Transcatheter tricuspid valve intervention (TTVI) has become a valuable treatment option and may be considered in inoperable patients with severe symptomatic tricuspid regurgitation (TR) and a suitable anatomy in whom improvement can be expected1. We present the long-term clinical outcomes of the Mistral device (Mitralix Ltd.), which is used to treat patients with severe functional TR.
The Mistral device is a helix-shaped nitinol wire and allows, in the ideal case, the approximation of all three tricuspid leaflets with one single implant through the inward pulling of the chordae tendineae and subsequent leaflet coaptation. The placement of more than one device is possible and might be preferred in some cases to reduce TR sufficiently. A detailed description can be found elsewhere2. Patients were scheduled to undergo follow-up visits at 30 days, and 3, 6, 12 and 24 months after device implantation. The change in New York Heart Association (NYHA) Class, 6-minute walk test (6MWT) distance, and Kansas City Cardiomyopathy Questionnaire (KCCQ) score was recorded. Echocardiographic data were analysed in an independent core lab.
Ethical oversight was secured for our study. The investigation is registered at ClinicalTrials.gov: NCT04071652 (MATTERS) and NCT04073979 (MATTERS II).
In this multicentre, single-arm, open-label study, 9 consecutive patients (between April 2019 and December 2020) underwent TTVI with the Mistral device and completed 12-month follow-up. The mean age was 76.5±5.5 years, and 5 patients were female (55.5%). In 3 (33.3%) patients a pacemaker had previously been implanted. The median Society of Thoracic Surgeons risk score was 6.3%. Severe TR was present in 3 (33.3%) patients, massive TR in 5 (55.5%) and torrential TR in one patient (11.1%). A total of 15 Mistral devices were used. In 3 (33.3%) cases one device was deployed; in the remaining patients two Mistral devices were used. No procedural- or device-associated adverse events were noted intraprocedurally. None of the patients died. In one patient with a pacemaker lead, in whom 2 Mistral devices had been implanted, embolisation of one device to the pulmonary artery was seen at 306 days after the procedure. The device was successfully snared and the event resolved without sequelae.
All patients improved by at least 1 TR grade from baseline, with 56% of patients presenting with mild TR and 44% presenting with severe TR at 1-year follow-up (Central illustration A). The vena contracta narrowed (baseline: 0.98±0.32 cm vs 1 year: 0.49±0.16 cm; p=0.072) and the effective regurgitant orifice area (EROA; baseline: 0.52 [interquartile range {IQR} 0.38-0.84] cm2 vs 1 year: 0.12 [IQR 0.10-0.13] cm2; p=0.007) decreased. Favourable right heart remodelling was observed (fractional area change [FAC] at baseline: 28.6±5.5 % vs 1 year: 45.9±10.9%; p=0.016) (Central illustration B). Tricuspid annular plane systolic excursion (TAPSE) improved numerically (baseline: 1.7±0.2 cm vs 1 year: 1.8±0.3 cm; p=0.74). All patients, except for one (11.1%), experienced a NYHA Functional Class improvement from baseline (Central illustration C). The 6MWT distance increased steadily over time (average difference of +105.14 m; p=0.003) (Central illustration D). The KCCQ overall summary score (OSS) numerically improved (baseline: 38.26±17.9 points vs 1 year: 58.8±24.52 points; p=0.093; average difference +20.55 points). Of note, KCCQ data at 12 months could only be collected in 7/9 patients. Four (44.4%) patients reached clinically meaningful improvements of >25 points. The most pronounced improvements were observed within the first 30 days and continued improvement was observed over the follow-up time.
With its unique design, the Mistral device offers a transcatheter repair option for TR which aims to reduce the coaptation gap without directly interfering with the leaflets or the annulus. In the future, the device may be interesting for standalone implantation techniques or combined procedures with other TR edge-to-edge repair systems or annular reduction procedures. Given the recency of percutaneous tricuspid repair technology, TTVI trials have rather short follow-up periods. The TRILUMINATE Study, including 85 patients who underwent TTVI with the TriClip (Abbott), showed effective and durable repair of the tricuspid valve at 1-year follow-up3. In our cohort, outstanding favourable right heart remodelling was observed, while other investigators report an improvement in FAC of only 6%4. However, the functional and prognostic value of TAPSE and FAC as surrogate parameters for right heart remodelling in patients undergoing TTVI is limited. Regarding the safety profile, we did not observe any deaths during the follow-up period, while other investigators3 report all-cause mortality of up to 7.1% at 12-month follow-up, which might be attributed to different baseline characteristics and patient preselection. With a 100% procedural success rate, our study compares favourably to other TTVI technologies5. During the 1-year follow-up of the TRILUMINATE Study, self-assessed heart failure symptoms, measured by the KCCQ, significantly improved from baseline3. Similar to our study, the largest improvements were seen within the first 30 days. This is an exploratory study with a very limited number of patients and no control group. Further adequately powered, randomised, blinded, sham-controlled trials are needed to investigate the safety and efficiency of the Mistral device.
In conclusion, a significant and sustained reduction in TR, along with favourable right heart remodelling, occurred at 12-month follow-up in patients treated with the Mistral device for functional TR.
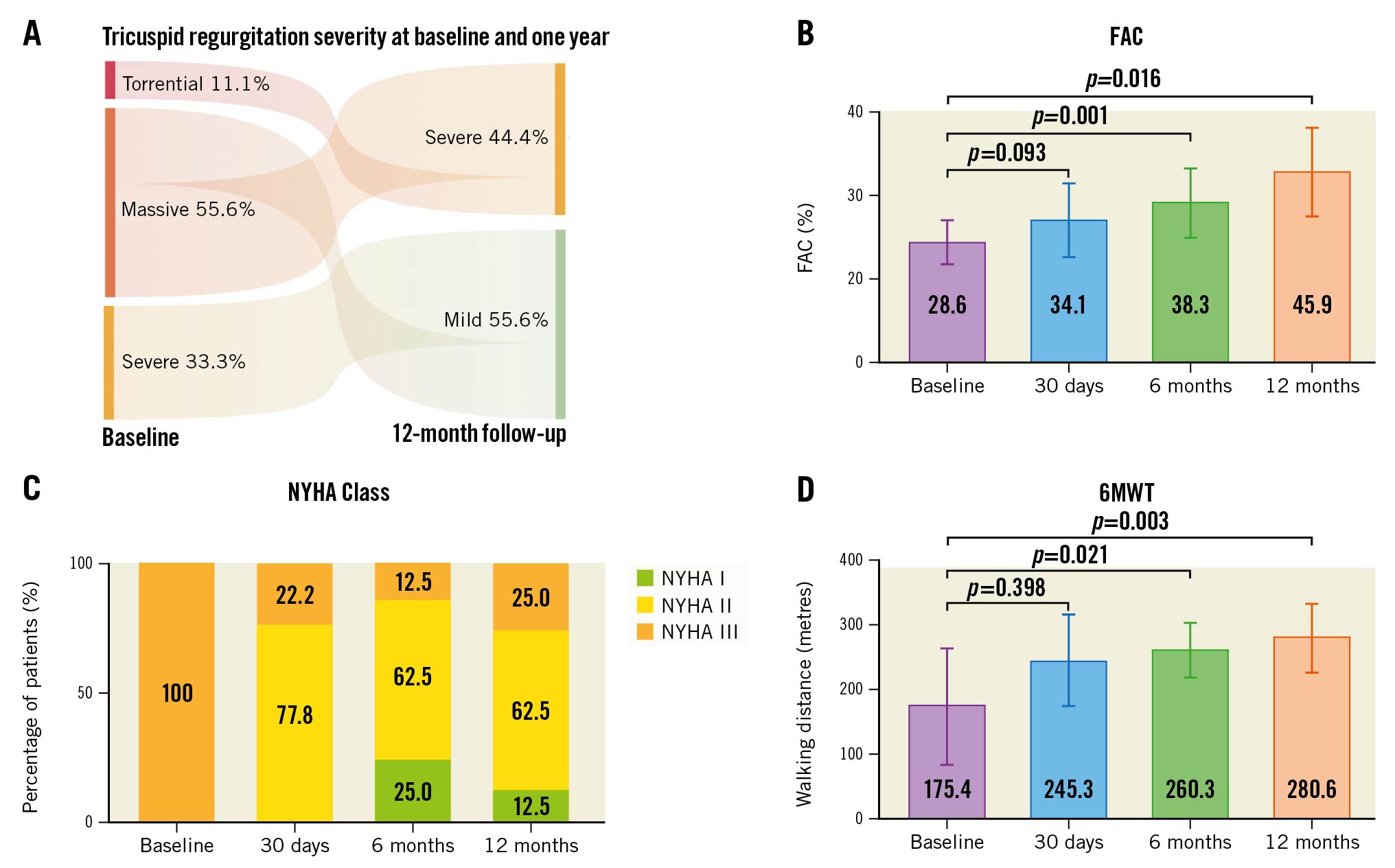
Central illustration. One-year outcomes of patients treated with the Mistral device for severe, functional tricuspid regurgitation. A) At 12-month follow-up all patients had either mild (55.6%) or severe (44.4%) residual tricuspid regurgitation (TR). All patients improved by at least 1 tricuspid regurgitation grade. B) Fractional area change (FAC) significantly improved over time. C) At baseline, all patients were in New York Heart Association (NYHA) Functional Class III, whereas at 1-year follow-up, 75% of patients reached NYHA Class I/II (p<0.001). D) The improvements in 6-minute walk test (6MWT) distances over time* (baseline: 175 [interquartile range {IQR} 75-300] m vs 1 year: 281 [IQR 210-342] m; p=0.0032; average difference of 105.1 m). *2 patients were excluded from the numerical analysis of the 6MWT (one broke their coccyx bone, and one patient aborted the test due to dyspnoea, brought on by wearing a COVID-19 face mask).
Conflict of interest statement
H. Sievert has received study honoraria to the institution, travel expenses and consulting fees from 4Tech Cardio, Abbott, Ablative Solutions, Adona Medical, Akura Medical, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Cardiac Dimensions, Cardiac Success, Cardimed, Cardionovum, Celonova, Contego, Coramaze, CroíValve, CSL Behring LLC, CVRx, Dinova, Edwards Lifesciences, Endobar, Endologix, Endomatic, Esperion Therapeutics, Inc., Hangzhou Nuomao Medtech, Holistick Medical, Intershunt, Intervene, K2, Laminar, LifeTech, Magenta, Maquet Getinge Group, Metavention, Mitralix Ltd., Mokita, Neurotronic, NXT Biomedical, Occlutech, ReCor, Renal Guard, Shifamed, Terumo, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, Vivasure Medical, Vital Biomed, and Whiteswell. R. Beeri and H.D. Danenberg serve as consultants for Mitralix Ltd. I. Yaron is an employee of Mitralix Ltd. The other authors have no conflicts of interest to declare.

