate categories based on fasting and post-load glucose are defined as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT)20,21,22. Diagnostic criteria and thresholds are summarised in Table 1.
SCREENING FOR HYPERGLYCAEMIA
Because of the high prevalence of glucose abnormalities, and their proven association with adverse prognosis, guidelines recommend screening for dysglycaemic states in patients with ACS and in CCS patients undergoing elective PCI18. In routine clinical practice, an accurate medical history allows the detection of the majority of cases of known diabetes in patients undergoing PCI. A determination of plasma glucose at hospital admission (irrespective of fasting) is mandatory for the detection of previously unknown diabetes. In the case of dysglycaemia at admission, further determinations of glycaemia at different times of the day will allow a better definition of the metabolic status of the patient. In case of dysglycaemia in patients without known diabetes, the determination of HbA1c allows the discrimination of diabetes from stress hyperglycaemia. An OGTT is recommended if HbA1c and/or fasting glucose are inconclusive18. Of note, in ACS the OGTT should not be performed earlier than 4-5 days after PCI to minimise false-positive results caused by stress hyperglycaemia, and may be performed during the polyclinic rehabilitation depending on the length of hospital stay23,24.
Indications for PCI in patients with T2DM
The indications for myocardial revascularisation, for both symptomatic and prognostic reasons, are the same in patients with diabetes as in patients without18,25. The anatomical pattern of CAD in diabetes influences prognosis and response to revascularisation. Patients with diabetes more frequently develop left main and multivessel critical stenoses, with diffuse disease also involving the small vessels17,26,27. Furthermore, common comorbidities of diabetes such as renal impairment and peripheral vascular disease adversely affect outcomes after coronary revascularisation28,29,30. Therefore, individual cardiac and extra-cardiac characteristics as well as patient preferences will determine when PCI is the appropriate revascularisation modality in patients with diabetes and CAD18,25. Figure 1 outlines an algorithm for the recommended revascularisation modality in patients with diabetes based on the current European guidelines18,25.
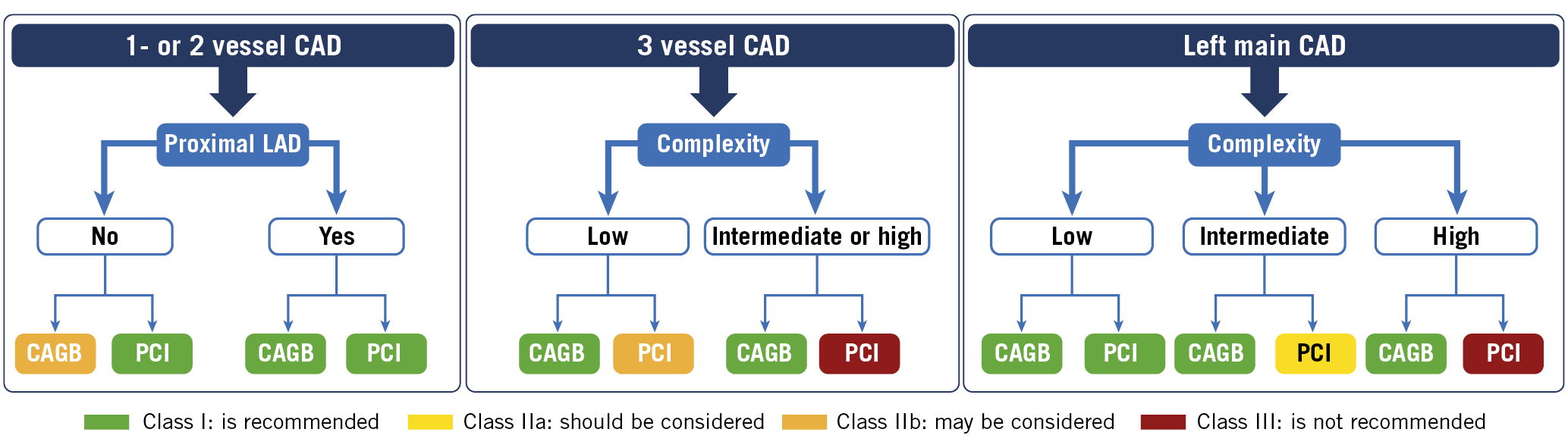
Figure 1. Recommendations for coronary revascularisation adapted from the 2019 ESC guidelines on CVD and pre-diabetes and diabetes18. Low disease complexity coronary anatomy (SYNTAX score 0-22), intermediate disease complexity (SYNTAX score 23-32) and high disease complexity (SYNTAX score ≥33). CABG: coronary artery bypass graft; CAD: coronary artery disease; PCI: percutaneous coronary intervention
Optimising glucose control during hospital admissions for ACS and elective PCI
AGGRESSIVE VERSUS NON-AGGRESSIVE GLUCOSE LOWERING
The benefits of an accurate glycaemic control in patients with diabetes and ACS was first demonstrated by the results of the DIGAMI trial, which showed that a more aggressive, insulin-based approach, compared with conventional treatment of hyperglycaemia at hospital admission and maintained long term, was associated with a significant reduction of mortality at 1, 3, and 5 years31. Notably, the trial results did not discriminate the benefits of accurate glycaemic control in the acute phase from those determined by the longer-term reduction of hyperglycaemia. Further, the differences in outcomes between the two arms could be attributed either to a positive effect of better glucose control, or to a beneficial action of insulin per se, or both. The DIGAMI-2 trial tried to address the limits of the DIGAMI trial by randomising patients to three treatment arms (intensified treatment in both the acute and chronic phase; intensified treatment in the acute phase only; conventional treatment)32. At the study's end however, glycaemic control was similar in the three groups and no difference in CV outcomes or mortality was demonstrated, leaving the question unanswered. Notably, the patients on intensified insulin treatment during hospitalisation and after discharge did not show better outcomes than the other two groups, where the majority of individuals received oral glucose-lowering drugs, suggesting that benefits of intensified insulin therapy are due to the improvement of glycaemic control, rather than to a direct effect of insulin per se31,32. Available data from trials in non-diabetic, normoglycaemic patients with ACS show that the intravenous infusion of insulin and glucose in the acute phase does not affect clinical outcomes33,34, confirming that the benefits of intensified insulin treatment in ACS are entirely attributable to the reduction of hyperglycaemia and not to the glucose-independent protective effects of insulin.
The improvement of glucose control still has potential benefits both in the acute and chronic phases of coronary syndromes. However, as discussed above, the assessment of the effects of accurate glycaemic control during the acute phase is limited by the paucity of available data from randomised trials. Observational studies suggest that both hyperglycaemia and hypoglycaemia in the acute phase are associated with a poorer prognosis35,36. Randomised trials, often performed on patients in intensive care units with different medical conditions, and usually including patients with stress hyperglycaemia together with those with diabetes, have provided discordant results, with either reduced37,38, unchanged39, or increased40 mortality. In addition, intensified glucose control reduced restenosis, without modifying mortality, in patients with diabetes undergoing PCI41. It is reasonable to believe that the results of each trial are determined by the balance between the benefits of improved glycaemic control and the risks of hypoglycaemic episodes induced by the intensification of therapy. For this reason, current guidelines recommend an accurate treatment of hyperglycaemia in ACS, providing therapeutic targets well above normoglycaemia, in order to minimise the risk of hypoglycaemia17,42,43.
GLUCOSE-LOWERING AGENTS DURING PCI
Insulin is considered the drug of choice for the treatment of hyperglycaemia in the acute setting because of its pharmacokinetic and pharmacodynamic profile, allowing prompt correction of blood glucose levels42. Patients undergoing elective PCI may be treated either with intravenous insulin infusion or with multiple daily subcutaneous insulin injections, depending on their dietary regimen and glucose control42. Oral glucose-lowering treatments could theoretically affect the prognosis after PCI in patients with diabetes and should be given careful consideration both in the acute setting and when planning elective PCI. In those patients who are already treated with glucose-lowering agents at admission, some of the non-insulin drugs, such as sulfonylureas and thiazolidinediones, should be withdrawn for safety reasons. Metformin increases the risk of lactic acidosis in case of heart failure or renal failure44. The current recommendation is that metformin should be stopped prior to elective PCI in patients with renal failure18,25. However, the actual risk of lactic acidosis is minimal45,46; therefore, concern for lactic acidosis in metformin-treated patients should not interfere with the clinical decision to perform primary PCI in patients with ACS18,25. Sulfonylureas are associated with a high risk of hypoglycaemia47; in addition, sulfonylureas reduce myocardial function in ischaemic conditions48. Thiazolidinediones induce fluid retention, exacerbating clinical manifestations of heart failure49. The possibility of maintaining a pre-existing treatment with DPP4 inhibitors, GLP-1 RAs or SGLT2 inhibitors is still controversial, with little available evidence of their effects in the acute phase. Pilot trials with GLP-1 agonists in the acute phase of coronary syndromes were inconclusive50,51, whereas intervention studies with SGLT2 inhibitors are still ongoing.
GLUCOSE-LOWERING AGENTS DURING HOSPITAL STAY
Non-critically ill ACS patients who are able to eat regular meals may be treated either with intravenous insulin infusion or with multiple daily subcutaneous insulin injections, depending on their dietary regimen and glucose control. For patients with ACS who are critically ill, hyperglycaemia is usually managed via intravenous infusion of regular human insulin42. The determination of the insulin infusion rate is based on measurements of blood glucose (from samples of arterial, venous, or capillary blood). Several algorithms have been developed for the calculation of appropriate insulin doses52,53,54.
Recovering patients who are able to eat regular meals can be shifted to standard basal-bolus insulin therapy with subcutaneous injections of rapid-acting insulin at meals and a single administration (usually at bedtime) of a long-acting insulin. In this latter approach, rapid-acting analogues are preferable to regular human insulin as bolus insulin, due to their superiority in post-prandial glucose control55,56; similarly, long-acting insulin analogues are preferable to NPH insulin for the lower risk of hypoglycaemia57. The accuracy in the determination of insulin doses depends on an appropriate frequency of glucose testing (usually, 5-6 tests daily); the use of devices for continuous monitoring of interstitial glucose could theoretically facilitate the management of insulin therapy, but it needs to be validated further58.
The flow chart for the management of glycaemia in patients with ACS and in patients undergoing elective PCI is shown in Figure 2. Notably, although a large majority of patients are treated with insulin during hospitalisation, the pharmacological therapy for diabetes must be revised at discharge or shortly afterwards, in order to warrant a satisfactory glycaemic control and an optimal cardiovascular protection in the longer term.
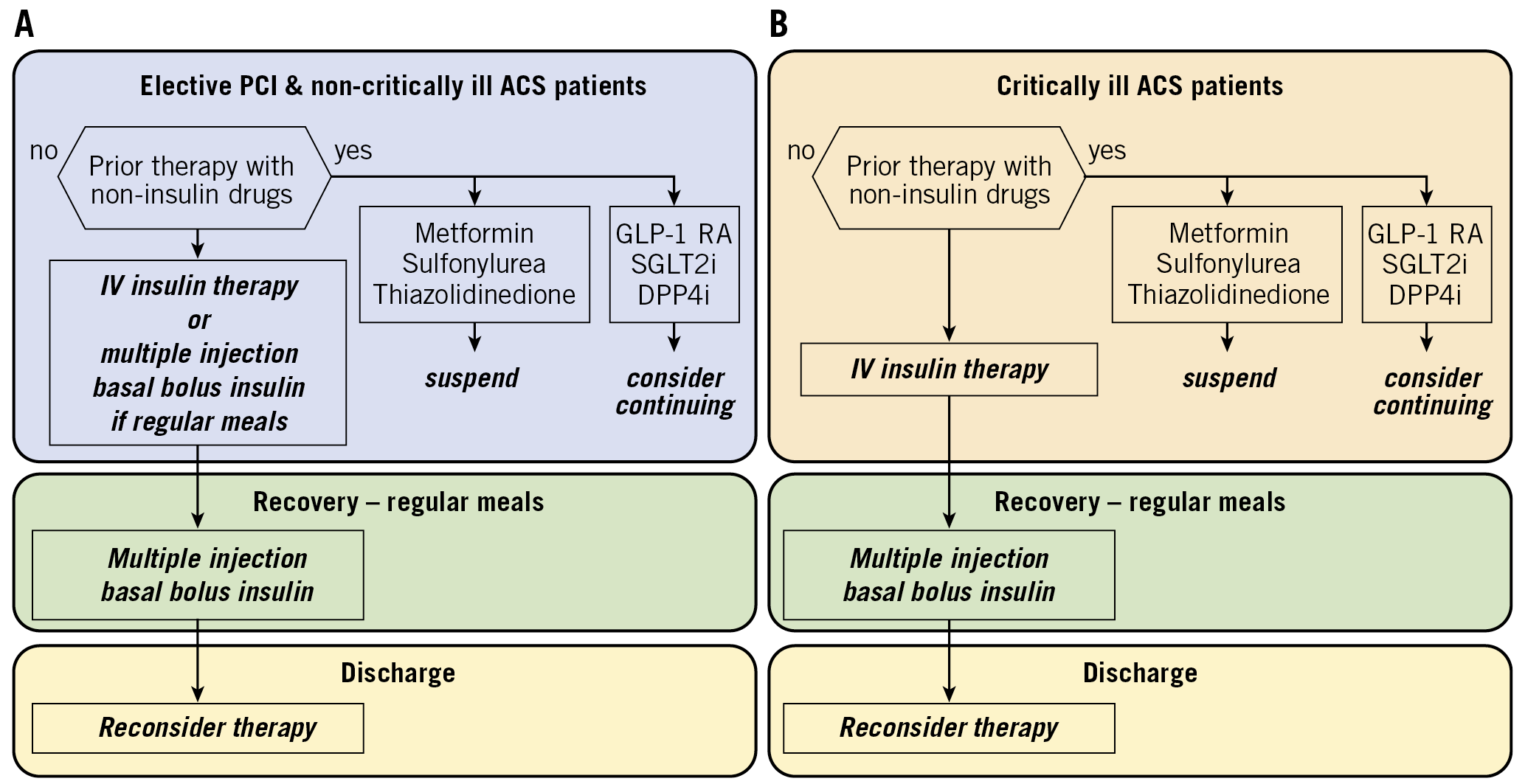
Figure 2. Flow chart for the management of hyperglycaemia. A) In non-critically ill ACS patients and patients undergoing elective PCI. B) In critically ill ACS patients. ACS: acute coronary syndrome; DDP4: di-peptidyl peptidase 4; GLP-1 RA: glucagon-like peptide-1 receptor agonist; PCI: percutaneous coronary intervention; SGLT2: sodium-glucose transporter 2 inhibitor
Diabetes management post PCI
MULTIFACTORIAL TREATMENT
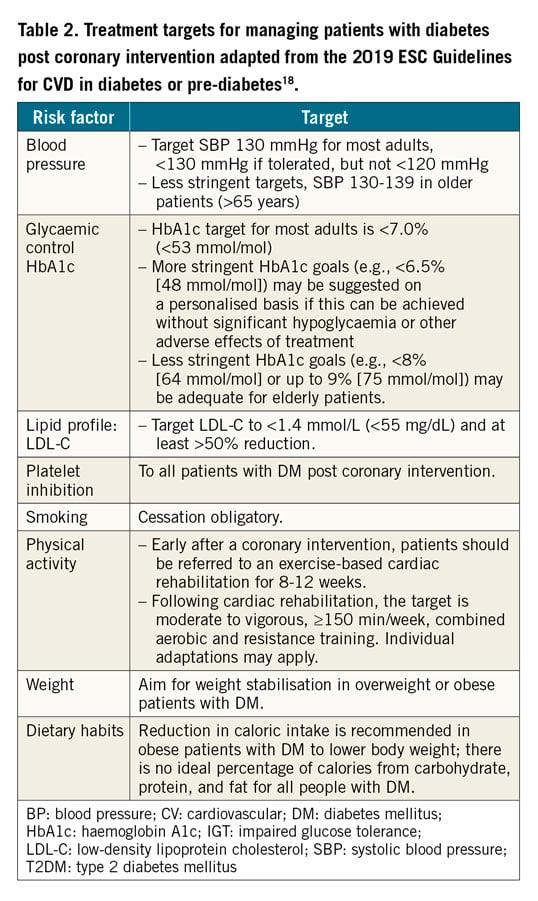
Following PCI, the rate of adverse events remains higher in patients with diabetes than in those with normal glucose metabolism. Hence, these patients will benefit from early identification and treatment of comorbidities and factors that increase CV risk59. Aggressive treatment of risk factors associated with hyperglycaemia is beneficial for long-term reduction of microvascular and macrovascular complications in T2DM as demonstrated by several trials, including the UKPDS, STENO 2, ADDITION and JDOIT3 studies60,61,62,63,64,65. From observational data provided by the Swedish National Diabetes Register, the excess risk of all-cause death, acute myocardial infarction (MI) and stroke, respectively, decreases by bringing each risk factor (HbA1c, low-density lipoprotein cholesterol [LDL-C], albuminuria, smoking, and systolic blood pressure [SBP]) within target range. Risk of heart failure (HF) hospitalisation consistently proved to be higher among patients with diabetes as compared with controls without (hazard ratio [HR] 1.45, 95% confidence interval [CI]: 1.34-1.57)66. The Euro Heart Survey found that, among 1,425 patients with known diabetes and CAD, the combination of aspirin, a beta-blocker, a renin-angiotensin-aldosterone system (RAAS) blocker, and a statin was associated with a significantly lower all-cause mortality (3.5 vs 7.7%; p=0.001) and incidence of CV events (11.6 vs 14.7%; p=0.05) after one year of follow-up67. Depending on individual risk level, the 2019 ESC guidelines on DM and CVD have set different recommended targets for risk factor control. Targets relating specifically to secondary prevention post revascularisation in patients with diabetes are outlined in Table 2,18.
GLUCOSE-LOWERING TREATMENT
Evidence indicates that improved glycaemic control defers the onset and reduces the progression of microvascular complications in diabetes. CV benefits from the use of glucose-lowering drugs in patients with macrovascular complications, including patients post PCI, have recently emerged from several cardiovascular outcome trials (CVOTs). Accordingly, early, effective, and sustained glycaemic control is advocated in the diabetes guidelines to mitigate the risks of hyperglycaemia18. The choice of treatment should be made with consideration of the balance between benefits induced by reduction of hyperglycaemia and harm determined by treatment side effects, particularly hypoglycaemia. Such a balance is determined by individual patient characteristics, and the pharmacological profile of the available treatment. In general, the more advanced the CVD, the older the patient, the longer the diabetes duration and the more comorbidities that are present, the less stringent the glucose control should be, because of the higher risk related to the adverse effects of treatment (Table 2).
ESTABLISHED ORAL GLUCOSE-LOWERING DRUGS
CV effects of glucose-lowering agents have been extensively evaluated in clinical trials for newer drugs, but not for some long-established drugs. For example, there are no recent large-scale randomised CVOTs assessing the effect of metformin or sulfonylureas on CV events. The cardiovascular safety of sulfonylureas has been discussed for decades, because of the risk of hypoglycaemia, which induces sympathoadrenergic activation68. In addition, sulfonylureas interact directly with myocardiocytes, blocking an ATP-dependent potassium channel involved in myocardial adaptation to ischaemia69. Available clinical trials with sulfonylureas failed to produce significant effects (either detrimental or beneficial) on the incidence of major adverse cardiovascular events (MACE)70,71,72. Of note, combined data from all available randomised trials show an increase of all-cause mortality associated with sulfonylureas71. The alpha-glucosidase inhibitor acarbose did not alter MACE in patients with IGT and CVD over five years in the ACE trial73. The thiazolidinedione pioglitazone was neutral for the primary composite outcome. Despite a signal of reduced risk of subsequent MI or recurrent stroke, this drug should be avoided in patients with HF because of an increased risk of HF incidence49,74,75,76. A large, unblinded randomised comparison (TOSCA.IT) of pioglitazone versus sulphonylurea as add-on to metformin showed similar rates of the composite of MACE endpoint as well as its individual components; however, the trial was stopped for futility and, hence, results should be interpreted with caution72. Among long-established drugs for diabetes, insulin has been studied in several CV outcome trials. The results of studies performed in patients with diabetes and MI (i.e., DIGAMI and DIGAMI-2) have been reported above. In the UKPDS, long-term insulin treatment failed to provide a significant CV protection70; in another trial in high-risk patients, glargine insulin did not modify the incidence of major cardiovascular events77.
NEWER ORAL GLUCOSE-LOWERING DRUGS
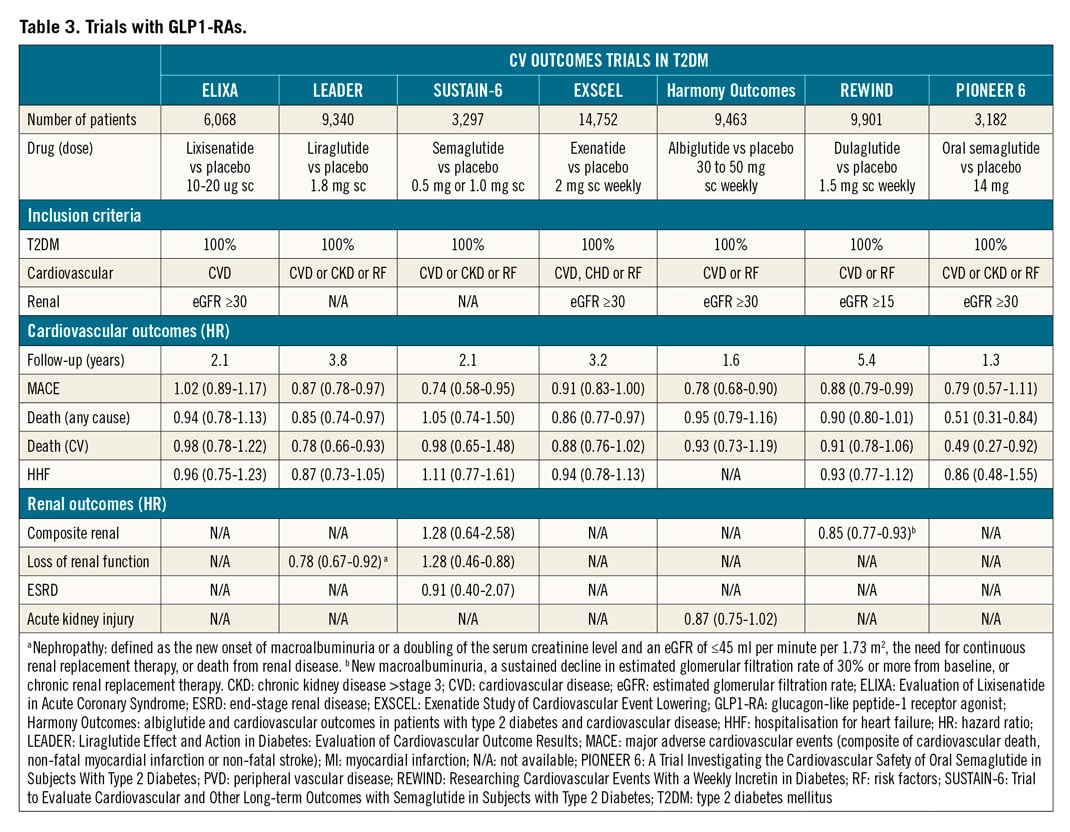
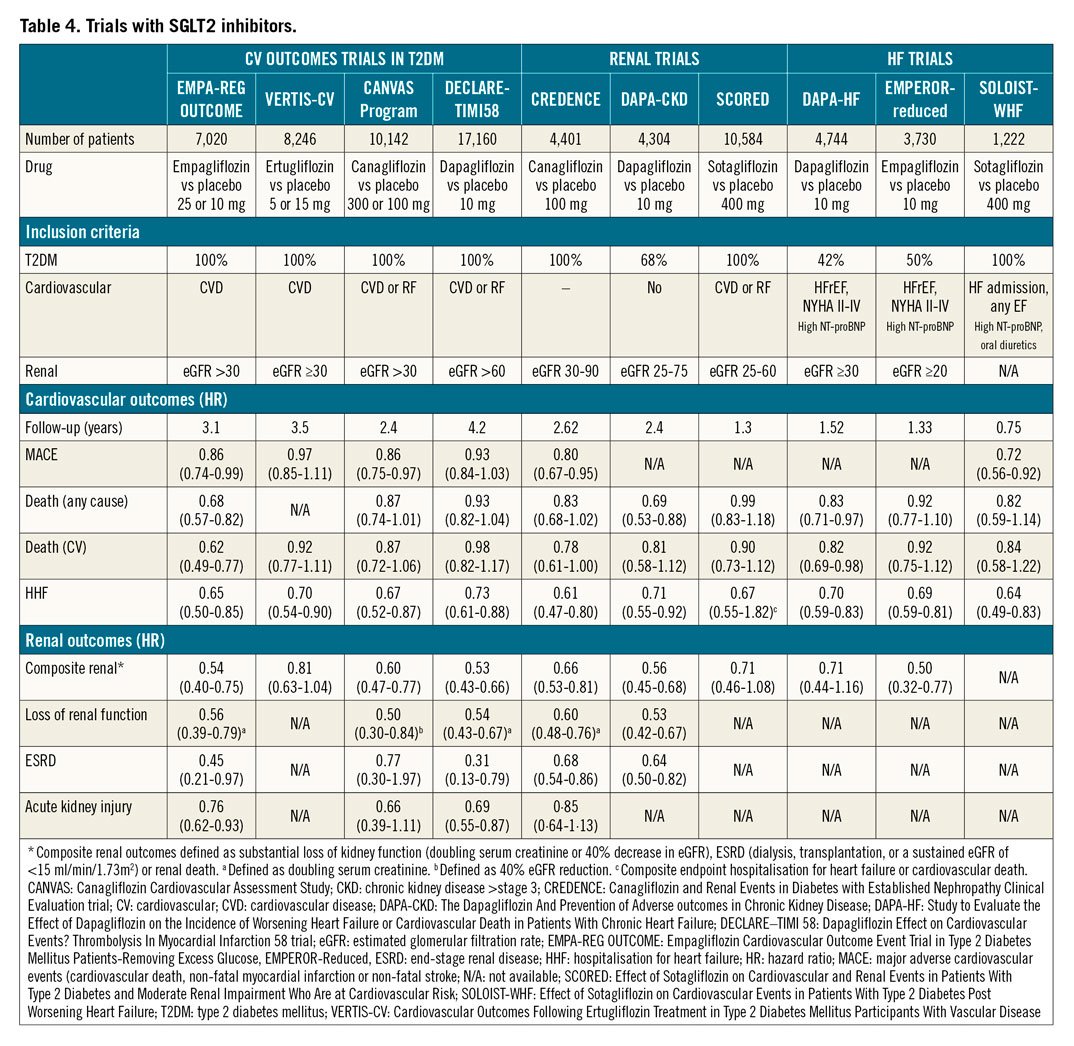
Following a meta-analysis of CV events with the thiazolidinedione rosiglitazone78, the regulatory landscape for diabetes drugs underwent a major change in 200979. Thereafter, all future diabetes drugs were required to demonstrate designated margins of CV safety to achieve or maintain regulatory approval. As a consequence, a large number of trials to assess CV outcome were performed80,81, most of which were designed to confirm non-inferiority of the experimental therapy versus placebo, added to background antihyperglycaemic treatment. Since the primary reason for performing these trials was the demonstration of safety, the study design was often not ideal for the detection of beneficial effects82. Despite these limitations, several CV safety trials have provided results suggesting that some of these drugs are capable of reducing CV complications substantially in patients with T2DM. These are summarised in Table 3 and Table 4.
Five large prospective trials in T2DM populations with different baseline risk have assessed the CV effects of dipeptidyl peptidase 4 (DPP4) inhibitors: saxagliptin (SAVOR-TIMI 53)70,83, alogliptin (EXAMINE)84, sitagliptin (TECOS)85, and linagliptin (CARMELINA and CAROLINA86) as reported to date. Four of the five trials confirmed statistical non-inferiority versus placebo (and alternative glucose-lowering therapy for glycaemic equipoise) for their primary composite CV outcome, but none of them showed significant CV benefits. Saxagliptin was associated with an increased risk of HF hospitalisation83, especially in those with a high baseline NT-proBNP, pre-existing HF, or chronic kidney disease (CKD)87, while there was a numerical, yet non-significant increase with alogliptin84.
Seven CVOTs have examined the effect of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) on CV events in patients with DM and CVD: lixisenatide (ELIXA)88, exenatide (EXSCEL)73, liraglutide (LEADER)89,90, injectable semaglutide (SUSTAIN-6)91, oral semaglutide (PIONEER-6)92, albiglutide (Harmony Outcomes trial, no longer marketed)93 and dulaglutide (REWIND)94. A reduction in CV outcomes has been documented for liraglutide, injectable semaglutide and albiglutide. Liraglutide was associated with a reduction in both CV death and total mortality. Reductions in renal outcomes have been documented for liraglutide and injectable semaglutide89,90. A recent meta-analysis of these trials suggests that GLP-RAs reduce three-point MACE by 13% (HR 0.87, 95% CI: 0.83-0.92; p<0.001)95. Although the mechanisms by which some long-acting GLP-RAs reduce CV outcomes are still unclear, their effect could be partly mediated by direct vascular and cardiac action, beyond the improvement of traditional risk factors such as blood pressure and body weight96. Further, the gradual separation of the event curves in the trials suggests that the CV benefit is mediated by a reduction in atherosclerosis-related events.
Four CVOTs with sodium glucose transporter-2 (SGLT2) inhibitors, empagliflozin (EMPA-REG OUTCOME)97, canagliflozin (CANVAS)98, dapagliflozin (DECLARE−TIMI 58 trial)99, and ertugliflozin (VERTIS-CV)100, plus three trials on renal events with canagliflozin (CREDENCE)101, dapagliflozin (DAPA-CKD)102 and sotagliflozin (SCORED)103 respectively, have been performed to date. CV benefits have been observed for three-point MACE for empagliflozin and canagliflozin; empagliflozin additionally showed mortality benefit, whereas all five agents have shown reductions in HF hospitalisation98,99,101,104,105. In addition, this class of drugs has been shown to have salutatory effects on renal function. It is believed that the CV benefits of SGLT2 inhibitors are mostly unrelated to the extent of glucose lowering and occur too early to be the result of weight reduction. Instead, the achieved beneficial effects more likely result from a reduction in HF-associated events. This is further supported by two recent superiority trials, DAPA-HF106 and EMPEROR-reduced107, in which patients with HF and reduced ejection fraction with and without DM were randomised to dapagliflozin or empagliflozin versus placebo, respectively. Both trials showed reductions of HF hospitalisation, irrespective of diabetes status106,107; in addition, a reduction of mortality was observed with dapagliflozin106.
Further, the SOLOIST-WHF trial108, although ended prematurely due to lack of funding, showed that sotagliflozin versus placebo was safe and beneficial in patients with diabetes and acute HF, irrespective of whether the patient had reduced or preserved ventricular function. The underlying mechanisms for SGLT2 inhibitor cardioprotective effects are not the object of the present article and remain to be fully elucidated109,110,111,112.
IMPLICATIONS OF RECENT CVOTs
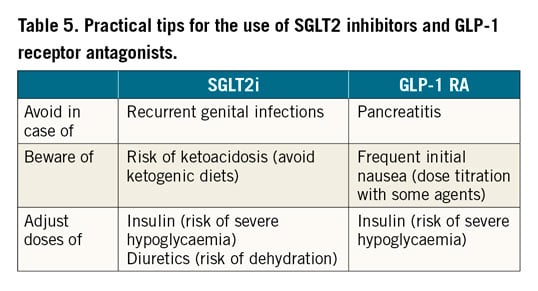
Based on the available evidence, SGLT2 inhibitors and GLP-1 RAs are considered the best options for the long-term treatment of T2DM in patients with established atherosclerotic CVD or at high/very high CV risk. These drugs are safe, effective, and generally well tolerated and can be started already during the hospitalisation for ACS or elective PCI, if indicated. Data from trials with liraglutide and empagliflozin suggest that at least some of the drugs of these two classes could also reduce mortality. Benefits with GLP-1 RAs seem to be related to an anti-atherosclerotic effect, whereas SGLT2 inhibitors appear to reduce HF-related endpoints and have specific advantages in patients with or at high risk for HF. Although the trial-based evidence for metformin monotherapy from UKPDS is not as strong as with the novel drugs tested in recent CVOTs, it is supported by extensive observations from everyday clinical practice70,113,114,115. There are a few precautions that should be kept in mind when selecting candidates for SGLT2 inhibitors and GLP1-RAs, respectively, in order to limit the risk of unexpected adverse events, as summarised in Table 5.
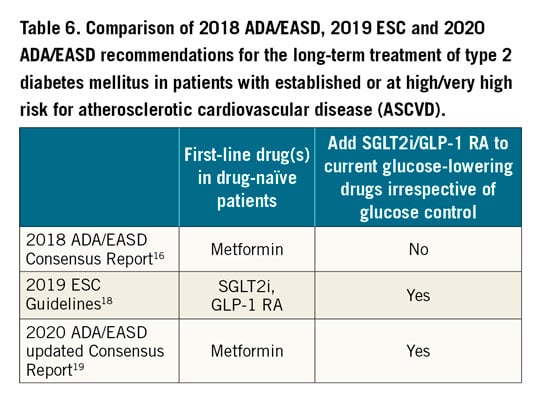
In a 2018 Consensus Report by the American Diabetes Association (ADA) and the EASD, metformin was confirmed as first-line drug, with SGLT2 inhibitors and GLP-1 RA as preferred add-on treatments in patients at high cardiovascular risk in case of inadequate control on monotherapy16. In 2019, ESC guidelines recommended the use of SGLT2 inhibitors or GLP-1 RAs as first-line drugs in CV patients at high or very high risk with T2DM who do not receive any glucose-lowering treatment and the addition to current glucose-lowering therapy of SGLT2 inhibitors or GLP-1 RAs in those patients regardless of glucose control (Figure 3),18. The 2020 ADA/EASD Consensus Report, in agreement with ESC guidelines, confirmed that current glucose-lowering therapy has to be integrated either with GLP-1 RA or SGLT2 inhibitors, irrespective of the achievement of glucose targets (Table 6),19. These apparent differences are mainly theoretical, considering that the vast majority of patients need more than one drug in order to achieve an acceptable glucose control.
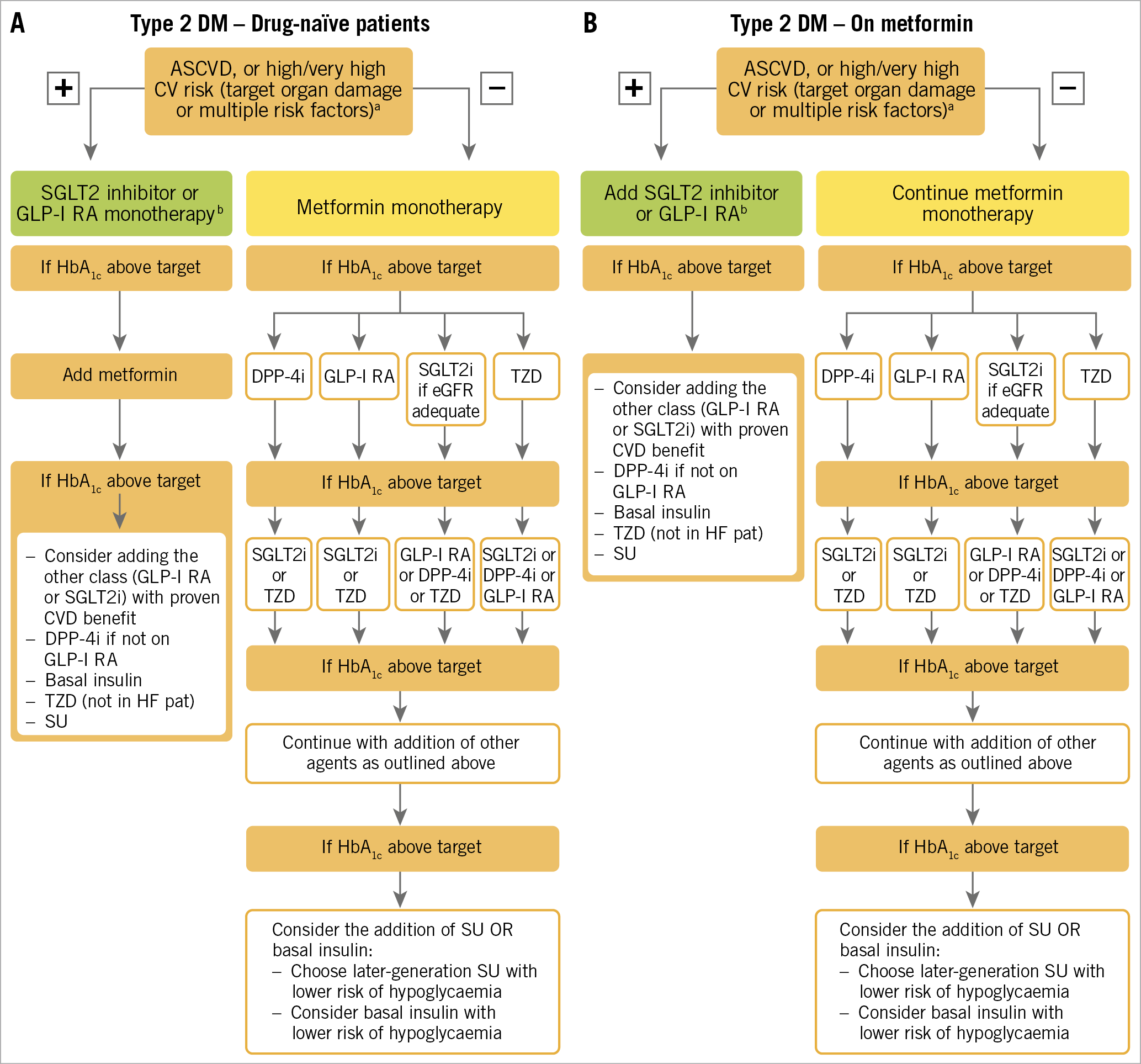
Figure 3. Treatment algorithm in patients with T2DM and ASCVD or high/very high CV risk. A) Drug-naïve patients. B) Metformin-treated patients. Treatment algorithm proposed by the 2019 ESC guidelines on CVD, pre-DM and DM, reproduced from reference 18 with permission from Oxford University Press on behalf of the European Society of Cardiology. ASCVD: atherosclerotic cardiovascular disease; CV: cardiovascular; CVD: cardiovascular disease; DPP4i: dipeptidyl peptidase-4 inhibitor; eGFR: estimated glomerular filtration rate; GLP1-RA: glucagon-like peptide-1 receptor agonist; HbA1c: haemoglobin A1c; SGLT2i: sodium-glucose co-transporter-2 inhibitor; T2DM: type 2 diabetes mellitus; TZD: thiazolidinedion
Conclusion
The number of individuals with diabetes and pre-diabetes is constantly increasing. Given that these conditions are overrepresented in patients with an indication for coronary revascularisation, it is important that colleagues stay up to date. We have outlined the current state of the art related to glucose lowering in patients with diabetes undergoing PCI. An accurate glycaemic control in the acute phase of ACS is a relevant factor for the improvement of longer-term outcomes. In addition, appropriate pharmacological therapy, including some newer drugs, for glucose control in the longer term can have a remarkable impact on recurrence of events, hospitalisations for heart failure and mortality and should be considered early in the patient’s disease trajectory. Extensive research efforts have led to improved outcomes for patients with dysglycaemic states in recent decades. Still, the rate of adverse events remains higher in patients with diabetes following PCI. Some important open issues that future research efforts must address are the following:
1. Optimal glycaemic control for the outcome of ACS, CCS and post-coronary revascularisation interventions remains to be established.
2. The role of hypoglycaemia in the occurrence of CV events/mortality remains to be fully elucidated.
3. Further trials with SGLT2 inhibitors and GLP-1 RAs in patients with coronary syndromes/undergoing PCI without diabetes would eventually provide further knowledge as to the potential benefits of these drugs irrespective of glucose control, possibly expanding their present indications.
Conflict of interest statement
I. Johansson has received research grants from the Swedish Heart Lung Foundation and from Stockholm County Council (Stockholms Lans Landsting). F. Consentino has received research grants from the Swedish Research Council, the Swedish Heart Lung Foundation, and the King Gustav V and Queen Victoria's Foundation, as well as fees from Abbott, AstraZeneca, Bayer, Bristol-Myers Squibb, Merck Sharp & Dohme, Mundipharma, Novo Nordisk, and Pfizer. E. Mannucci has received consultancy fees from Merck and Novartis, speaking fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Sanofi, and Novartis, and research grants from Merck, Novartis, and Takeda. I. Dicembrini has received speaking fees from Merck, Novo Nordisk, Eli Lilly, Abbott, Sanofi, and Boehringer Ingelheim.
Supplementary data
To read the full content of this article, please download the PDF.

