
Abstract
Aims: A significant number of patients with non-valvular atrial fibrillation (NVAF) are ineligible for non-vitamin K oral anticoagulants (NOACs) due to previous major bleeding or because they are at high bleeding risk (HBR). In this setting the indication for percutaneous left atrial appendage closure (LAAO) is a valuable alternative. We aimed to evaluate the efficacy and safety of NOACs versus LAAO indication in NVAF patients at HBR (HAS-BLED ≥3).
Methods and results: All consecutive patients who underwent successful LAAO (n=193) and those treated with NOACs (n=189) (dabigatran, apixaban or rivaroxaban) were included. A 1:1 propensity score matching (PSM) was used to match LAAO and NOACs patients. At baseline, patients in the LAAO group had higher HAS-BLED (4.2% vs 3.3%, p<0.001) and lower CHADS-VASc (4.3% vs 4.7%, p=0.005) scores. After 1:1 PSM, 192 patients were enrolled in the final analysis (LAAO n=96; NOACs n=96). At two-year follow-up, no significant differences in thromboembolic (7.3% vs 6.3%, p=0.966) and ISTH major bleeding event rates (6.7% vs 4.8% p=0.503) were found between the two unmatched groups. All-cause death was significantly higher in the LAAO group (18.7% vs 10.6%; p=0.049). After PSM, all-cause death, thromboembolic and ISTH major bleeding event rates were similar between the groups. Significant independent predictors of all-cause death were dialysis (HR 5.65, 95% CI: 2.16-14.85, p<0.001) and age (HR 1.08, 95% CI: 1.05-1.13, p<0.001).
Conclusions: In NVAF patients at HBR, LAAO and NOACs performed similarly in terms of thromboembolic and major bleeding events up to two-year follow-up. Our findings warrant further investigation in randomised trials and therefore can be considered as hypothesis-generating.
Introduction
Non-vitamin K oral anticoagulants (NOACs) are the mainstay of stroke prevention in patients with non-valvular atrial fibrillation (NVAF), showing at least the same efficacy as warfarin but less intracranial bleeding1. Nonetheless, there is still a significant number of patients who do not receive anticoagulation (up to 40%) despite being eligible for it2 or who cannot benefit from this therapy because they are ineligible due to previous major bleeding (especially intracranial haemorrhage [ICH])3,4 or high bleeding risk (HBR).
In this setting, current AF guidelines suggest considering percutaneous left atrial appendage occlusion (LAAO)5. Indeed, antiplatelet therapy cannot be recommended, being inferior to warfarin for stroke prevention6 and also increasing the bleeding risk7. Albeit thrombi from the LAA account for 90% of ischaemic strokes8, there are other sources of thrombi (i.e., left atrium, left ventricular apex, aorta and carotid arteries) that may be treated better with anticoagulant therapy (due to its systemic effects) which are not prevented by LAAO.
A head-to-head comparison between the indications for NOACs and LAAO is still lacking. A recent network meta-analysis of randomised controlled trials and observational studies9 compared the efficacy and safety of LAAO and NOACs, showing that LAAO performed better than NOACs in avoiding major bleeding events but was less effective for ischaemic stroke prevention. Considering this pathophysiological background, we sought to evaluate the efficacy and safety of NOACs versus LAAO in a tertiary care real-world setting of NVAF patients at HBR.
Materials and methods
STUDY POPULATION
This was a single-centre, observational prospective study conducted at San Raffaele Hospital between July 2009 and December 2016. In the LAAO group, all consecutive patients with NVAF who underwent successful percutaneous LAAO in the Arrhythmia and Electrophysiology Unit, and at the Interventional Cardiovascular Unit were included. A successful LAAO procedure was defined as an effective device implantation in the LAA in the absence of serious adverse events (such as cardiac perforation and pericardial effusion causing cardiac tamponade, procedure-related stroke, other major bleeding and cardiac death).
In the NOACs group, all consecutive patients with NVAF (either anticoagulation naive or switched from a vitamin K antagonist) who had started a NOAC (dabigatran, apixaban or rivaroxaban) were included. Patients with valvular AF (moderate-to-severe mitral stenosis and mechanical prosthetic valves) were excluded. All patients were prospectively followed, with a minimum of one-year follow-up being required in order to be included in the final analysis. Patients treated with edoxaban (introduced in September 2016) were not included in the present analysis due to a short period of follow-up. LAAO patients who shifted towards anticoagulant therapy before one year and those in the NOACs group who underwent percutaneous LAA closure before one year after drug initiation were excluded (Figure 1).
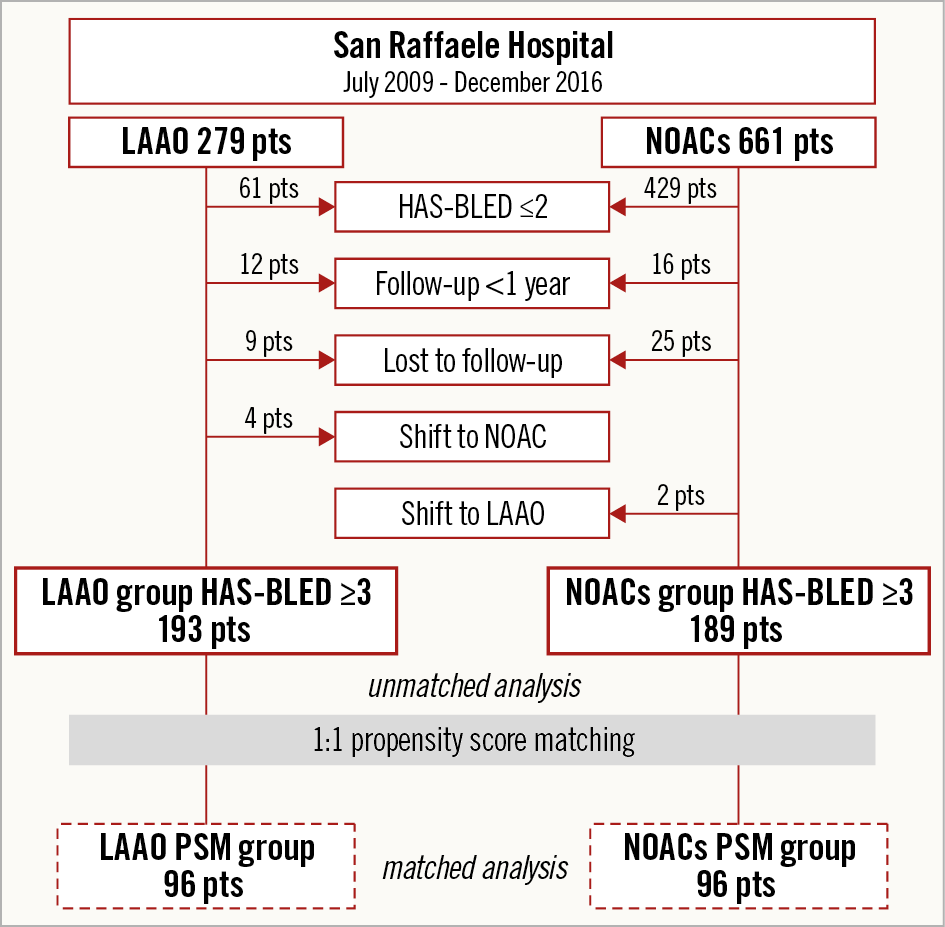
Figure 1. Study flow chart.
All NOACs were used at reduced doses approved for stroke prevention in NVAF in elderly people and in patients with chronic kidney disease (CKD), when indicated10. A patient was defined as having an HBR profile if the HAS-BLED score was ≥3. To achieve an equal distribution of baseline clinical characteristics, a 1:1 matched analysis (NOAC vs LAAO groups) without replacement was performed using propensity score matching (PSM).
The study was approved by the Hospital Ethics Committee and each patient provided written informed consent for the procedure, data collection and subsequent analysis. No external source of funding supported this study.
ENDPOINT DEFINITION
The primary safety endpoint was major bleeding, defined according to International Society of Thrombosis and Haemostasis (ISTH) classification: decrease in the haemoglobin level of at least 2 g/dL, transfusion of at least two units of packed red blood cells, occurring at a critical site or resulting in death. The primary efficacy endpoint included thromboembolic events: ischaemic stroke, transient ischaemic attack (TIA), systemic embolism (SE), acute myocardial infarction (AMI). The combined efficacy and safety endpoint was a composite endpoint of thromboembolic events (ischaemic stroke, TIA, AMI, SE) and ISTH major bleeding. The individual categories of the efficacy endpoint, as well as all bleedings, intracranial bleedings, gastrointestinal bleedings, overall death and cardiac death were analysed as secondary endpoints.
DATA COLLECTION (Supplementary Appendix 1)
STATISTICAL ANALYSIS
Continuous variables were reported as mean±standard deviation (SD). Categorical variables were compared with the χ² or Fisher’s exact test, as appropriate. Clinical outcomes and adverse events were prospectively monitored by direct visit, phone interview or contact with the referring physician, and specific hospital files were requested when needed. Event-free survival was evaluated according to the unadjusted Kaplan-Meier method and survival among groups was compared using the log-rank test (Cox-Mantel test). Finally, multivariable Cox regression analysis was performed to analyse the influence of relevant variables (diabetes, prior myocardial infarction, prior intracerebral haemorrhage, dialysis and age) on mortality. To avoid multi-collinearity, a “low-noise model”, in which each predictor variable correlates at most only minimally with the other, was researched. Only covariates that were significantly associated with the risk of death at univariate analysis (p<0.10) were included, and the convention of limiting the number of independent variables to one for every 10 events was followed. A propensity score-based sensitivity analysis method for uncontrolled confounding between groups was performed and obtained by fitting a logistic regression model with the type of treatment (NOAC vs LAAO) as binary outcome and other predictor variables for the primary endpoint11. Patients of the two groups were matched 1:1 through a greedy algorithm based on a calliper defined as having a maximum width of 0.2 standard deviation (SD) of the logit of the estimated propensity score. The final calliper was 0.06112. Finally, the success of PSM was judged by analysing the baseline clinical characteristics in the propensity-matched groups; absence of difference in all variables related to the endpoint (p-value >0.05) supports the assumption of a balance between matched groups13. Hosmer and Lemeshow (H-L) and c-statistic tests were used to assess the goodness of fit for logistic regression models and the predictive model discriminatory power, respectively. Data for patients lost to follow-up were censored at the time of the last contact. Two-sided p-values <0.05 were considered statistically significant. The statistical analyses were performed using SPSS, Version 23 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CLINICAL DATA
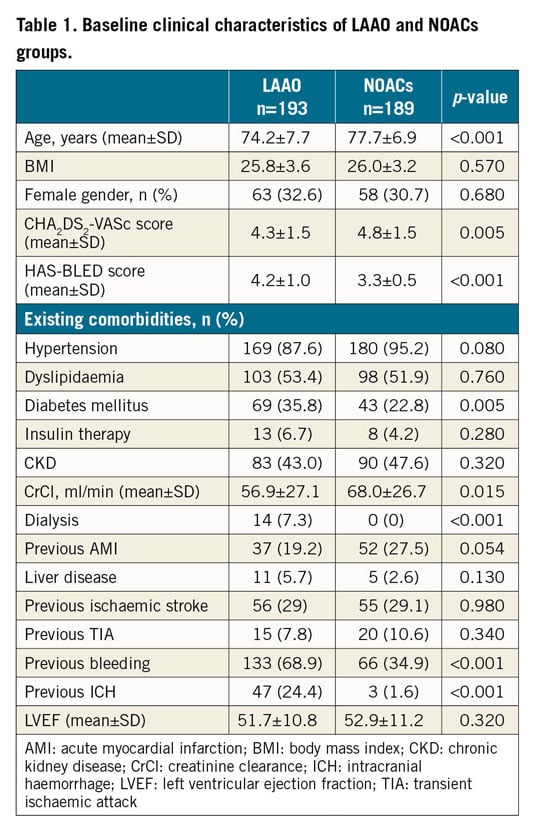
During the index period, from a total cohort of 940 patients with NVAF, 382 patients presented a HAS-BLED risk score ≥3 and were included in the present study. Of these, 193 patients (193/940, 20.5%) underwent successful LAAO (LAAO group) and 189 patients (189/940, 20.1%) were treated with NOACs (NOACs group) (Figure 1). In the LAAO group, the WATCHMAN™ device (Boston Scientific, Marlborough, MA, USA) was used in 65 cases (33.7%), the AMPLATZER™ Cardiac Plug (St. Jude Medical [now Abbott Vascular], St. Paul, MN, USA) in 43 cases (22.3%), while in the remaining 85 cases (44%) the AMPLATZER™ Amulet™ device was used. After the procedure, 40 patients (20%) were discharged with an indication for only anticoagulation therapy up to two months, 141 patients (64%) with an indication for dual antiplatelet therapy up to six months, and 12 patients (6%) on single antiplatelet therapy up to two months. In the NOACs group, 78 patients were treated with dabigatran (41%), 77 with apixaban (41%) and 34 with rivaroxaban (18%). Baseline clinical characteristics of the two groups (unmatched population) are reported in Table 1. Patients who underwent percutaneous LAAO had more comorbidities such as diabetes and lower creatinine clearance, and more previous bleeding and intracranial bleeding, reflected in a higher HAS-BLED score (4.2 LAAO vs 3.3 NOACs, p<0.001). Fourteen LAAO patients (7%) were on dialysis, while, as expected, there were none in the NOACs group. NOACs patients were older and at higher ischaemic risk as estimated by CHA2DS2-VASc score (4.8 vs 4.3, p=0.005). After 1:1 PSM, 192 patients (96 forming the NOACs group and 96 the LAAO group) were adjusted for variables included in the CHADS-VASc and HAS-BLED scores. Finally, the two groups were homogeneous in terms of age (73.8±7.1 vs 75.3±6.8 years, p=0.15), ischaemic and bleeding risk (CHADS-VASc 4.3±1.5 vs 4.3±1.5, p=0.88 and HAS-BLED 3.5±0.7 vs 3.5±0.6, p=0.83), as reported in Supplementary Table 1.
CLINICAL OUTCOMES
Clinical outcomes of the two unmatched groups are reported in Table 2. At two-year follow-up (median/IQR 2.4/2.1-2.9 years), no significant differences in the primary efficacy endpoint (thromboembolic events) were observed between the LAAO and NOACs groups (7.3% vs 6.3%, p=0.966). Similarly, the ischaemic stroke (3.1% vs 3.2%), TIA (2.1% vs 1.1%), SE (0% vs 1.1%) and AMI rates (1.6% vs 1.6%) were comparable between the two unmatched groups. Regarding the primary safety endpoint (ISTH major bleeding events), this occurred in 13 LAAO patients and in nine NOACs patients, with no statistical difference (6.7% vs 4.8% p=0.503, respectively). Among LAAO patients, an ISTH major bleeding event occurred in 2/13 (15.3%) and 6/13 (46.1%) patients during the first three and six months, respectively. Also, the gastrointestinal bleeding rate did not differ between the NOACs and LAAO groups (8.5% vs 5.2%, p=0.203). In terms of overall bleedings, more patients on NOACs experienced an event (20.6% vs 11.9%; p=0.021). Unmatched Kaplan-Meier curves for the primary efficacy endpoints, primary safety endpoints and for the combined endpoint are shown in Figure 2. The combined efficacy and safety endpoint occurred in 27 patients (14%) in the LAAO group and in 21 patients (11%) in the NOACs group (p=0.881). Compared to the NOACs group, the all-cause death rate was significantly higher in the LAAO group (18.7% vs 10.6%; p=0.049). When considering cardiac death alone, no difference was evident. The incidences of minor procedural complications, leaks, device-associated thrombus and stroke at follow-up were low and similar among the three different occluder devices used for LAAO procedures (Supplementary Table 2).
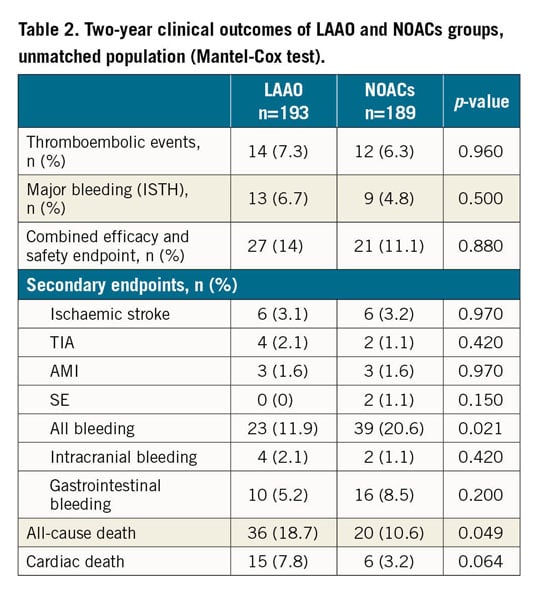
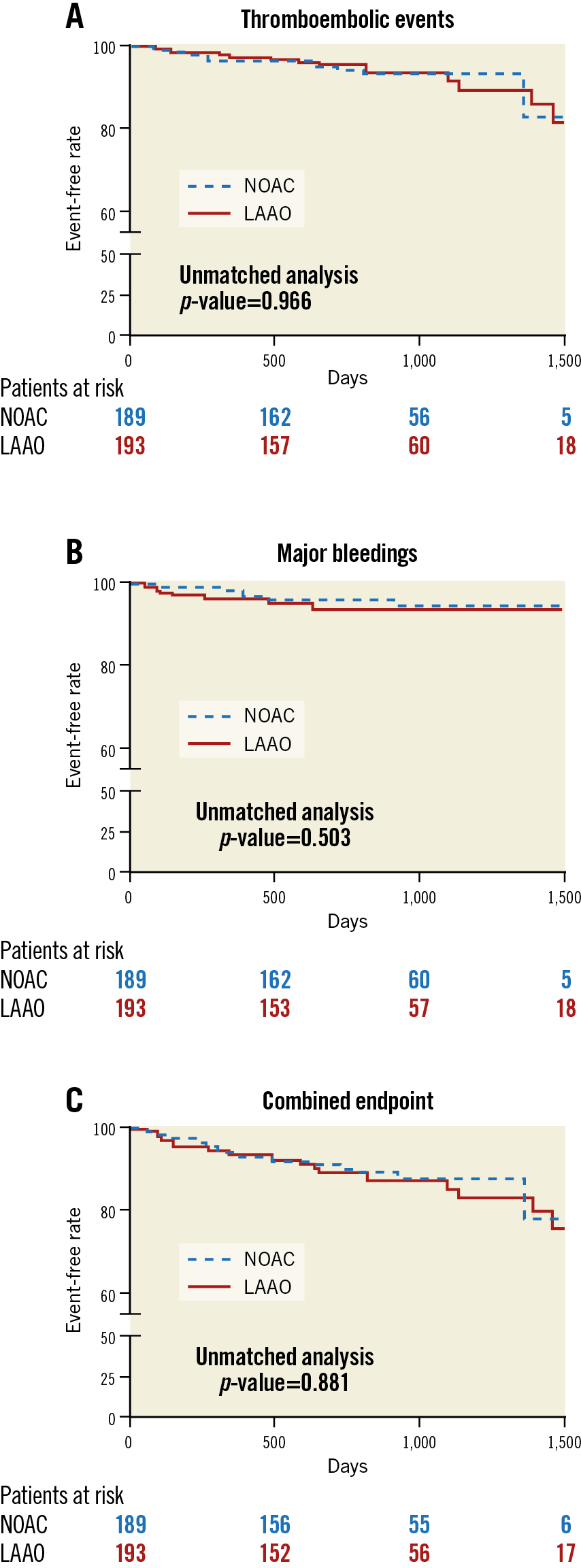
Figure 2. Unmatched Kaplan-Meier analyses. A) Thromboembolic events. B) ISTH major bleeding events. C) Combined thromboembolic and ISTH major bleeding events (log-rank [Mantel-Cox] test).
After PSM, no difference in all-cause death was evident between the two matched groups (10.4% vs 15.6%, p=0.284, NOACs vs LAAO) and the rates of thromboembolic and ISTH major bleeding events were still comparable (7.3% vs 6.3%, p=0.77, and 6.3% vs 6.3%, p=1, respectively). Matched Kaplan-Meier curves for the matched analysis are shown in Supplementary Figure 1.
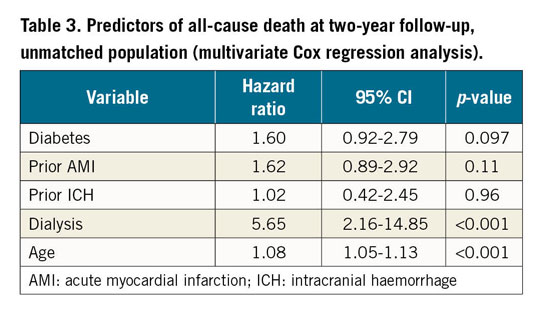
According to the multivariate analysis, significant independent predictors of all-cause death (Table 3) were dialysis (HR 5.65, 95% CI: 2.16-14.85, p<0.001) and age (HR 1.08, 95% CI: 1.05-1.13, p<0.001), c-statistic value (0.72), Hosmer-Lemeshow (0.830).
Discussion
This study is one of the first comparisons between percutaneous left atrial appendage occlusion and the use of direct oral anticoagulants for thromboembolic event prevention in HBR patients with non-valvular atrial fibrillation. The main findings of our study are the following:
1. In this tertiary care real-world series of NVAF patients, one third of patients were at HBR (HAS-BLED ≥3) and were treated with NOACs or LAAO equally.
2. At two-year follow-up, the primary efficacy and safety endpoints were equal for the two groups of indication (thromboembolic events: 7.3% in the LAAO group vs 6.3% in the NOACs group, and ISTH major bleeding: 6.7% vs 4.8%, respectively).
3. In the unmatched analysis, the LAAO group showed a higher rate of all-cause death, reflecting the real-world high-risk profile of patients having this indication.
4. After 1:1 PSM, in both the NOACs and LAAO groups comparable outcomes were confirmed for the primary endpoints and also for all-cause death.
Large randomised clinical trials and real-world evidence1,14,15,16 have shown NOACs to be at least as effective as warfarin in the prevention of stroke in patients with NVAF, with fewer intracranial haemorrhages. This has led current ESC AF guidelines to recommend NOACs over warfarin for stroke prevention in NVAF patients5.
However, although NOACs have a better safety profile than warfarin, use of anticoagulant therapy carries a non-negligible risk of serious bleedings. This is a major concern, especially for patients with a high HAS-BLED score or for patients who have experienced previous major bleedings (especially ICH) due to apprehension of possible recurrence of bleeding related to anticoagulation. At the same time, the ischaemic risk might even outweigh the bleeding risk, as described in some patients with previous ICH17.
In this setting, as suggested by current ESC guidelines5, LAAO might be an option (grade IIb, level B). In the PROTECT AF trial, LAAO with the WATCHMAN device met criteria for both non-inferiority and superiority, compared to warfarin at one year18 and four years19 of follow-up, in terms of prevention of the combined outcome of stroke, systemic embolism, and cardiovascular death. Nonetheless, the PREVAIL trial20 failed to achieve the pre-specified criteria for non-inferiority, raising some concerns about the efficacy of this procedure, at least in patients eligible for OAC therapy. Although randomised clinical trials have evaluated LAAO in patients who were still eligible for anticoagulation, in real-world practice there was a shift towards LAAO in higher-risk patients with a contraindication to anticoagulation or deemed at prohibitive risk of bleeding21.
Thromboembolic event prevention in these patients is challenging but necessary: the main objective of our research was to evaluate whether, in a setting of patients with HBR or contraindication to oral anticoagulant therapy, LAAO represented a valuable alternative to standard care with NOACs, especially regarding effectiveness.
A network meta-analysis of both randomised clinical trials and observation studies9 evaluated the safety and efficacy of LAAO and NOACs in anticoagulation-eligible patients, showing that LAAO performed better than NOACs in avoiding major bleeding events but was less effective for ischaemic stroke prevention (randomised trials analysis). In the present study, in the unmatched analysis, patients with an indication for LAAO experienced comparable rates of both thromboembolic and ISTH major bleeding events compared to patients with an indication for NOACs. Although we expected a better efficacy profile with NOACs, our findings can be partially explained by taking into account 1) the increased ischaemic risk of patients with an indication for NOACs, as estimated by the CHADS-VASc score (4.8 vs 4.3, p=0.005), 2) the relatively short follow-up time (mean two years), and 3) the fact that two thirds of patients with an indication for LAAO (64%) continued the antithrombotic treatment up to six months after the procedure. On the other hand, the finding of comparable safety (major bleeding events) between groups, albeit with an increased bleeding risk in patients with an indication for LAAO, highlights the safety profile of NOACs and encourages their use in a setting of HBR patients, provided no clear contraindications exist. Notably, the LAAO group showed an increased rate of all-cause death; however, this finding should be evaluated considering the baseline differences in clinical profile and possible unmeasured confounders between groups. In fact, this difference was lost after the matched analysis, adjusting for variables included in the CHADS-VASc and HAS-BLED scores. The matched groups, with similar comorbidities and ischaemic and bleeding risks, showed comparable all-cause death rates as well as thromboembolic, ISTH major bleeding events and combined endpoint rates. Thus, both indications (NOAC and LAAO) seem to be valuable strategies for thromboembolic event prevention in patients at HBR.
Finally, we aimed to identify predictors of all-cause death in this cohort of NVAF patients at HBR. Dialysis was the strongest predictor of all-cause death, further confirming that the reduced survival after LAAO could reflect the high-risk profile of patients who undergo this kind of procedure in the real world.
Recently, in a propensity-matched study in patients with prior ICH treated with oral anticoagulants (one third with NOACs) or LAAO (one third receiving no antithrombotic treatment during follow-up), the primary composite endpoint (all-cause mortality, ischaemic stroke and major bleeding) was lower in the LAAO group22. The estimated risk of the included population was similar to our study, but rates of ischaemic stroke (8.7%/year in the OAC group and 2.6%/year in the LAAO group) were higher compared to our findings, while major bleeding (4.1%/year in the OAC group and 2.5%/year in the LAAO group) were similar. The increased stroke risk in that study may be partially explained by the time gap between the start of observation and the initiation of anticoagulation (within 180 days after their index ICH). In another study on LAAO with the AMPLATZER Plug in patients with previous major bleeding and an estimated bleeding risk profile similar to our patients, the major bleeding rate was higher (6%/year), while the stroke rate was similar (2.1%/year)23. The recent two-year follow-up of the EVOLUTION trial (WATCHMAN device) reported similar incidences of major bleeding (2.7%/year) and stroke (1.3%) events24. Conversely, both major bleeding and stroke rates were higher in the one-year follow-up of the prospective AMPLATZER Amulet registry (10.3%/year and 2.9%/year, respectively); the high proportion of patients with previous major bleeding may probably account for the particularly high major bleeding rate in this registry25.
The ongoing PRAGUE-1726, prospective, multicentre, randomised non-inferiority trial will determine whether LAAO is non-inferior to treatment with NOACs in moderate- to high-risk AF patients, and the ongoing OPTION (NCT03795298) trial will determine whether LAAO with the WATCHMAN FLX™ device is a reasonable alternative to oral anticoagulation in patients after AF ablation.
In conclusion, despite the high ischaemic risk of the present population, NOACs and LAAO showed comparable and reassuring efficacy in thromboembolic event prevention. Indeed, our initial hypothesis of finding more cerebral ischaemic events in the LAAO group (because of the procedure’s ineffectiveness on the sources of thromboembolism other than left atrial appendage) was not confirmed.
On the other hand, NOACs did not show an increase in ISTH major bleeding events as compared to the LAAO group, highlighting the good safety profile of these drugs in this challenging setting. Thromboembolic event prevention should always be pursued in patients with AF, even if the bleeding risk is high. Our results contribute to the evidence about the encouraging safety of non-vitamin K oral anticoagulants (NOACs) and effectiveness of LAAO for this purpose. Nevertheless, our findings warrant further investigation in larger randomised trials and therefore can be considered only as exploratory and hypothesis-generating.
Study limitations
The principal limitation of this study is its observational nature. On the other hand, it represents a snapshot of a high-volume tertiary care centre practice in this challenging setting. The PSM analysis, with the ability of balancing groups, contributed to a more precise estimation of treatment response. The relatively small size of the population studied might have led to a possible underestimation of the events during follow-up. In order to evaluate the event rate fully, a longer follow-up would have been useful. Moreover, the discharge antithrombotic regimen used was not standardised, but was different according to the clinical characteristics of the patient and the device used.
Conclusions
This tertiary care single-centre observational study showed good safety and efficacy outcomes after LAAO and NOACs in NVAF patients at HBR (HAS-BLED ≥3), with no differences in thromboembolic events or in major bleeding between groups even after propensity score-matching analysis. The inherent limitations of the observational study design require that these results be confirmed in a randomised clinical trial.
|
Impact on daily practice Prevention of thromboembolic events in patients with NVAF at high bleeding risk is challenging. This single-centre real-world 1:1 propensity-matched study showed that LAAO was as effective as NOACs in preventing ischaemic events. On the other hand, NOACs were extremely safe with no excess of major bleeding. Thus, both treatments can be considered valuable in this high-risk patient setting. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

