“Those that fail to learn from history are doomed to repeat it.”
− Winston Churchill, House of Commons, 1948
(attributed originally to George Santayana, The Life of Reason, 1905)
More than 12 years have elapsed since the first bioresorbable scaffold (BRS) received the European conformity (CE) mark of approval for use in Europe. The concept behind BRS technology is intuitively attractive – to provide temporary support to the vessel wall following percutaneous dilatation of atherosclerotic lesions, resisting acute and chronic constrictive forces of the vessel wall, as well as to promote vascular restoration in terms of vasomotion1. The Absorb Bioresorbable Vascular Scaffold (Absorb BVS; Abbott) was the first BRS to receive the CE mark and the only such device to receive U.S. Food and Drug Administration (FDA) approval for use. It showed favourable outcomes in early clinical trials for selected coronary lesions, comparable to second-generation drug-eluting stents (DES)2. Subsequently, randomised trials comparing this technology to contemporary DES in broader patient populations revealed inferior long-term outcomes for Absorb BVS, especially related to late thrombotic events and myocardial infarction34. As a consequence, Abbott withdrew Absorb BVS from the market in 2017, and the field of BRS technology remained in hibernation from that point forward.
In the current issue of EuroIntervention, Gao and colleagues report on the 3-year outcomes of the first-in-human trial of a novel thin-strut sirolimus-eluting iron bioresorbable stent in non-complex coronary artery lesions5. Inclusion criteria allowed use of this device in simple de novo lesions in 45 subjects who were equally grouped into 2 cohorts with different follow-up timepoints: specifically, cohort 1 (with follow-up at 6 months and 2 years) and cohort 2 (with follow-up at 1 year and 3 years). The primary outcomes were target lesion failure (TLF) rates at 6 months and late lumen loss (LLL), as evaluated with quantitative coronary angiography (QCA). The main findings were that the rate of TLF was 2.2%, and LLL was 0.33 mm at 6 months and 0.37 mm at the 3-year timepoint. Three cases of clinically indicated target lesion revascularisation were observed. At 12 months, 2 patients presented with recurrent angina and restenosis, as evaluated in the 12-month angiography. No deaths, myocardial infarctions or scaffold thrombosis were seen in the 3-year follow-up window.
The authors must be commended for pursuing important work in the aftermath of the failure encountered with first-generation polymeric BRS. Against this background, BRS using metallic compounds may hold several advantages – for example, relatively high radial force6 – making them attractive platforms for future BRS development. The two most widely tested metals in the field of biomedical engineering of BRS are magnesium and iron. Magnesium tends to corrode rapidly and requires protection against premature corrosion in vascular applications, though its corrosion products are relatively benign with regard to biocompatibility. Iron, on the other hand, tends to corrode very slowly and requires accelerating technologies to promote corrosion in vascular applications (Figure 1). The corrosion products of iron-based compounds are bioreactive, and protection against toxic corrosion products7 is required.
In a physiological environment, iron (Fe) exists in two principal oxidation states, ferrous (Fe2+) and ferric (Fe3+) iron; the rate of degradation of iron to react to ferrous hydroxide (Fe[OH]2) is highly dependent on the amount of dissolved oxygen present. Iron can be the initiator of chemical reactions that result in radical formation. Moreover, redox-active iron also catalyses the generation of reactive oxygen species, which can promote oxidation and the subsequent modification of proteins and nucleic acids8. To protect against corrosion, the technology studied by Gao et al used nitriding technology, a thin-strut design with a protective zinc layer, and a proprietary poly-DL-lactic acid coating with a sirolimus structure design9. While the 600 nm thick zinc layer is aimed at protecting the stent from premature onset of corrosion within the first 3 months, the nitriding of iron is intended to accelerate corrosion after this time period. Moreover, the low strut thickness – 70 μm – reduces thrombogenicity and is associated with faster endothelialisation in comparison with thicker-strut devices10.
A careful preclinical investigation of iron-based scaffolds is of high importance before investigating such technology under human disease conditions. In this regard, Zheng and colleagues previously reported the outcomes of a large preclinical study investigating the safety of a novel iron-based bioresorbable scaffold (IBS) in a porcine model up to 180 days. Histological analysis revealed the absence of excessive inflammatory reaction, compared to control everolimus-eluting stents, and absence of scaffold thrombosis9. Against this background, the most salient learning from preclinical investigations of first-generation Absorb BVS should not be forgotten. True long-term assessment, up to 2 or even 3 years of follow-up, in the implant location may be required to detect signals of increased inflammation or other adverse effects resulting from scaffold degradation11. Along these lines, corrosion in iron bioresorbable stents was only observed in 2 out of 10 cases at 180 days in the study by Zheng et al, emphasising the outstanding need for long-term evaluation of this technology. In a separate study using rabbits’ abdominal aorta, iron bioresorbable stents were proven safe by the absence of excessive inflammation up to 13 months following implantation, while significant peri-strut corrosion was observed at long-term follow-up at 53 months in porcine coronary arteries12.
The safety and performance data reported by Gao et al are certainly encouraging. Using angiographic surveillance and optical coherence tomography, there was a high rate of stent strut coverage and a low rate of TLF at 3 years. However, it must be remembered that the assumed time frame of bioresorption exceeds 3 years, warranting careful scrutiny of safety at long-term follow-up. Although the current study provides initial evidence supporting the safety of this novel technology, ultimately only randomised comparison against contemporary benchmark devices, in appropriately powered trials with longer-term follow-up, will tell us whether the promise of this technology will become reality.
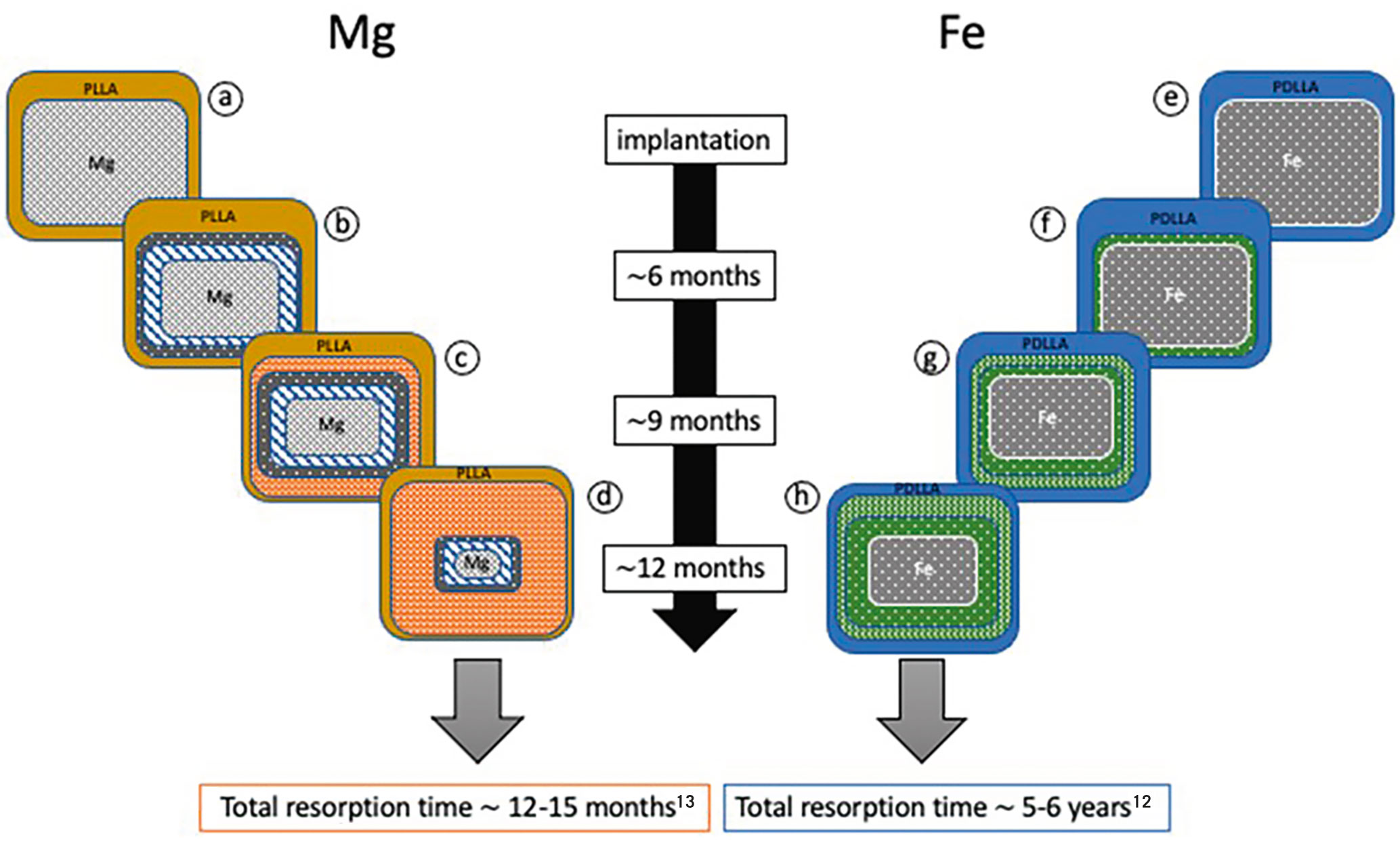
Figure 1. Reabsorption of magnesium-based versus iron-based vascular scaffolds. Schematic degradation steps during putative in vivo degradation of magnesium-based (a-d) and iron-based (e-h) bioresorbable vascular scaffolds over 12 months. Bioresorption of magnesium-based versus iron-based vascular scaffolds. Schematic degradation steps during putative in vivo degradation of magnesium-based (a-d) and iron-based (e-h) bioresorbable vascular scaffolds over 12 months. a) innermost core of magnesium alloy surrounded by a PLLA coating carrying sirolimus, b) innermost core of magnesium alloy surrounded by a thin layer of magnesium hydroxide, covered by a thin layer of magnesium phosphate; c) innermost core of magnesium alloy surrounded by a thin layer of magnesium hydroxide, then a thin layer of magnesium phosphate, and finally, covered by a layer of amorphous calcium phosphate; d) innermost remnant of magnesium alloy surrounded by a thin layer of magnesium hydroxide, covered by a thin layer of magnesium phosphate, followed by a large layer of amorphous calcium phosphate; note, the time period for complete degradation is 12-15 months13; e) innermost core of iron surrounded by a PDLLA coating carrying sirolimus; f) innermost core of iron surrounded by a thin layer of iron oxides and hydroxides; g) innermost core of iron surrounded by a thin layer of iron oxides and hydroxides, surrounded by a layer of calcium and phosphorous; h) innermost core of iron surrounded by a layer of iron oxides and hydroxides, surrounded by large layer of calcium and phosphorous; note, the time period for complete corrosion is 5-6 years12. Fe: iron; Mg: magnesium; PDLLA: poly-DL-lactic acid; PLLA: poly-L-lactic acid
Conflict of interest statement
M. Joner reports personal fees from Orbus Neich, AstraZeneca, and ReCor; grants and personal fees from Biotronik, Boston Scientific, and Edwards Lifesciences; and grant support from Amgen, outside the submitted work. He has also received funding from the German Center for Cardiovascular Research: DZHK (FKZ 81Z0600502; FKZ 81X2600526) and from the Leducq Foundation (grant agreement number 18CVD02). R.A. Byrne reports research/educational grants to the institution of employment from Abbott Vascular, Biosensors, Boston Scientific, and Translumina. G. Klosterman has no conflicts of interest to declare.

