- drug-coated balloon
- drug-eluting stent
- restenosis
- contrast media
Abstract
Aims: EPC and hCASMC play an important role in the pathogenesis of restenosis and stent thrombosis. Drug-coated balloon catheters exert a local, short-term application of antiproliferative agents. This study investigates the time-dependent influence on growth and motility of paclitaxel and sirolimus alone and combined with the coating additive iopromide on EPC and hCASMC.
Methods and results: Treatment of cultured human EPC and hCASMC with paclitaxel and sirolimus 1.5 and 15 µM for three seconds, three minutes and 24 hours, alone or combined with iopromide 0.197 M, resulted in a concentration- and time- dependent inhibition of proliferation and of migration. Paclitaxel and sirolimus increase apoptosis in either cell type. However, the effects of paclitaxel and sirolimus differed between the cell types: short-term exposure with paclitaxel leads to stronger inhibition of cell-density and apoptosis of hCASMC compared to EPC. In comparison to paclitaxel, short-term incubation with sirolimus showed a more effective inhibition of cell-density and migration as well as increased apoptosis in EPC in contrast to hCASMC. The effects of paclitaxel and sirolimus were increased in combination with iopromide. Interestingly, the antiproliferative effect of the paclitaxel-iopromide formulation on hCASMC was more potent compared to its effect on EPC. Endothelialisation in a porcine coronary stent model was similar with drug-coated balloons and uncoated controls, whereas it was delayed with drug-eluting stents.
Conclusion: After short-term application, paclitaxel and sirolimus show differential, cell-specific effects on EPC and hCASMC. Iopromide used as a coating agent intensifies these effects.
Introduction
Drug-eluting stents (DES) were introduced for the prevention and treatment of intracoronary restenosis, a major problem of interventional cardiology. Restenosis is caused by neointimal hyperplasia due to hCASMC proliferation possibly as response to injury1. DES potently prevent restenosis by locally delivering an antiproliferative drug for a long time period. On the other hand, recent studies show that the anti-restenotic effects of drugs eluted from coated stents and their polymers could limit safety. The sustained drug release into the tissue and the polymer itself can lead to sustained delay in endothelial healing, inflammation and subsequently to late thrombotic complications2.
More recent data indicate that local non-stent based short-term application of anti-restenotic drugs may be similarly effective to prevent restenosis in coronary arteries3-6. For example, the drug-delivering balloon (Genie®) allows a local, short-term application of drugs in a suitable solvent while occluding the distal and the proximal vessel segment with a double balloon5. The drug-coated balloon catheter (PACCOCAT®) delivers a homogeneous drug concentration to the target lesion during short inflation time of the balloon. For this technique, the X-ray contrast medium iopromide is an essential component of the coating method because of its properties as effective solvent for lipophilic drugs such as taxanes in aqueous media. Preclinical trials demonstrated the efficacy of the drug-coated balloon to inhibit neointimal proliferation3,4,6. These results were confirmed by clinical evidence in patients with coronary in-stent restenosis7-9 and peripheral artery disease10,11.
It has been shown that two cell types play opponent roles in the pathogenesis of in-stent restenosis and potentially also in late stent thrombosis, namely endothelial progenitor cells (EPC) that help in re-establishing the integrity of the endothelial layer, and might protect from restenosis12,13, and coronary artery smooth muscle cells (hCASMC) which enhance neointima proliferation14-18. A differential influence of short-term application of paclitaxel or sirolimus alone or in combination with iopromide on EPC and hCASMC may play a role in the prevention of restenosis and stent thrombosis. To investigate this hypothesis we studied cell growth, apoptosis and cell function in EPC and hCASMC after incubation with paclitaxel and sirolimus alone and in combination with iopromide. The time points were chosen according to the short-term exposition with the drug-coated balloon and the more long term exposure by drug-eluting stents. The concentration of 15 µM paclitaxel alone or in combination with iopromide 0.197 M represents the average paclitaxel tissue concentration which is known to be effective in cell culture and in the porcine coronary arteries after treatment with the drug- coated PACCOCATH® balloon3,19. Furthermore, in vivo endothelisation was investigated in the porcine coronary stent model.
Methods
Cell isolation and cell culture
Mononuclear cells (MNC) were isolated by density gradient centrifugation with Biocoll from peripheral blood of a healthy human volunteer (BS) and cultured on fibronectin-coated dishes in endothelial basal medium (Cell Systems Corporation, Kirkland, WA, USA) supplemented with endothelial growth medium SingleQuots and 20% fetal calf serum. Blood sampling conformed with the principles outlined in the Declaration of Helsinki for use of human tissue or subjects. After three days in culture, adherent cells were incubated with DiLDL and stained with FITC-labeled Ulex europaeus agglutinin I (lectin, 10 µg/mL; Sigma-Aldrich Corp., St. Louis, MO, USA)20,21. Following the isolation, 106MNCs/ml medium were plated on culture dishes coated with fibronectin (Sigma) and starved in Roswell Park Memorial Institute Medium (RPMI, Invitrogen; Life Technologies Corporation, Carlsbad, CA, USA). After three days in culture, non-adherent cells were removed by washing with phosphate-buffered saline and adherent cells were incubated in fresh medium with various concentrations (1.5 and 15µM) of paclitaxel or sirolimus alone and in combination with iopromide 0.197M for different incubation-times (three seconds, three minutes and 24 hours exposition). The concentrations were selected according to prior experiments showing direct pharmacokinetic evidence for uptake of the active compound in the vessel wall of opacified porcine arteries and adjacent tissue at concentrations in the range of 3 to 15 mol/l19,45,46.
Paclitaxel was purchased from Indena (Indena S.p.A., Milan, Italy); sirolimus, from Fujian Kerui (Fujian Kerui Pharmaceutical Co., Ltd., Cangshan District, Fuzhou, Fujian, China); iopromide from Bayer Schering (Bayer Schering Pharma AG, Berlin, Germany). Paclitaxel and sirolimus were dissolved in ethanol in a final dissolution of ethanol 0.2%. MNC were cultured for seven to 14days in endothelial basal medium supplemented with EGM Single Quots and 20% fetal calf serum.
HCASMC, as well as cell culture medium (SmBm Smooth Muscle Cell Basal Medium + SmGM-2 BulletKit), were purchased from Lonza (Clonetics; Lonza AG, Basel, Switzerland). The 104hCAMCs/ml medium were plated on culture dishes coated with fibronectin (Sigma) in suitable medium and starved for three days. HCASMC passages were 4 to 7. Non-adherent cells were removed by washing with phosphate-buffered saline. Adherent cells were incubated in fresh medium with various concentrations (1.5 and 15µM) of paclitaxel or sirolimus alone and in combination with iopromide 0.197M for different incubation-times (three seconds, three minutes and 24 hours exposition). Cells were cultured for seven to 14 days. All experiments were carried out at the very least in triplicates.
Cell density and proliferation
Cells were cultured in dishes coated with human fibronectin, washed and fixed in 4% formaldehyde. EPC and hCASMC were stained with 4.6-diamidinophenylindole (DAPI; 0.2g/ml in 10mmol/l Tris–HCl, pH 7.0, 10mmol/l EDTA, 100mmol/l NaCl) for 20 minutes. EPC were additionally characterised by dual staining for 1.1-dioctadecyl-3.3.3 3 - tetramethylindocarbocyanine labelled acetylated low-density lipoprotein (DiLDL) and Lectin. Three culture dishes per condition were counted under fluorescence microscopy by two independent blinded investigators.
For the measurement of proliferation, the cells were seeded at a density of 3x106 cells/well (MNCs) and 1x104 cells/well (HCASMC) in a 24-well plate. The cells were cultivated in EGM and SMGM for three days. At day three, the cells were treated with sirolimus and paclitaxel in a concentration of 15µM for seconds and 24 hours. At day seven (four days after stimulation), cell proliferation was evaluated by MTS Assay (Cell Titer 96® AQueous One Solution Cell Proliferation Assay; Promega Corporation, Madison, WI, USA). The media was replaced by serum-free media (500µl) and the MTS Solution (100µl) was added to each well. After incubation for 3½h the absorbance was detected at 450nm using an elisa reader (Xfluor; Tecan Group Ltd., Männedorf, Switzerland). Assays were performed in triplicates.
Apoptosis
Detection of EPC apoptosis by Annexin-FACS analysis: adherent cells were detached with trypsin, washed in PBS, and incubated with fluorescein isothiocyanate conjugated annexin22. IgG 2a-fluorescein isothiocyanate (PharMingen, San Diego, CA, USA) served as negative control. FACS analysis was performed immediately after staining using a FACS Calibur instrument (BD [Becton, Dickinson and Company], Franklin Lakes, NJ, USA) and Cell Quest software (BD Biosciences).
Detection of apoptosis in EPC and hCASMC by DAPI staining: cells were cultured on culture dishes coated with human fibronectin, fixed in 4% formaldehyde, and stained with 4.6-diamidinophenylindole (DAPI; 0.2g/ml in 10mmol/l Tris–HCl, pH 7.0, 10mmol/l EDTA, 100 mmol/l NaCl) for 20 minutes. Additionally EPC were stained with DiLDL-Lectin. Morphological features of apoptosis were defined as shrinking of cytoplasm, membrane blebbing, irregular surface and chromatin condensation. Five hundred cells were counted by two independent blinded investigators, and the percentage of apoptotic cells per total number of cells was determined.
Migration
Migratory capacity of EPC and hCASMC was determined in a modified boyden chamber22. Cells were cultured and incubated as described above. Cells were harvested by centrifugation and re-suspended in 500µl medium, counted and placed in the upper chamber of a modified boyden chamber (BD Bioscience; 1×105 EPCs per chamber, 1×104 hCASMCs per chamber). The chambers were placed in 24 well culture dishes containing medium and VEGF (50ng/ml). After incubation at 37°C for 24 hours the lower side of the filter was washed, fixed and stained with DAPI/DiLDL. Migrated cells at the lower surface of the filter were counted manually in four random microscopic fields.
Animal model
All animal experiments were conducted after approval of the local animal protection committee in accordance with their guidelines for animal experiments (animal protection committee of the Sachsen-Anhalt government, Germany) and the European Commission Directive 86/609/EEC. After the animals reached an adequate anaesthetic level with ketamin, xylazinhydrochlorid and propofol, they were intubated and general anaesthesia was maintained with an inhaled mixture of 30 volume % of pure oxygen, 70 volume % N2O and 1-2 volume % of isoflurane. Thirty-eight stents were implanted in the left anterior and circumflex coronary arteries of nineteen domestic pigs with an oversize ratio of ≈1.2. The animals were randomised to four different treatments:
1) control, consisting of uncoated bare metal stents crimped onto uncoated PTCA catheters (n=8 vessels);
2) DCB+BMS, consisting of uncoated bare metal stents crimped onto PTCA catheters coated with a paclitaxel dose of 3 µg/mm2 of balloon surface (n=10 vessels);
3) Taxus®, paclitaxel-eluting stents (n=10 vessels);
4) Cypher®, sirolimus-eluting stents (n=10 vessels).
After three days quantitative angiography (QCA) and histomorphometry of the stented arteries was performed. Hearts were rapidly excised and the arteries fixed by perfusion with 4% buffered formalin. The target segments were carefully dissected and stained with anti-vWF antibodies (polyclonal rabbit anti-human von Willebrand Factor, DakoCytomation; Dako, Glostrup, Denmark). After antibody staining the segments were embedded in methyl-methacrylate (Heraeus Kulzer, Wehrheim, Germany). Five cross-sections per segment were evaluated for stent strut endothelialisation in comparison to the negative control (semi-quantitative score in which 0 means no coverage, 1 partial coverage, 2 complete coverage of stent struts, and three complete coverage of stent struts by neointima).
Statistical analysis
Results are presented as mean ± standard deviation. Unpaired Students’s t-test for single comparison and ANOVA analysis (Student-Newman-Keuls post hoc test) were performed by SPSS 15.0 software. Values of p <0.05 were considered significant.
Results
Cell density and proliferation
After exposure to paclitaxel and sirolimus (1.5 and 15µM) for three seconds and culture of the cells for seven to 14 days, endothelial progenitor cells (EPC) as well as coronary artery smooth muscle cells (hCASMC) showed a concentration-dependent decrease of cell density. Short-term treatment for three seconds with 1.5µM sirolimus exerted a decrease of EPC density to 19±4% of control treated cells. Treatment with paclitaxel in 1.5µM did not decrease EPC density and 15µM resulted in a lesser decrease of EPC density (77±5%) compared to sirolimus. In contrast to EPC, the reduction of hCASMC density was stronger after paclitaxel treatment compared to sirolimus after incubation for three minutes. In particular after treatment with the 15µM concentration, paclitaxel showed amore potent effect on hCASMC density then sirolimus (45±10% vs. 74±7%; p<0.05). Exposure with paclitaxel or sirolimus (1.5 and 15µM) for 24 hours intensified the effect of paclitaxel on hCASMC density. Longer incubation time of 14 days shows a long-lasting effect of sirolimus on EPC density (Figure1). Cell proliferation was reduced by sirolimus and paclitaxel.
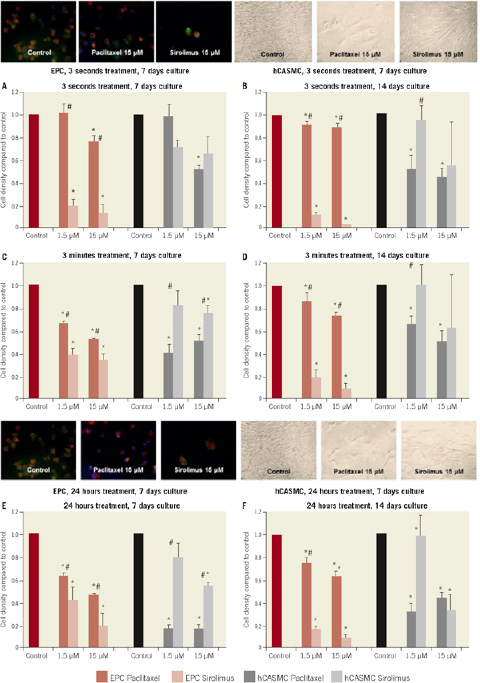
Figure 1. Cell density after exposure to 1.5 and 15 µM paclitaxel or sirolimus compared to control treated cells for 3 seconds with 7 days culture (A), 3 seconds with 14 days culture (B), 3 minutes with 7 days culture (C), 3 minutes with 14 days culture (C), 24 hours with 7 days culture (D) and 24 hours with 14 days culture (E). Each panel depicts the concentration-dependent effect on cell density in both cell lines. EPC: human endothelial progenitor cells; hCASMC: human coronary artery smooth muscle cells; * p<0.05 compared to control; # p<0.05 sirolimus vs. paclitaxel. Photographs of cell density after 3 seconds (above) and 24 hours (below) exposure with 15 µM paclitaxel or sirolimus compared to control, 7 days culture.
Apoptosis
Annexin FACS analysis was carried out after incubation of EPC with paclitaxel or sirolimus (15µM) for three seconds or 24 hours. The long term effect of sirolimus on EPC apoptosis after 14 days in culture was more potent compared to paclitaxel (250±13% vs. 158±21%; p<0.05 / 15µM, 24hours exposition). Detection of apoptosis in hCASMC was quantitated by DAPI staining. After short-term exposure of hCASMC to paclitaxel an increased apoptosis rate was observed (426±62% / 15µM, three seconds exposition). Compared to paclitaxel, sirolimus required 24 hours exposure to induce long-lasting apoptosis in hCASMC (Figure 2).
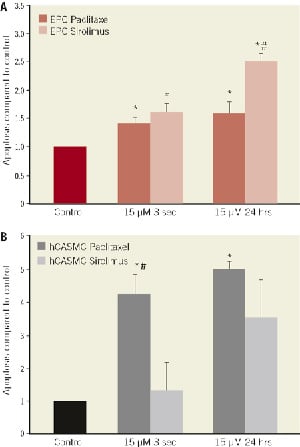
Figure 2. Rate of apoptosis after exposure to 1.5 and 15 µM paclitaxel or sirolimus compared to control treated cells for 3 seconds and 24 hours with 14 days culture in (A) human endothelial progenitor cells (EPC) and (B) in human coronary artery smooth muscle cells (hCASMC) determined by DAPI staining. *p<0.05 compared to control; #p<0.05 sirolimus vs. paclitaxel
Migratory potency
A critical event in both prevention and pathogenesis of restenosis constitutes the migration of EPC and hCASMC into the injured area23. Decreased migratory capacity was observed after incubation with both paclitaxel and sirolimus for 24 hours in both EPC (79±7% vs. 76±7%; ns) and hCASMC (68±10% vs. 69±1%; ns). In contrast, short-term exposure with paclitaxel did not inhibit EPC migration (112±31%) (Figure 3).
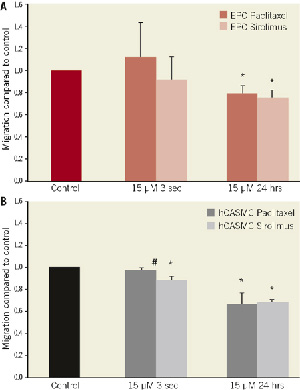
Figure 3. Cell migration after exposure of EPC and hCASMC with paclitaxel or sirolimus, 15 µM, compared to control. Exposure for 3 seconds and 24 hours of (A) human endothelial progenitor cells (EPC) and (B) human coronary artery smooth muscle cells (hCASMC) determined by Boyden chamber assays. *p<0.05 compared to control; #p<0.05 sirolimus vs. paclitaxel
Addition of iopromide
The contrast medium iopromide is an effective solvent for lipophilic drugs such as paclitaxel and sirolimus and an essential component of the coating method of the drug coated balloon-catheter. Iopromide 0.197 M alone did not alter ECP or hCASMC density, apoptosis or migration. Combination of iopromide 0.197 M with low concentration of a paclitaxel (1.5µM) decreased cell density of hCASMC to 47±6% compared to 99±12% without iopromide; p<0.05 / three seconds exposition). In contrast to the hCASMC, the addition of iopromide to paclitaxel had no additional impact on EPC cell density after exposure for three seconds. The short term effect of sirolimus on hCASMC and EPC was not influenced by the addition of iopromide (Figure 4).
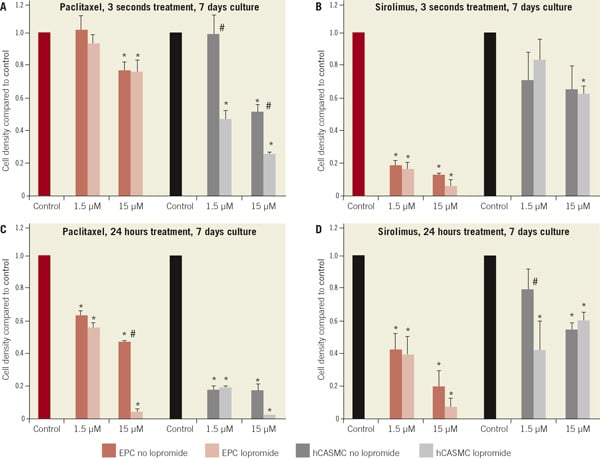
Figure 4. Cell density after exposure to 1.5 and 15 µM paclitaxel or sirolimus in the presence and absence of iopromide 0.197 M compared to control after 7 days. (A) paclitaxel or paclitaxel-iopromide, exposure for 3 seconds, (B) sirolimus or sirolimus-iopromide compared to control, exposure for 3 seconds. (C) paclitaxel or paclitaxel-iopromide, exposure for 24 hours, (D) sirolimus or sirolimus-iopromide compared to control, exposure for 24 hours. *p<0.05 compared to control; #p<0.05 iopromide vs. no iopromide
Cell proliferation was reduced by sirolimus and paclitaxel. However, the addition of iopromide did not lead to a consistent impact on proliferation rate (Figure 5). In contrast, the addition of iopromide 0.197M to paclitaxel and sirolimus lead to an increased hCSAMC apoptosis rate after long-term exposition (1.5 fold increase for paclitaxel, 2.4 fold increase for sirolimus). After exposition of EPC, this effect was only observed for the combination of sirolimus and iopromide (Figure 6).
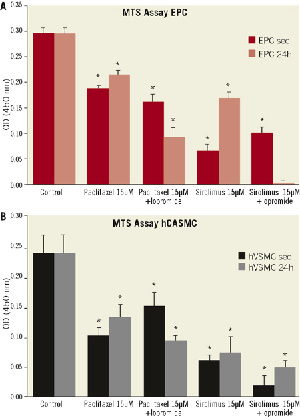
Figure 5. Cell proliferation after exposure to 1.5 and 15 µM paclitaxel or sirolimus in the presence and absence of iopromide 0.197M compared to control after 7 days. Exposition for 3 seconds or 24 hours compared to control. (A) MTS proliferation assay of EPC, (B) MTS proliferation assay of hCASMC. *p<0.05 compared to control.
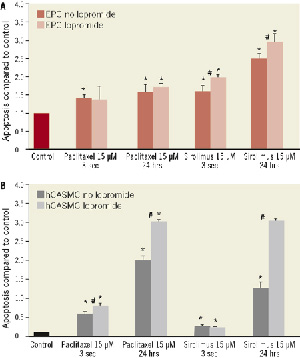
Figure 6. Apoptosis of EPC and hCASMC after exposure to paclitaxel and sirolimus, 15 µM, in the presence and absence of iopromide 0.197M. Exposition for 3 seconds or 24 hours compared to control. (A) Annexin FACS of EPC, (B) Dapi staining of hCASMC. *p<0.05 compared to control; #p<0.05 iopromide vs. no iopromide
Inhibition of migratory capacity after exposure to paclitaxel-iopromide was stronger in hCASMC than in EPC (99±1% without vs. 68±2% with iopromide; p<0.05 / hCASMC, paclitaxel 15µM, three seconds exposition). Short-term exposure of paclitaxel-iopromide did not inhibit EPC migration (Figure 7).
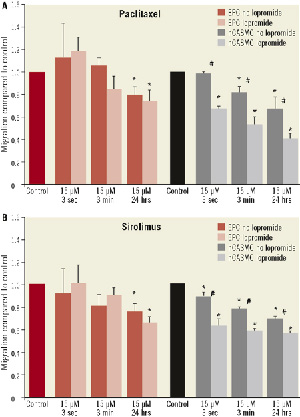
Figure 7. Migratory potency of EPC and hCASMC. (A) Exposure to paclitaxel, 15 µM, or paclitaxel-iopromide, 0.197 M, and (B) to sirolimus, 15 µM, or sirolimus-iopromide, 0.197 M, compared to control for 3 seconds, 3 minutes, and 24 hours. *p<0.05 compared to control; #p<0.05 iopromide 0.197 M vs. no iopromide
Endothelialisation in vivo
Endothelialisation after three days in the porcine coronary stent model was similar in the DCB+BMS group compared to the uncoated control group, whereas it was delayed with the Cypher® stent versus the control group (p=0.02) and DCB+BMS group (p<0.01). However, the difference between the Taxus® group and control or DEB+BMS did not reach the level of statistical significance (>0.05) (Table 1 and Figure 8).
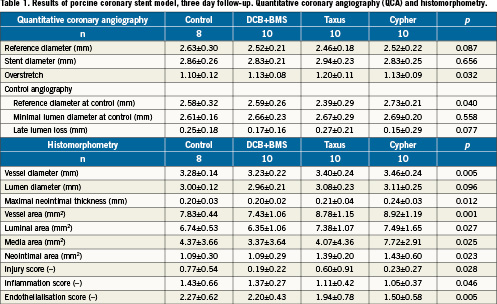
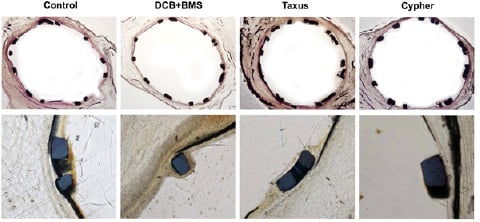
Figure 8 . Representative examples of histology, 3 day follow-up. Upper panel: HE staining; lower panel: endothelialisation assessed by von Willebrand antibody staining.
Discussion
Endothelial progenitor cells (EPC) and coronary artery smooth muscle cells (hCASMC) play differential roles during restenosis and vascular healing after angioplasty. While EPC contribute to healing of the injured area of the vessel wall, hCASMC cause neointima proliferation and accelerate the development of restenosis15,17,18,24. The main finding of this study is that short-term application of paclitaxel exerts a more pronounced inhibition of growth and motility in hCASMC, while its effects on EPC were smaller. Addition of iopromide increases the effects on cell density, migration and apoptosis in both cell types. Iopromide particularly increases the inhibitory effects of paclitaxel on hCASMC. The results of the in vivo study in pigs support the findings from the cell culture experiments. Endothelialisation of coronary stents was similar after a short time application of paclitaxel in combination with iopromide (drug-coated balloon plus bare metal stent) as compared to uncoated bare metal stents. In contrast, long-term local drug application with a paclitaxel-eluting stent, and even more pronounced with a sirolimus-eluting stent, leads to an inhibition of early endothelialisation.
Sirolimus and paclitaxel inhibit cellular growth and function by different mechanisms. Sirolimus creates a sirolimus-FKBP-12 complex by binding to the FK-506 binding protein-12 (FKBP-12). This complex inhibits the kinase activity of mammalian target of rapamycin (mTOR) which reduces kinase activity. This leads to increased levels of p27 and subsequent cell cycle arrest at the G1/S transition point27,28. Sirolimus was shown to reduce proliferation of vascular smooth muscle cells without affecting smooth muscle cell migration acutely29. However, prolonged exposure (22 to 48 hours) reduced smooth muscle cell migration through FKBP-12 interaction and p27 expression29,30. Newer studies suggest reversible effects of sirolimus on smooth muscle cell proliferation without inducing apoptosis and either weak or no effect on coronary artery smooth muscle or endothelial cell migration31. While sirolimus affects the cell cycle indirectly by interacting with the signalling pathway, paclitaxel directly interferes with the cell cycle. Once paclitaxel binds to the aminoterminus of b-tubulin it prevents microtubule depolymerisation, promotes microtubule stabilisation and enhances microtubule assembly32,33. The effect of paclitaxel on b-tubulin suppresses spindle microtubule dynamics directly and irreversibly32,34. Consistent with the observations that different cellular processes involving microtubules have different sensitivity to paclitaxel, the concentration of paclitaxel appears to be the major determinant of its antiproliferative, anti-migratory and pro-apoptotic effects35. Response or resistance to taxanes depends on the state of microtubules and the phase of cell-cycle35-37. Higher paclitaxel concentrations (>20 nM) caused mitotic arrest by inhibiting cells in the G2/M phase with subsequent cell death. Lower paclitaxel concentrations (3-6 nM) arrested cells in G1, S, G2 or M phase and resulted in delayed apoptotic cell death39,40. In former studies, acute and prolonged inhibition of arterial smooth muscle cell proliferation and migration was observed after exposure to paclitaxel in low concentrations41. Recent studies suggest that the antiproliferative activity of paclitaxel in vascular smooth muscle cells is due to primary and post-mitotic G1 arrest without induction of apoptosis42,43.
Our data show long-lasting effects on decreasing hCASMC density after short-term exposure with paclitaxel in a concentration-dependent manner. These findings are consistent with studies observing complete and prolonged inhibition of smooth muscle cell proliferation for up to 14 days after short-term exposure with paclitaxel even at low concentrations31,41. Compared to paclitaxel, short-term incubation with sirolimus showed a more potent inhibition of EPC. Similarly, short-term exposure with low-concentrations of paclitaxel leads to a stronger induction of apoptosis of hCASMC as compared to EPC. These findings point towards different modes of action of the two antiproliferative drugs after short-term exposure, depending on cell type, drug concentration and the phase of cell-cycle35-37. In contrast to sirolimus, the paclitaxel induced effects on cell density and apoptosis appear after drug exposure for three seconds. Furthermore, short-term exposure with low-concentration paclitaxel leads to increased apoptosis of hCASMC compared to EPC. Creating comparable effects of sirolimus on hCASMC requires considerably longer exposition time and higher drug concentrations. These results are consistent with studies suggesting reversibility of acute short-term effects of sirolimus on smooth muscle cell proliferation in the inhibition of restenosis29,31.
While sirolimus impairs cellular migration on a signalling pathway mediated by p2729, paclitaxel exerts irreversible effects on cellular function by direct interactions with the cytoskeleton41. Accordingly, newer studies suggested reversible effects of sirolimus on smooth muscle cell proliferation31. It was shown that EPC migration was suppressed after long-term incubation with increasing dosage of paclitaxel (1 - 1000 ng/ml)23. Our data show decreased levels of migration after incubation with paclitaxel and sirolimus for up to 24 hours in both EPC and hCASMC. In contrast, short-term exposure with paclitaxel, even in high concentrations, does not inhibit EPC migration.
The non-ionic contrast medium iopromide can be used as a solvent for several antiproliferative, lipophilic drugs45,46. Paclitaxel and sirolimus show concentration- and time-dependent effects on cell growth, cell function and apoptosis in both cell lines. The effects were increased in combination with iopromide 0.197 M, even at low-concentration drug applications. Importantly, the effect of the paclitaxel-iopromide formulation on hCASMC was more potent as compared to the effect on EPC.
Drug-eluting stents (DES) provide long-term drug delivery for the treatment of restenosis. However, high drug concentrations on the stent struts are necessary to compensate the inhomogeneous drug distribution in the peri-strut area of the vessel wall47, which may be associated with unwanted effects. Sustained drug release and high drug concentrations might impair endothelialisation causing thrombogenic complications48.
In vivo, short-term drug application is subject to rapid dilution and elimination. Endothelial cells and their precursor cells migrate to the injured vessel segment. Re-endothelialisation may not be inhibited after short-term application of the antiproliferative drug because EPC entering the lesion from distant locations had no previous exposure to the drug and therefore maintain their capability to proliferate49,50. Therefore, the findings of our cell culture study may explain some of the positive effects of paclitaxel coated balloons. In combination with iopromide, paclitaxel is able to inhibit hCAMSC growth and migration in low concentrations, and after exposure for only seconds. At the same time, paclitaxel allows EPC to maintain their capability to proliferate and to promote endothelial recovery and re-endothelialisation. Indeed, our animal studies showed at the re-endothelialisation was similar after short-term application of paclitaxel in combination with iopromide (drug-coated balloon plus bare metal stent) compared to uncoated bare metal stents. On the other hand, long-term drug application with a paclitaxel-eluting stent, and even more pronounced with a sirolimus-eluting stent, leads to an inhibition of early endothelialisation.
The results of this study suggest that the disadvantages of long-term polymer based drug delivery for the treatment of restenosis may be overcome by short-term application of antiproliferative drugs. The short-term application of antiproliferative drugs such as paclitaxel in combination with the solvent iopromide may represent a promising future method for the treatment of restenosis.
Acknowledgements
We thank Nicole Hollinger and Bianca Rastoul for excellent assistance in performing the cell-culture experiments.
Conflict of interest statement
Ulrich Speck and Bruno Scheller report being named as co-inventors of a patent application by Charité University Hospital, Berlin describing contrast media and drug coated balloon catheters for local drug delivery. The other authors have no conflicts of interest to declare.
References

