
Abstract
The neuroprotective effects of hypothermia have been demonstrated in experimental models and clinical trials. Experimental studies indicate that improved efficacy and broadened indications can be achieved with moderate to deep hypothermia. The TwinFlo catheter was designed to provide very rapid, deep and selective brain cooling with faster cooling rates, and temperatures much lower than those which can be achieved by any other hypothermia device and technique. This report describes the experimental in vivo studies and initial clinical experience with the TwinFlo catheter.
Introduction
Neuroprotection –protecting the brain from the devastating effects of ischaemia and reperfusion injury– remains as an important unmet clinical need today. The neuroprotective effects of hypothermia following acute stroke and cardiac arrest have been demonstrated in numerous experimental models and clinical trials1,2,3,4,5. In the laboratory, hypothermia has been shown to be the most robust neuroprotectant identified to date, because it acts on many of the pathways that lead to cell death; its role in mitigating reperfusion injury is of particular importance. In the clinical setting, although questions regarding degree of temperature reduction and other procedural matters still remain, therapeutic hypothermia is currently recommended for most out-of-hospital cardiac arrest (OHCA) patients; however, in acute stroke, the hypothermia techniques that have been attempted failed to demonstrate benefit6, probably because they were unable to cool the brain safely to temperatures shown to be efficacious in experimental models. The TwinFlo™ catheter (ThermopeutiX, Inc. San Diego, CA, USA) was designed to address and potentially reverse these serious health problems by providing a means to cool the brain very rapidly, deeply and selectively, with faster cooling rates, and temperatures much lower than those which can be achieved by any other hypothermia device and technique.
Methods
THE TWINFLO CATHETER
The TwinFlo catheter was introduced by standard percutaneous transfemoral technique, and was used to isolate and selectively perfuse the carotid artery with cold blood (Figure 1-Figure 3). It comprises two concentric tubular shafts (8.5 Fr and 14 Fr, respectively) that define inflow and outflow lumens, and an atraumatic occlusion balloon positioned at the distal end of the inflow lumen. Figure 2 illustrates the operation of the catheter.
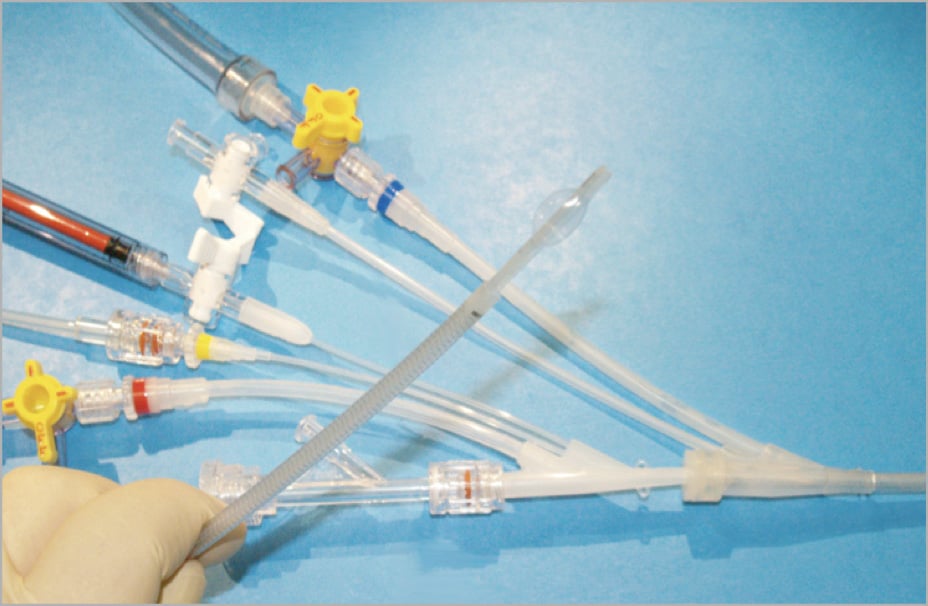
Figure 1. The TwinFlo catheter. The warm blood outflow is identified by the blue marker band, and the cooled blood inflow is identified by the red marker band. The yellow band denotes the pressure measurement lumen. Ports at the proximal ends of the inner and outer catheter shafts provide means for flushing, contrast injection, and selective delivery of diagnostic and therapeutic agents.
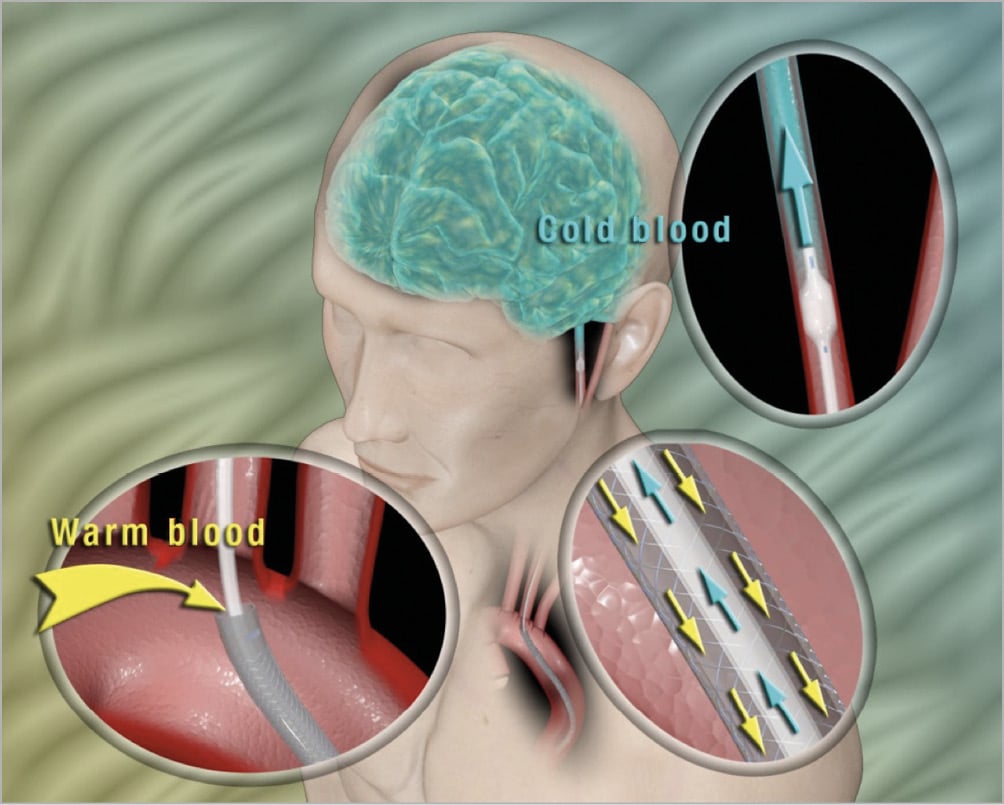
Figure 2. Schematic of operation in selective cerebral cooling. Using a standard percutaneous technique, the TwinFlo is introduced into the femoral artery and, under fluoroscopic control, advanced to the common or internal carotid artery. Blood is withdrawn from the distal end of the outer catheter located in the aortic arch, directed to an extracorporeal circuit where it is cooled, and then pumped directly to the brain through the inner catheter beyond a very atraumatic occlusion balloon, so that the brain only receives cold blood. The counter-current blood flow design (arrows in centre insert) very effectively insulates the body from the cold blood during the prolonged perfusion through the aorta to the brain. As such, significantly lower temperatures can be achieved in the brain with minimal or no systemic reduction of temperature.
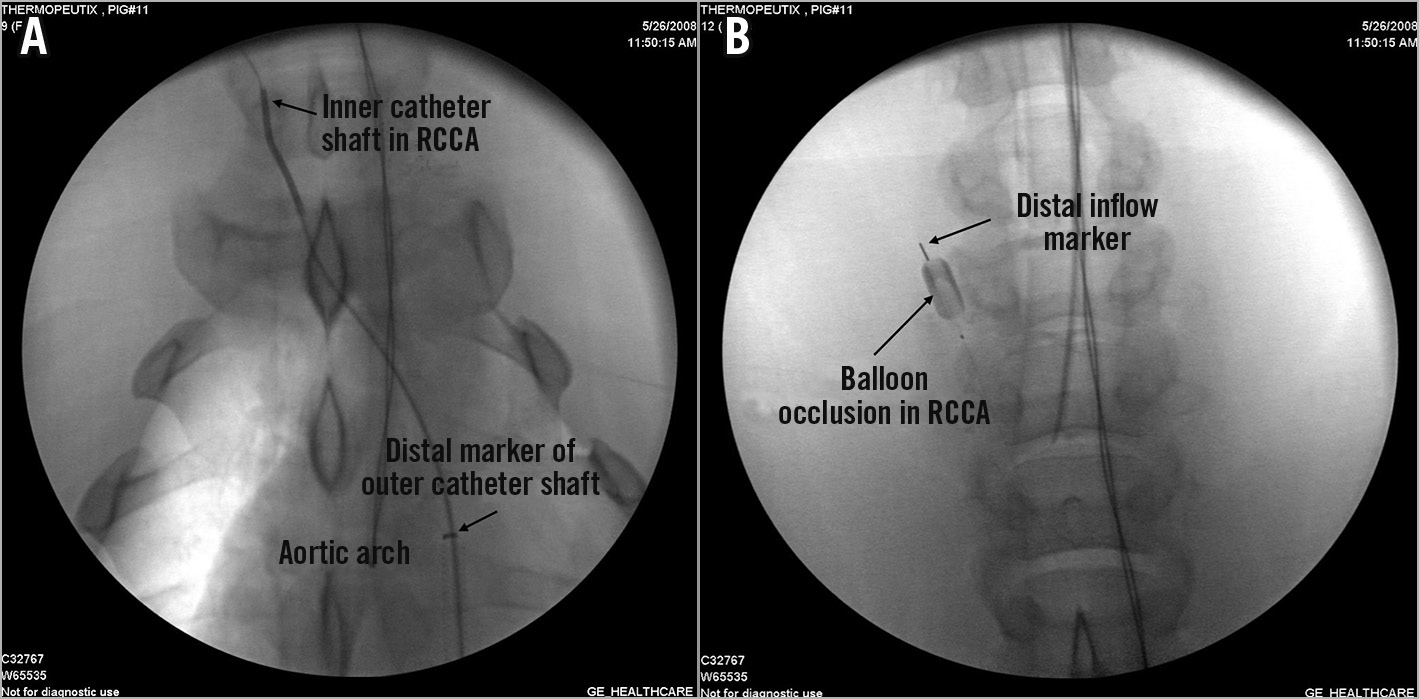
Figure 3. Fluoroscopic imaging of the TwinFlo catheter in use. A) TwinFlo catheter positioned in right common carotid artery (RCCA). The outer shaft of the catheter is situated in the aortic arch. B) Occlusion balloon inflated in the right common carotid artery.
IN VIVO STUDIES
In vivo studies were performed in 47 pigs weighing 50-72 kg. All procedures and care of animals were in accordance with institutional guidelines. All animals were anaesthetised, intubated and heparinised. They were placed on a water-heating mattress preset to 38°C and covered from the neck to the feet with either a forced-air heating blanket or warmed blankets (Figure 4A). Temperature was measured in bilateral frontal lobes, nasopharynx, ear, oesophagus, rectum, jugular vein and descending aorta. Standard cardiopulmonary perfusion circuits and equipment, or alternatively a standard dialysis pump plus a stainless steel heat exchanger coil in a bucket of iced water, were used to cool and pump the blood. Figure 4B shows the set-up of the TwinFlo catheter. Three series of studies evaluated selective cerebral cooling and parameters, surrogate non-invasive brain temperature measurement, and the effect of selective endovascular cooling in a focal stroke model. Haemodynamic parameters (heart rate, mean arterial blood pressure) and arterial blood gases (oxygen, haemoglobin, glucose, pH) were continuously evaluated. Outflow blood was cooled to 5-20°C, and reperfused at rates of 80-250 ml/min for 30-180 minutes. Animals were euthanised at the end of each study. In a porcine stroke model, focal ischaemia was created by surgically clipping one of the middle cerebral arteries7. After three hours of ischaemia, the clip was removed, and a three-hour period of reperfusion followed. During the three-hour reperfusion period, 25 pigs were randomised to receive selective hypothermia with the TwinFlo catheter or serve as a normothermic control. After sacrifice, the brain was fixed and removed for magnetic resonance imaging (MRI) and histological analysis, which was performed by experts who were blinded to the study groups.
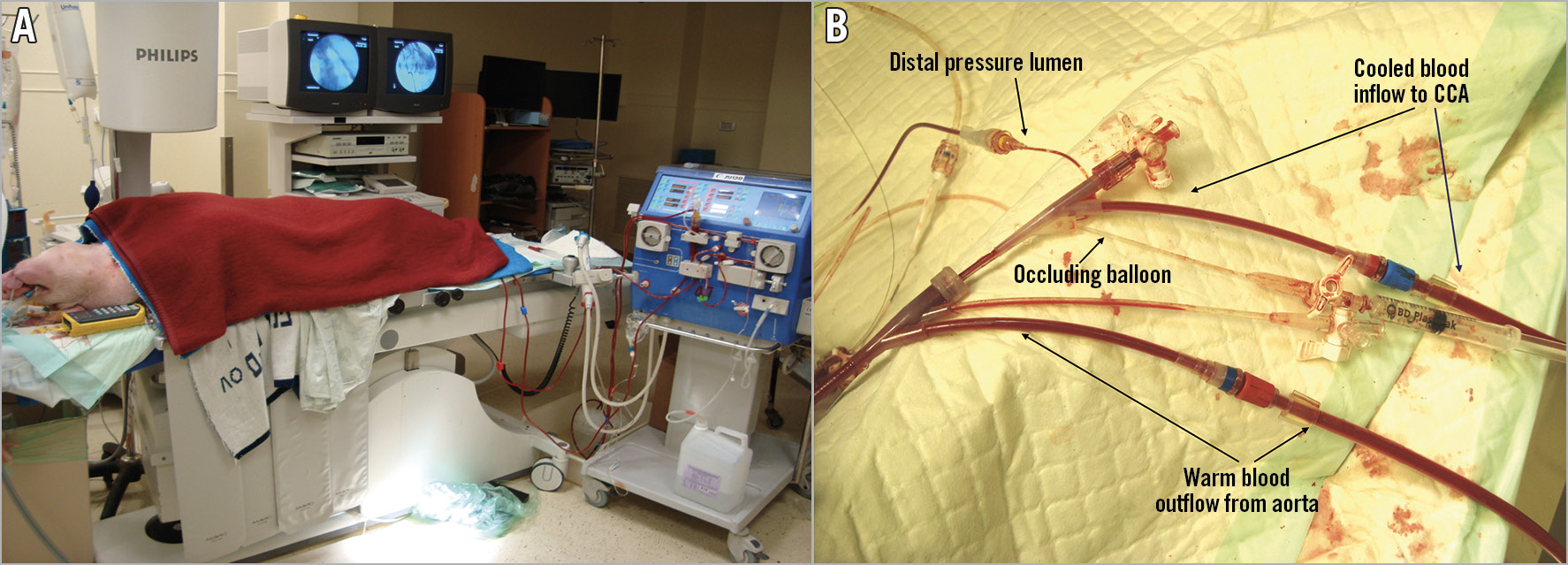
Figure 4. In vivo study set-up. A) General procedural set-up during in vivo studies. In this study, the extracorporeal circuit comprised a standard dialysis pump and a stainless steel heat exchanger coil in a bucket of iced water. B) TwinFlo catheter set-up.
Results
The TwinFlo catheter was successfully positioned in the common carotid artery in all animals. In the ipsilateral hemisphere, temperatures as low as 15°C were attained, and passive rewarming did not result in rebound hyperthermia (Figure 5). Initial cooling rates up to 1.8°C/min were reached. These were dependent on the flow rate and temperature of the perfused blood (Figure 6). No haemolysis was observed for any of the blood temperatures and flow rates used in these studies. There was a strong temperature correlation between the two hemispheres, with contralateral hemispheric temperature reaching equivalence to the treated side within 60 minutes in most animals (Figure 7A, Figure 7B). Systemic temperature did not fall below mild hypothermic levels; in a substudy of nine pigs, covering with an additional warming blanket reduced the systemic temperature drop from a mean of 3.9±1.9°C to only 2.0±1.0°C during selective cerebral cooling to 28-30°C (Figure 7A, Figure 7B). Results of the brain temperature surrogate investigation (Figure 8A, Figure 8B) showed strong correlation with both cerebral hemispheres and all surrogate measurement sites. Nasal temperatures tracked the respective cerebral hemispheric temperatures, measuring 1-3 degrees warmer than cerebral temperature.
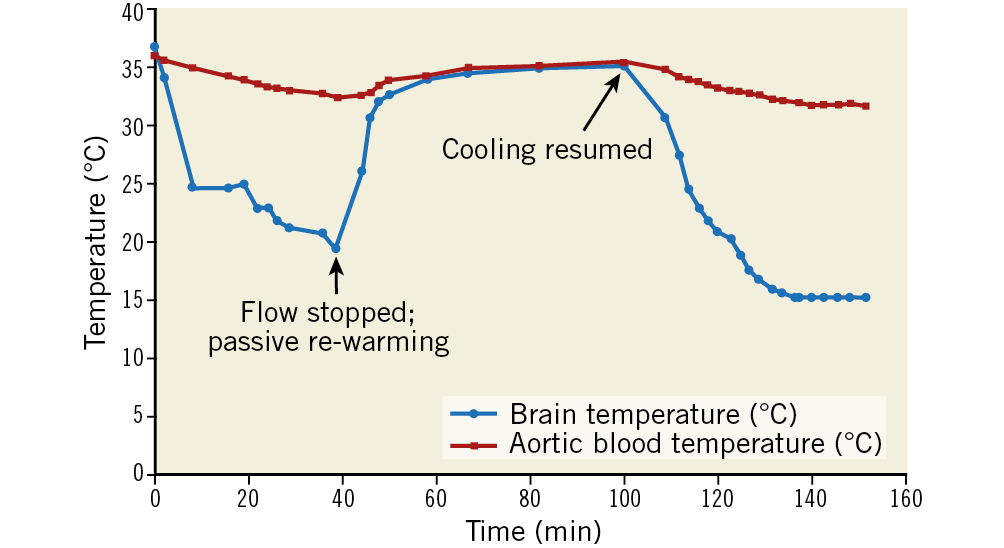
Figure 5. Illustrative case demonstrating passive re-warming of the brain upon cessation of cold blood perfusion and deflation of occlusion balloon. Temperature of the cooled hemisphere equilibrated to systemic temperature in approximately 25 minutes.
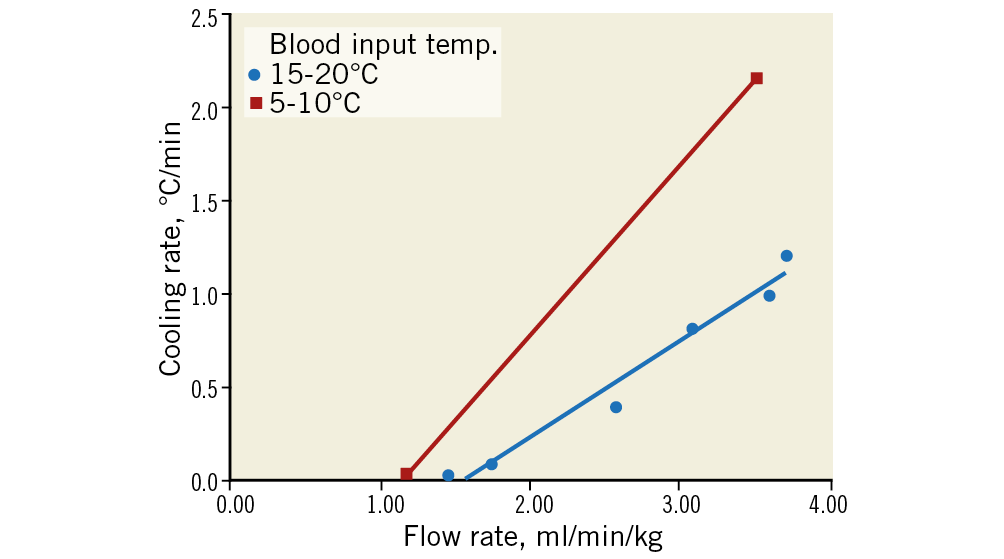
Figure 6. Rate of cooling of the treated hemisphere as a function of the flow rate of the perfused blood normalised by subject body weight for two ranges of temperature of the perfused blood (n=8 pigs). Higher flow rates and lower temperatures of the perfused blood resulted in faster rates of cooling.
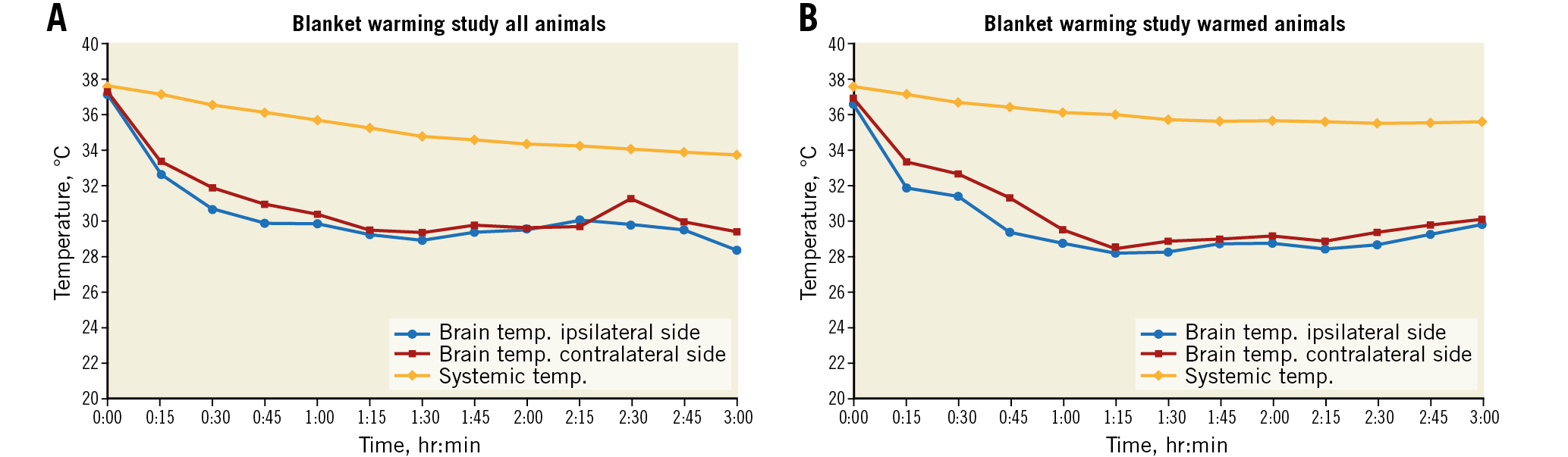
Figure 7. Substudy of nine pigs designed to evaluate further minimalisation of systemic cooling. Selective cerebral cooling was performed for three hours, with a target ipsilateral cerebral temperature of 28-30ºC. Each data point is an average of the temperatures at that site at that time point in all animals of the study group. There was a strong correlation between the two hemispheres over the three-hour study period (R=0.830, p<0.0001). A) Brain and systemic temperatures of all pigs in the substudy. Systemic temperatures, as measured by a rectal thermistor, fell by a mean of 3.9±1.9°C to 34±1.6°C. B) Brain and systemic temperatures of four pigs which were covered with an additional blanket during cerebral cooling. Systemic temperatures fell by only 2.0±1.0°C to a mean of 35.5±0.9°C compared with the standardly warmed animals (n=5) where it fell by 5.4±0.6°C to 32.4±0.5°C, p<0.005).
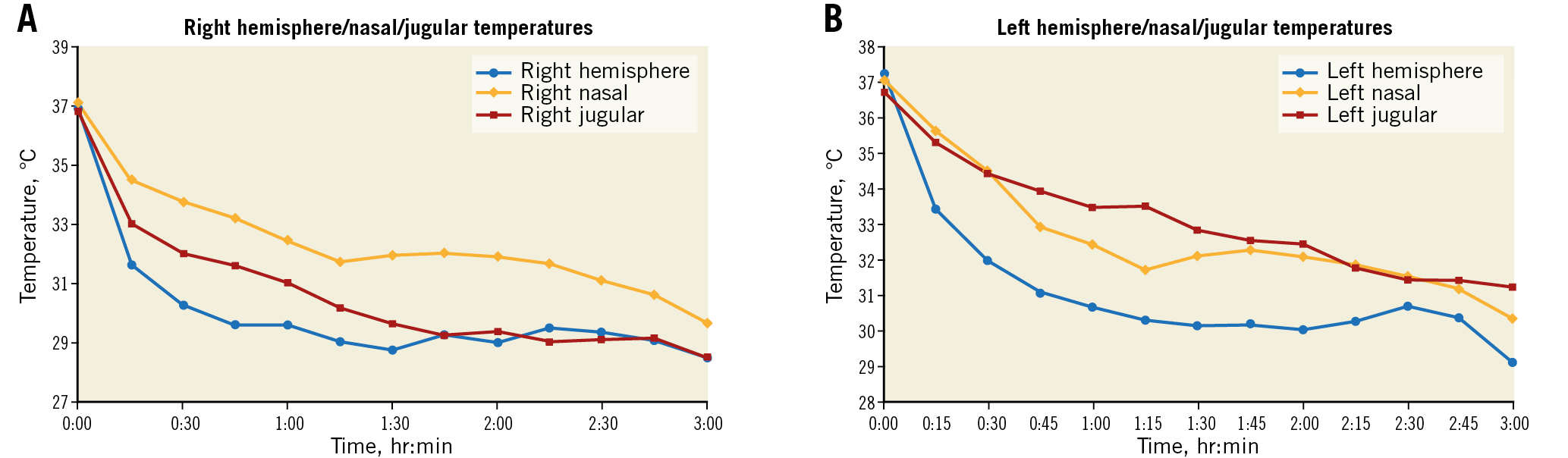
Figure 8. Brain temperature surrogate study (n=9 pigs; same subjects as in substudy shown in Figure 7A). A) Ipsilateral cooled hemisphere. B) Contralateral cooled hemisphere.
In the porcine stroke study, nasal temperatures were used as a surrogate for brain temperature. The mean ipsilateral nasal temperature decreased from 38°C to 26.5°C during selective hypothermic perfusion. By MRI imaging, the ratio of stroke volume to hemispheric volume was 10 times lower in the hypothermic group compared to the control group (0.005±0.011 vs 0.050±0.059; p<0.05), and the mean histological stroke volume of the hypothermic group trended to nearly half that of the controls (0.57±0.76 cm3 vs 0.99±1.00 cm3; p=0.256). With the exception of a decrease in pH in the hypothermia group (7.34±0.18 vs 7.44±0.03; p<0.001), systemic parameters including mean arterial pressure, blood oxygenation and haemoglobin remained stable and did not differ from the normothermic cohort.
FIRST-IN-HUMAN USAGE OF THE TWINFLO CATHETER
Our initial human experience with TwinFlo was in the setting of neurosurgery where prolonged occlusion time was required for surgical repair of a giant aneurysm of the middle cerebral artery in a 59-year-old man. The TwinFlo selectively cooled the brain to 26°C, providing neuroprotection while maintaining systemic normothermia (36.7°C). Cooling was initiated 65 minutes before temporary arterial occlusion, continued during the 39 minutes of occlusion and 16 minutes after, for a total selective cooling time of two hours. The patient’s recovery, with no neurological deficit, appeared to be much quicker than we have typically seen in this setting.
Discussion
The challenge in protecting the brain from the effects of hypoxia is that there are numerous pathways that lead to cell death. Hypothermia addresses and prevents cell death through many mechanisms, including a lowering of the metabolic rate, reduction of excitotoxic neurotransmitters, prevention of blood-brain barrier disruption, decrease in the destructive oxygen free radical production, reduction in inflammatory markers, mitigation of reperfusion injury, and an overall upregulation of cell survival mechanisms1,3,4,5. Experimental studies of hypothermia in acute ischaemic stroke showed greatest efficacy when cooling to below 31°C, suggesting that there is a potential temperature threshold in acute stroke3. Unfortunately, in the clinical setting to date, hypothermia did not improve recovery or mortality, and it was associated with more complications6. The techniques employed could only cool to a median target temperature of 33°C (range 32-35), and the time to reach target temperature was a median of four hours6. The inability of these techniques to effect cooling times and temperatures that were shown to be most efficacious in the experimental studies would provide a reasonable explanation for the discrepancy between bench and bedside.
Use of the TwinFlo addresses the time and temperature issues and, by perfusing cold autologous blood, not only is the metabolic demand decreased, but an adequate supply of oxygen and nutrients to the brain is ensured. In the setting of refractory cardiac arrest, Wang et al demonstrated that infusing cold blood with the TwinFlo for 12 hours was not only safe but highly efficacious in improving survival and neurological outcomes8. The authors reported that adding selective cerebral hypothermia at 27±3°C to their standard extracorporeal cardiopulmonary resuscitation (ECPR) protocol increased survival from 35% to 75%, and the rate of Cerebral Performance Category 1 (CPC 1 – no neurological deficit) increased from 12% to 50%. The encouraging outcome from this difficult patient population provides optimism that the TwinFlo may be effective in acute ischaemic stroke and other cerebral ischaemic conditions.
Limitations
Findings from the in vivo studies will need to be verified in the clinical setting. The first-in-human clinical reports include very small sample sizes and, although the preliminary data are encouraging, larger studies and studies in acute stroke indications will be required.
Conclusion
Selective endovascular cooling of the brain with the TwinFlo catheter shows promise in providing rapid, selective, deep cerebral hypothermia, and may offer an improved method for neuroprotection during neurosurgery, cardiac arrest, acute stroke and other ischaemic insult. Larger clinical investigations in these most challenging and important clinical settings are warranted.
|
Impact on daily practice The ability of hypothermia to reduce brain injury is well established in animal models and in some human scenarios of global ischaemia. Cerebral metabolism reductions, thought to be the mechanism behind observed neuroprotection in robust animal models, are substantial with moderate hypothermia, but the systemic complications have limited clinical applications to only very mild temperature reductions. Selective endovascular cooling via the TwinFlo catheter represents a more efficient and effective application of therapeutic hypothermia specific to the organ of interest, e.g., the brain. With this technology, rapid and substantial temperature reductions may finally allow clinicians to realise the potential neuroprotective benefits of therapeutic hypothermia in global ischaemia (cardiac arrest) and focal ischaemia (ischaemic stroke and aneurysm). Questions regarding the optimal depth and duration of hypothermia may now be relevant in the human clinical realm as lower temperature limits appear to be circumvented through the percutaneous selective approach provided by the TwinFlo catheter. |
Acknowledgements
We are indebted to Glen Lieber, Lynn Denning, Karen Siroen, Barb Lehrbass, Rivka Farkash, Rachel Meerkin Pachino and the teams in London, Ontario, Canada, and Israel for their enthusiastic and tireless dedication in obtaining the exciting in vivo data.
Funding
This work was supported by the Heart and Stroke Foundation of Canada (GIA NA 7273). ThermopeutiX, Inc. provided material support consisting of TwinFlo catheters.
Conflict of interest statement
R. Solar and D. Meerkin are shareholders of ThermopeutiX, Inc. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

