Abstract
Low flow, low gradient aortic stenosis is a highly challenging condition in terms of diagnosis and therapeutic management. With regard to prognosis and to management decisions, it is essential to distinguish those patients with preserved systolic left ventricular ejection fraction from patients with impaired systolic left ventricular ejection fraction, and in particular those with true severe aortic stenosis from patients who have non-significant aortic stenosis associated with reduced transvalvular flow for other reasons and who present with a functionally small valve area. In addition, measurement errors deserve particular consideration in order to avoid a misdiagnosis. Echocardiography, including low dose dobutamine stress studies, is the key diagnostic tool. Magnetic resonance imaging, invasive assessment of haemodynamics by catheterisation and quantification of valve calcification by computed tomography calcium scoring can provide additional information that helps to assess aortic stenosis severity accurately, predict outcome and guide treatment decisions. Percutaneous aortic valve implantation may provide an intervention with lower periprocedural risk in this challenging patient subset; however, further studies are required to define its exact role in this setting.
Introduction
Calcific aortic stenosis has become the most frequent type of valvular heart disease in Europe and North America. Echocardiography is the key diagnostic tool. According to current guidelines, severe aortic stenosis is defined by a peak velocity >4 m/sec, mean pressure gradient >40 mmHg, and aortic valve area (AVA) <1.0 cm2 (<0.6 cm2/m2 when indexed for body surface area) as provided by the continuity equation1,2. A subset of patients presents with discrepant findings with regard to these echocardiographic criteria. Most often patients might have a small calculated AVA below 1.0 cm2 but nevertheless present with gradients <30-40 mmHg. When measurement errors are excluded, “low flow conditions” must be considered as the cause of this combination of measurements.
These patients with low flow, low gradient (LF-LG) aortic stenosis (AS) are among the most challenging ones encountered in the diagnosis and treatment of valvular heart disease. They can present with impaired (i.e., classical LF-LG AS) or preserved (i.e., “paradoxical” LF-LG AS) left ventricular ejection fraction (i.e., LVEF ≥50%) (Figure 1). Definition of low flow is mainly based on cardiac index (CI) and stroke volume index (SVI). Recent studies suggest that stroke volume is an important independent predictor of mid-term all-cause mortality compared to patients with normal flow and should consequently be included in the assessment of patients with severe AS3. Although the definition of low flow is not standardised in the literature a SVI ≤35 ml/m2 has been commonly used3-5.
Classical low flow, low gradient aortic stenosis
Patients with classical LF-LG severe aortic stenosis who represent 5-10% of the AS population mostly present with a dilated LV and impaired LV systolic function and consequently reduced transvalvular flow6. The entity has been defined by the combination of an aortic valve area (AVA) <1.0 cm2, a mean transvalvular pressure gradient <40 mmHg, an LV ejection fraction (LVEF) ≤40%, and a transvalvular stroke volume index ≤35 ml/m2 (cardiac index <3.0 l/min/m2).
In this situation dobutamine stress echocardiography (DSE) has been demonstrated to help distinguish between true severe AS with a fixed small valve area and non-severe AS with a functionally small valve area due to reduced driving forces caused by other underlying myocardial disease (pseudo-severe AS)6-8. After obtaining baseline measurements, echocardiography is repeated while administering dobutamine. Starting at 5 µg/kg/min the dobutamine dose is gradually increased up to a maximum dose of 20 µg/kg/min by steps of 5 µg/kg/min per three minutes trying not to exceed a heart rate of 100 beats/minute. Reasons for early dobutamine discontinuation, other than developing tachycardia, include hypotension and ventricular arrhythmias. A comparable protocol can also be applied in the catheterisation laboratory9.
Both modalities allow the determination of the presence of a “flow reserve” (also referred to as contractile reserve) that is defined as an increase in stroke volume of >20%. The presence of flow reserve has been shown to be of important prognostic value with regard to operative and postoperative mortality8.
In the presence of flow reserve, DSE permits a further differentiation between pseudo-severe (PS) stenosis, which is characterised by a marked increase in valve area to >1.0-1.2 m2 with increasing flow (or ≥0.3 cm2) but only minor changes in gradients (mean gradient remains <30-40 mmHg), and true severe (TS) stenosis, characterised by a significant increase in gradients with little change in valve area (Figure 1)8,10.
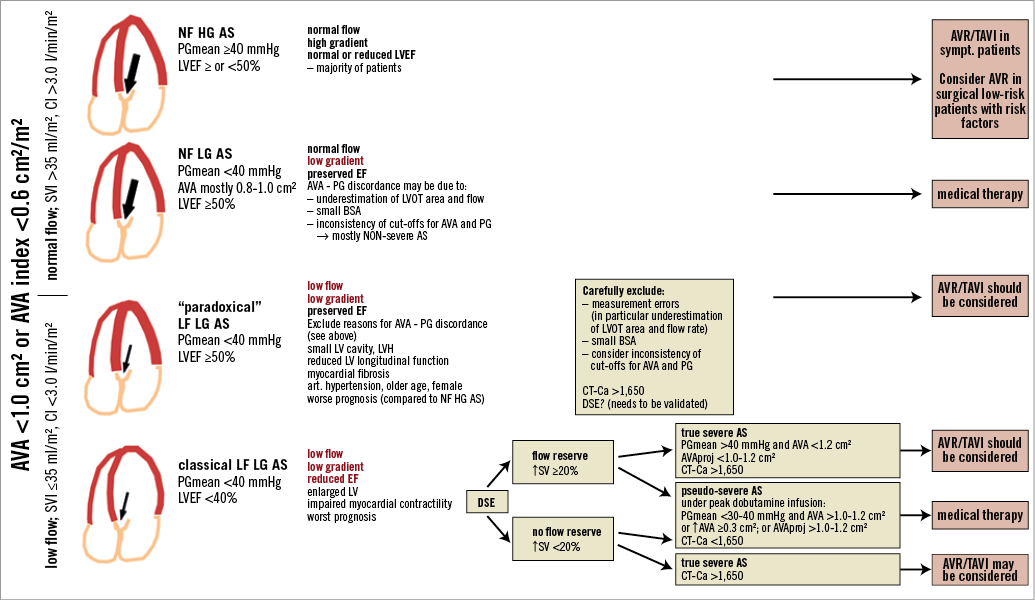
Figure 1. Classification of patients with aortic stenosis (AS) and aortic valve area (AVA) <1.0 cm2 (AVA index <0.6 cm2/m2) depending on pressure gradient level (low gradient [LG] vs. high gradient [HG]), flow state (normal flow [NF]; low flow [LF]) and left ventricular ejection fraction (LVEF). The subgroup of patients with low flow, high gradient was not taken into account due to limited study data. AVAproj: projected aortic valve area; BSA: body surface area; CI: cardiac index; CT-Ca: computed tomography calcium score; DSE: dobutamine stress echocardiography; PG: pressure gradient; LVH: left ventricular hypertrophy; LVOT: left ventricular outflow tract; SVI: stroke volume index
The distinction between true severe versus pseudo-severe AS is essential because symptomatic patients with TS AS will generally benefit from surgical aortic valve replacement (SAVR) or from transcatheter aortic valve implantation (TAVI), whereas patients with PS AS may not respond favourably to aortic valve replacement but rather may need intensive medical therapy and close follow-up as recommended by current guidelines1,10. A recent study reporting data from a European registry supports this hypothesis. In 107 patients with classical LF-LG AS who were followed conservatively, five-year mortality was 43±11% in patients with PS AS compared to 91±6% in patients with TS AS and 100% in patients without flow reserve. In addition, the survival of patients with pseudo-severe AS was similar to that of a propensity matched group of heart failure patients without aortic stenosis10.
One major difficulty in the interpretation of DSE results is that the changes in gradient and valve area are highly dependent on the actual flow rate increase, which varies widely from one patient to another. Therefore, Clavel et al11 recently proposed a new index of AS severity derived from DSE, the projected aortic valve area (AVAproj) extrapolating from the measured data the valve area at a normal transvalvular flow rate of 250 ml/sec (flow increase >15% during DSE), and demonstrated that it is superior to traditional echocardiographic parameters (i.e., change in gradients and AVA) for differentiation between TS and PS AS as well as for the prediction of outcome in patients with classical LF-LG AS. However, there is a group of patients in whom the increase in flow rate during DSE remains <15%, and the calculation of AVAproj is then not practical.
Distinguishing TS AS from PS AS in patients without or with limited flow reserve remains challenging. Cueff et al12 recently reported that the quantification of aortic valve calcification using multislice computed tomography may be helpful in this setting. A value of >1,651 Agatston units was found to have 82% sensitivity, 80% specificity, 88% negative-predictive value and 70% positive-predictive value for the diagnosis of severe AS in the setting of low flow, low gradient AS.
OUTCOME AND TREATMENT
In “classical” low flow, low gradient AS, outcome is in general poor with survival rates <50% at three-year follow-up if treated medically. Therefore, aortic valve replacement should be considered in TS AS but the operative risk remains high1,13. Several studies have shown that especially patients with severe AS and low flow due to left ventricular systolic dysfunction have a similar or worse prognosis than those with classical normal flow, high gradient AS both with and without surgery3,5,14-16.
The assessment of LV flow reserve is useful to estimate operative risk but does not permit prediction of recovery of LV function, improvement in symptomatic status, or late survival after operation14. For this reason, the absence of LV flow reserve should not preclude consideration of AVR in these patients8,14,17. Distinction between TS and PS AS remains a problem in this subset of patients and quantification of valve calcification by computed tomography may currently be the only clue in this setting. Because of the high operative mortality, the risk and benefit of surgery must be carefully weighed. In patients with concomitant coronary artery disease, assessment of myocardial viability should be considered as an additional basis for the decision to intervene.
A recent analysis of the PARTNER trial showed that even patients with severe AS and low flow as well as those with concomitant low ejection fraction and low gradient treated with TAVI had improved survival as compared to patients treated medically3. An additional finding of this study was that early survival improved with TAVI versus surgical aortic valve replacement (SAVR) in LF severe AS. As a possible explanation, the less invasive nature of TAVI and the harmful effects of cardiopulmonary bypass in these patients were proposed. In addition, patient prosthesis mismatch was significantly less frequent in TAVI compared to SAVR18 and has previously been shown to affect outcome negatively in LF-LG AS19. Thus, TAVI may be an attractive alternative to SAVR in LF-LG AS but its exact role in this setting still needs to be determined by further research.
In a recent study by Flett et al, diffuse myocardial fibrosis measured by CMR was the strongest determinant of functional status at baseline and seemed to be not reversible in a follow-up of six months after AVR20. Although further studies are required, the quantification of myocardial fibrosis may gain importance for therapeutic decisions in the future.
Low flow, low gradient aortic stenosis with normal LV ejection fraction
Low flow, low gradient aortic stenosis with preserved LV ejection fraction, also called “paradoxical” LF-LG (PLF-LG), has recently been introduced as a new entity. It is characterised by a hypertrophied, small LV and a restrictive physiology leading to impaired LV filling, low flow state, and higher valvulo-arterial impedance14,21,22. It is defined as aortic stenosis with a mean gradient <40 mmHg (or peak velocity <4 m/sec), an AVA <1.0 cm2 with preserved LVEF (>50%) but stroke volume index <35 ml/m2. The cause of reduced stroke volume has been attributed to intrinsic myocardial dysfunction and elevated arterial afterload as a manifestation of a stenotic valve and systemic arterial effects4,23,24. However, this entity has to be diagnosed with particular care, since other reasons –in particular measurement errors– for the finding of a small valve area and low gradients in the presence of normal left ventricular function have to be excluded. The continuity equation may underestimate the valve area because of flow underestimation caused by the underestimation of the LVOT area when assuming a circular shape while it is in fact rather oval25,26. Transoesophageal 3-D echocardiography may prove to be useful in measuring the definite LVOT cross-sectional area to obtain more accurate values27. Confirmation of a low flow state as well as gradient and valve area measurements may in some patients require additional non-invasive modalities (magnetic resonance, computed tomography) or invasive evaluation.
Furthermore, it has to be emphasised that current cut-offs for valve area and velocity/gradient are not really consistent to begin with. To generate a mean gradient of 40 mmHg with a normal stroke volume, the valve area must be closer to 0.8 than to 1.0 cm2 28-31. Finally, a patient’s small stature may be another reason for a small valve area and low gradient in the presence of normal EF if AVA is not indexed for body surface area.
Furthermore, the vast majority of these PLF-LG AS patients have in general been described as elderly patients with concomitant presence of arterial hypertension14,21. LV hypertrophy, myocardial fibrosis and consecutive reduced longitudinal LV function may in a particular patient be caused by long-standing hypertension rather than by the aortic stenosis32,33.
As in classical LF-LG severe AS, some patients may have a pseudo-severe AS due to incomplete opening of a moderately stenotic valve. Although Clavel et al also suggested in a recent study that DSE measuring the AVAproj may distinguish between these two patient groups in this entity34, more data are required to confirm these results. In any case, one would assume that these small volume ventricles with normal EF would respond differently to dobutamine as far as potential flow increase is concerned.
Again, the degree of valve calcification as assessed by multislice computed tomography may be an important hint to identify true severe AS although further studies are required to confirm this12.
OUTCOME AND TREATMENT
Patients with a PLF-LG severe AS have been reported to have a worse prognosis compared with patients with moderate AS as well as those with “classical” high gradient severe AS35. Several studies have also suggested that symptomatic patients with a PLF-LG severe AS have a markedly impaired survival among those treated medically compared to patients who undergo aortic valve replacement (TAVI or SAVR)3,21,35. A combination of more advanced age, more frequent comorbidities such as hypertension, worse intrinsic myocardial damage, and lesser referral to surgery were mentioned as reasons for the worse outcome35. As discussed above, a recent study by Clavel et al34 suggested that measurement of AVAproj may allow discrimination of TS versus PS AS in patients with PLF AS and may better predict clinical outcome of patients with PLF-LG AS.
In PLF-LG AS, surgery should be performed only in symptomatic patients and requires special attention because of the limited amount of data on the natural history and outcome after surgery30,36. Other reasons for the combination of a small valve area with a low gradient at normal LVEF (as described above) must be carefully excluded to confirm the diagnosis of severe AS before surgery is considered1. The difficulty in clinical practice is that symptoms, LV hypertrophy and BNP elevation, which may otherwise help in the decision for intervention, may in many of these patients also be due to hypertensive heart disease. Thus, PLF-LG AS remains currently challenging with regard to both certain diagnosis of severe AS and optimal patient management.
Patients with normal flow, low gradient aortic stenosis
Patients with a PLF-LG AS have to be distinguished from patients with normal flow, low gradient AS patients with preserved LV function. It is likely that these patients have in general only moderate AS. They may be primarily those with AVA - gradient discordance due to the inherent inconsistency in the guidelines criteria, inaccurate echocardiographic measurement of the left ventricular outflow tract and/or a small body size14. This entity is characterised by a normal afterload, and preserved LV longitudinal myocardial function and low BNP levels4,5. Although the outcome of these patients is currently debated5,35, it appears questionable in particular whether patients with this constellation can indeed have severe AS. In the study by Jander et al this patient group is likely to be predominant and they reported an outcome identical to those with moderate AS30,37. Valve replacement will therefore in general not be justified.
Summary
LF-LG AS remains a challenging condition both in terms of diagnosis and treatment. In classical LF-LG AS with reduced ejection fraction, dobutamine echocardiography should be the first step. It allows the detection of flow reserve which has prognostic implications and provides differentiation between pseudo-severe and true severe AS. Patients with true severe AS are the best candidates for valve replacement while those with pseudo-severe AS should rather be treated medically until studies have demonstrated a clear benefit of intervention in this subset. Patients without flow reserve have the worst outcome and a high operative mortality. Since survivors have been reported to benefit with regard to improvement in LV function as well as functional status, they may be considered for valve replacement on an individual basis. Assessment of myocardial viability in patients with coronary artery disease providing important information with regard to potential improvement of LV function and the quantification of valve calcification by multislice CT confirming severe AS may support the decision to intervene in this difficult patient subset. Although this has to be proven in further studies, TAVI with lower interventional risk and higher likelihood of avoiding patient-prosthesis mismatch may offer advantages in this context.
LF-LG AS with preserved LVEF represents the most challenging subset of patients. The first step should be to ensure a low flow status. Patients with an echocardiographically calculated normal flow are most likely to have moderate AS. Measurement errors, inconsistency of currently recommended cut-offs for mean gradient and valve area, and small patient stature may be other reasons for the combination of a small valve area with a low gradient.
In the case of echocardiographically calculated low flow (<35 ml/m2 stroke volume), paradoxical LF-LG AS with preserved LVEF should be considered. Again, measurement errors causing underestimation of flow need to be carefully excluded. This may require additional diagnostic modalities such as catheterisation or cardiac MRI. Although dobutamine echocardiography has also been proposed in this patient subset, currently it remains unclear how to distinguish best between pseudo-severe and true severe AS. Again, quantification of valve calcification by multislice CT may add important information in this context. In any case, severe AS must be carefully confirmed before deciding on intervention. The co-existence of LV hypertrophy and fibrosis, reduced longitudinal LV function as well as symptoms and elevated neurohormone levels may help in this context. However, it has to be kept in mind that these findings may also be due to hypertensive cardiac disease, which is frequently present in this patient subset. How to identify patients with PLF AS with preserved LVEF who benefit from valve replacement still needs to be defined.
Conflict of interest statement
The authors have no conflicts of interest to declare.

