Abstract
Aims: Metformin is widely prescribed for the treatment of type 2 diabetes mellitus and is associated with areduction in diabetes-induced cardiovascular morbidity and mortality. Concerns about metformin-associated lactic acidosis (M-ALA) in patients undergoing contrast-based angiographic procedures have led to the development and publication of a number of guidelines to improve the management of this patient cohort.
Methods and results: This review focuses on the evidence behind these guidelines and, in particular, that concerning metformin discontinuation in diabetic patients undergoing coronary angiography and percutaneous intervention. This review addresses and compares guideline-directed management of such patients and includes the results of a UK physician survey to highlight variations in clinical practice.
Conclusions: We conclude that evidence for M-ALA in diabetics on metformin undergoing coronary intervention is lacking and existing guidance on the management of such patients is inconsistent. More robust evidence is needed in the form of a large, adequately-sized randomised trial or extensive registry so that we can optimally manage those patients requiring contrast-based coronary interventions who are also taking metformin.
Introduction
Metformin, a biguanide, was introduced in 1957 for the treatment of non-insulin dependent diabetes mellitus1. Metformin may also be used in combination with insulin and is the most widely prescribed oral agent in diabetes2. It exerts its effects primarily by decreasing hepatic gluconeogenesis and glycogenolysis3, and by increasing skeletal muscle glucose uptake4. Metformin is also associated with a reduction in cardiovascular morbidity and mortality, when compared to insulin or sulfonylureas5. The plasma half-life of metformin is between four and 8.7 hours in patients with normal renal function6, with 90% being eliminated via renal excretion within 24hours7. The biguanide agent that preceded metformin, phenformin, was withdrawn from clinical practice in 1978, since it was associated with an unacceptable risk of lactic acidosis8, ranging from 40 to 64 cases per 100,000 patient-years9. Phenformin had been shown to impair oxidative phosphorylation in the liver, thereby increasing lactate production through anaerobic pathways10. Metformin has an estimated risk of lactic acidosis ten to twenty times less than that of phenformin11, as a result of different pharmacokinetics12.
Metformin use in the setting of coronary angiography and percutaneous coronary intervention
Concerns about metformin-associated lactic acidosis (M-ALA) have led to the practice of metformin discontinuation prior to diagnostic angiography and percutaneous coronary intervention (PCI), since lactic acidosis is a serious condition, with an estimated mortality rate of approximately 50%13. The incidence of M-ALA has recently come into question, such that the case could be made that routine discontinuation of metformin could carry the converse risk of it not being recommenced, which may lead to deleterious effects on glycaemic control and increased cardiovascular risk14.
Evidence for metformin-associated lactic acidosis
The evidence that metformin use is associated with lactic acidosis (Table1) has evolved from case reports on metformin treatment15,16. The mechanism for M-ALA has been associated with decreased gluconeogenesis from lactate, which could in theory cause lactate accumulation under circumstances such as acute renal failure17. Metformin itself is not directly nephrotoxic18. In patients predisposed to acute deterioration in renal function after contrast administration, i.e., contrast-induced nephropathy (CIN), it has been postulated that there is the potential for metformin to accumulate, leading to lactic acidosis. However, evidence for this is poor. Firstly, although CIN occurs in 2% to 25% of patients undergoing coronary intervention19, not every patient on metformin that develops CIN develops M-ALA18. Secondly, most of the reported cases of lactic acidosis in patients taking metformin have been in patients with severe underlying conditions, including renal dysfunction, septicaemia, hepatic failure and acute left ventricular failure, any of which could in themselves contribute to the lactic acidosis20-23. Among the first million patients (approximately) to have received metformin in the United States, the Food and Drug Administration received 47 confirmed reports of lactic acidosis, and of these only four patients had no other apparent risk factors for lactic acidosis: 13 had pre-existing renal insufficiency; 30 had pre-existing cardiac disease, of whom 18 had congestive cardiac failure; three had chronic pulmonary disease and hypoxia; and eight were older than eighty23. Furthermore, lactic acidosis has been reported in diabetics not taking metformin, typically secondary to underlying conditions in which there was significant tissue hypoxia, such as acute left ventricular failure24. No specific study has addressed the impact of chronic metformin therapy versus recently started metformin on M-ALA.
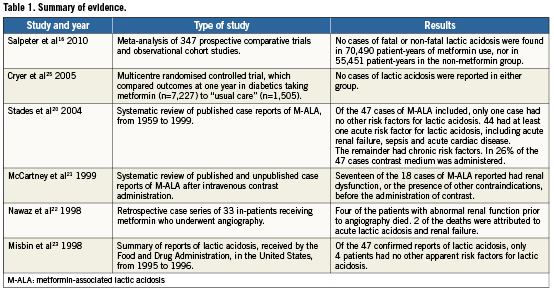
Evidence for the safety of metformin has been reported in a large randomised controlled trial (the Comparative Outcomes Study of Metformin Intervention Versus Conventional Approach [COSMIC] study), which compared outcomes at one year in diabetics taking metformin (n=7,227), to “usual care”, i.e., diabetics treated with asulfonylurea, thiazolidinedione, insulin, or any other non-metformin monotherapy or combination therapy (n=1,505)25. No cases of lactic acidosis were reported in either group.
A recent meta-analysis, by Salpeter et al16 on M-ALA, using pooled data from 347 prospective comparative trials and observational cohort studies, found no cases of fatal or non-fatal lactic acidosis in 70,490 patient-years of metformin use nor in 55,451 patient-years in the non-metformin group. Using Poisson statistics with 95% confidence interval the authors reported that the upper limit for the incidence of M-ALA was 4.3 cases per 100,000 patient-years, and in the non-metformin group the upper limit for the incidence of lactic acidosis was 5.4 cases per 100,000 patient-years. The mean blood lactate level measured during metformin treatment (1.24±0.31mmol/l), was not significantly different from that in patients on non-metformin therapies (weighted mean difference 0.04mmol/l, 95% confidence interval 0.00 to 0.13, p=0.07)16. Additionally, the net change from baseline lactate levels (1.13±0.25mmol/l), was no different in patients on metformin compared to non-metformin therapies. The authors concluded that there was no evidence that metformin was linked to an increased risk of lactic acidosis, or with increased lactate levels, compared to other anti-hyperglycaemic treatments16. However, most patients in these studies received metformin in the absence of routinely recommended contraindications such as renal failure. Furthermore, the meta-analysis16 did not address the issue of M-ALA in patients receiving contrast agents or in the setting of coronary angiography. The risk of M-ALA in patients undergoing coronary interventions is yet to be determined, with no published trial or registry data, but it is likely to be influenced by baseline renal function and volume of contrast agent used26.
Guidelines
Despite the findings of a previous randomised controlled trial25 and meta-analysis16, concerns regarding M-ALA have led to the development of strategies designed to reduce the potential incidence of M-ALA in patients on metformin undergoing coronary angiography and/or PCI. Existing guidelines on the management of such patients have been published by several professional organisations (Table2)27-33.
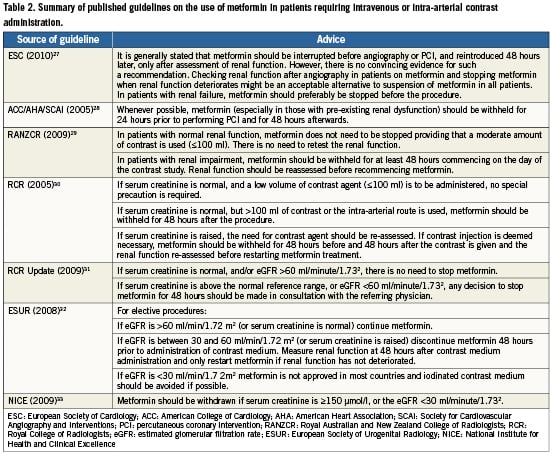
According to guidelines from the National Institute for Health and Clinical Excellence in the UK33, metformin should be withdrawn if serum creatinine is ≥150 μmol/l, or the estimated glomerular filtration rate (eGFR) is <30 ml/minute/1.732. Recommendations on the timing of discontinuing metformin prior to contrast administration vary depending on which guidelines are studied, and range from discontinuation 48 hours prior to the procedure30,32, 24hours prior to the procedure28, or on the day of the procedure29. According to the American College of Cardiology (ACC)/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions (SCAI) statement28, metformin need only be withheld for 24hours prior to performing PCI, “especially” in those with pre-existing renal dysfunction. By way of contrast the 2005 Royal College of Radiology (RCR) guidelines30 in the UK indicate that if serum creatinine is raised and contrast injection is deemed necessary, metformin should be withheld for 48hours prior to contrast administration. These guidelines30 do not specify the level of creatinine at which renal function is deemed “abnormal”, however, the 2009 RCR updated guidelines31 advise that if serum creatinine is above the normal reference range, or eGFR <60ml/minute/1.731, any decision to stop metformin for 48 hours should be made in “consultation with the referring physician”. The European Society of Cardiology (ESC) Guidelines on myocardial revascularisation27 recommend that in patients with renal impairment, metformin should be stopped before the procedure and suggest that an acceptable alternative to withholding metformin in all patients might be to check renal function after angiography and to stop metformin if renal function deteriorates. However, even these guidelines lack arobust evidence base, and have classed the recommendation of stopping metformin 48 hours prior to PCI in patients with known renal impairment as level of evidence C.
If metformin is discontinued, the ACC/AHA/SCAI28 and RCR30 guidelines advise restarting it 48 hours post contrast administration. However, existing guidelines are inconsistent in their recommendations on whether there is a need to recheck renal function prior to recommencing metformin15, and if so when is the best time, or whether this should be conducted according to dye load. The ACC/AHA/SCAI28 guidelines do not specify whether or not renal function should be reassessed prior to recommencing metformin, whereas the RCR30 guidelines advise that if baseline renal function is impaired, renal function should be reassessed prior to recommencing metformin.
Current practice in the UK: results of aphysician survey
We have collected information from UK physicians on their understanding of the management of patients on metformin booked to undergo coronary interventions, to better understand any variation in everyday clinical practice, and to determine to what degree local practice/strategies concur with published guidelines. In our study, an electronic questionnaire (Table3) was sent to 1,240 cardiovascular physicians from a central database throughout the UK, in November 2009. The questionnaire set out to determine views on the role of guidelines in the management of patients booked for contrast exposure on chronic metformin therapy and to determine, if any, the variation in practice. It included sections on: (i) presence and use of local guidelines, (ii) understanding of discontinuation criteria including definitions of renal impairment, (iii) the timing of metformin cessation, and its recommencement, (iv) renal function testing, and (v) views on the value of current guidelines. A total of 121 fully completed questionnaires were returned, representing a10% response rate. Responses were received from centres throughout the UK, including: London; Oxford; Cambridge; East Midlands: North and South; West Midlands; Kent; Surrey and Sussex; Leeds; Sheffield; Newcastle; Manchester; Liverpool; Severn; the South West Peninsula; Scotland; Ireland; and Wales. Therefore, this spread of regional responses from an unbiased physician approach may indeed provide a snapshot of the variance in what is currently being practised in the UK.


Local guidelines were in place in 89% of units. In those following local guidelines, the majority of respondents (51.9%) did not know which professional body guidelines the local recommendations were based on. In those following guidelines published by professional bodies, approximately 50% reported that they followed the RCR guidelines and 50% reported that they followed the ACC/AHA/SCAI guidelines.
Of all respondents, 94% routinely check renal function prior to elective coronary intervention. However, there is widespread uncertainty as to the definition of impaired renal function, with 36% of respondents using estimated glomerular filtration rate (eGFR) alone, 28% using creatinine alone and only 20% using eGFR, creatinine and urea, to define renal impairment.
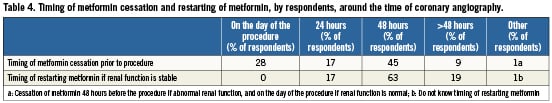
Eighty-eight percent of physicians routinely discontinue metformin prior to coronary angiography, irrespective of baseline renal status. Twenty-eight percent felt that discontinuing metformin did not make a significant difference to outcome. Of those who discontinue metformin, there was no consistency in the discontinuation period with 9% discontinuing over 48 hours prior to procedure, 45% 48 hours prior to procedure, 17% 24 hours prior to procedure, and 28% on the day of procedure (Table4). Of the total respondents in our study, 94% do not routinely check renal status post-procedure unless there is an abnormal pre-procedural result, for instance in a pre-admission clinic measurement. Recommencement timing ranged from 24 hours (17%) to more than 48 hours (19%) post-procedure. The overriding message borne out from this survey was that, when specifically asked, many (43%) did not think that any of the various current guidelines were clear, and overall (62%) of respondents felt that clearer guidance was needed. Our study of current practice on the management of patients on metformin undergoing coronary interventions demonstrated wide variations in clinician uptake and implementation of guidelines and overall clinical practice on a national scale.
Inconsistencies in existing guidelines
Current guidelines are inconsistent in their recommendations on the need to discontinue metformin, the timing of its cessation and the need to retest renal function prior to restarting metformin15. These inconsistencies are partly due to poor available evidence underpinning the guideline recommendations. It could of course be that the clinical issue is less important than believed by some, leading to inconsistency. However, the weak evidence base available raises the question of how to develop guidelines in the absence of good quality evidence and whether consensus-based expert opinion is robust enough and should have a role in such a setting. The ACC/AHA/SCAI statement on PCI in patients on metformin28 (Table2) is derived from expert consensus, however it is referenced with asingle citation on biguanide-related lactic acidosis by Aguilar et al34 from 1992. This was a retrospective observational cohort study, which concluded that biguanides in general are not associated with a high risk of metabolic acidosis and that severe systemic dysfunction in diabetics is the main determinant for lactic acidosis. Such consensus-based guidelines may not be as highly regarded as robust evidence emanating from randomised clinical trials, and therefore may not be rigorously followed in clinical practice, leading to variations in management. It may be that clinicians “recognise” that the risk of M-ALA is low, and may therefore not adhere rigorously to published guidelines on metformin discontinuation in patients requiring coronary interventions. If an adverse outcome is rare and unlikely to occur, the guidelines are less likely to be widely adhered to in clinical practice.
Recommendations
Following review of the guidelines for metformin discontinuation in the setting of elective coronary intervention, and in view of the lack of robust evidence, we would recommend metformin discontinuation when:
1)Serum creatinine is above the normal range prior to coronary intervention;
2)If this is the case, withdraw metformin for 48 hours before contrast administration and only restart metformin, if renal function measured 48 hours after contrast administration has not deteriorated.
If a patient with renal impairment has taken metformin within 48hours before contrast administration, the decision to postpone the procedure should be considered in the context of the urgency of the procedure, and after discussion with renal physicians. It is the authors’ opinion that if coronary intervention is required, and in the absence of definitive evidence of harm, the procedure should take place with adequate hydration and intravenous fluids, to minimise the risk of renal failure. Consensus through the appropriate guideline bodies and regulatory agencies would be needed to endorse such recommendations. Adequately-sized trials with sufficient power to detect a significant difference are clearly needed.
Conclusion
In summary, review of the published dataaddressingthe discontinuation of metformin for cardiac interventional procedures and alive snapshot of UK clinical practice has highlighted that robust evidence for M-ALA in diabetics on metformin undergoing coronary intervention is lacking. Existing guidelines on the management of such patients are inconsistent in their recommendations on the need to discontinue metformin, the timing of its cessation and the need to re-test renal function prior to recommencing metformin. Additionally, current practice varies widely. New data is necessary to determine whether there is a significant problem with lactic acidosis in those patients on metformin who undergo coronary angiography, and if so its scale. Furthermore, more data is needed to establish the potential hazard of inappropriate metformin discontinuation.
Conflict of interest statement
The authors have no conflict of interest to declare.

