Abstract
In patients with aortic stenosis (AS), a low-flow state may occur with reduced LV ejection fraction (LVEF) (i.e., classic low flow) or with preserved LVEF (i.e., paradoxical low flow) and it is often associated with low gradient because the gradient is highly flow-dependent. Low-flow, low-gradient (LF-LG) AS is a frequent clinical entity generally associated with worse outcomes. A multimodality imaging approach, including comprehensive resting echocardiography, dobutamine stress echocardiography (DSE), and multidetector computed tomography (MDCT), is the key to successful management of patients with LF-LG AS, who represent a highly challenging subset from both a diagnostic and a therapeutic standpoint. DSE and quantification of aortic valve calcification by MDCT provide important information that is crucial to differentiate true-severe from pseudo-severe AS and therefore select the most appropriate therapy (i.e., AVR vs. medical). The assessment of LV flow reserve by DSE is useful to stratify the operative risk and guide decision making between surgical and transcatheter AVR. Other imaging biomarkers, such as the global LV longitudinal strain measured during DSE or the amount of myocardial fibrosis assessed by cardiac magnetic resonance imaging, may provide incremental information for risk stratification and therapeutic management in LF-LG AS, but additional studies are needed to validate and refine these emerging biomarkers further.
Introduction
Most patients with severe aortic stenosis (AS) present with a small aortic valve area (AVA <1 cm2) and a high mean transvalvular gradient (MG ≥40 mmHg)1. If such patients are symptomatic or have a reduced LV ejection fraction (LVEF), aortic valve replacement (AVR) is recommended (Class I)2,3. However, in about one third of patients with AS, there is a discordance among echocardiographic or cardiac catheterisation markers of AS severity, the most frequent being an AVA <1 cm2 and a MG <40 mmHg (or peak aortic jet velocity, Vmax <4 m/s)4-6. This discordance may raise uncertainty about the actual severity of the stenosis and thus about the indication of AVR. This AVA (small) - gradient (low) discordance may be related to several factors including: i) measurement errors, ii) small body surface area, iii) inherent discrepancy in the AVA and gradient cut points proposed in the guidelines to define severe stenosis, and/or iv) the presence of a low-flow state7-10. A low-flow state may occur with reduced LVEF (i.e., classic low flow) or with preserved LVEF (i.e., paradoxical low flow) and it is often associated with low gradient and worse outcomes5,8,11-13. Hence, low-flow, low-gradient (LF-LG) AS is a highly challenging entity from both a diagnostic and a therapeutic standpoint. The purpose of this article is to describe the key role of multimodality imaging, and in particular of Doppler echocardiography and multidetector computed tomography (MDCT) for the assessment of stenosis severity, the risk stratification, and the therapeutic management in patients with LF-LG AS.
Different patterns of low-flow, low-gradient AS
Low flow is generally defined by a stroke volume indexed to body surface area <35 mL/m2 and/or a cardiac index <3 l/min/m2 and low gradient by a MG <40 mmHg2,3. There are two main different patterns of LF-LG AS: classic and paradoxical LF-LG AS (Figure 1, Table 1, Moving image 1). Classic LF-LG occurs in 5 to 10% of patients with AS14,15 and it is characterised by a reduced LVEF and often enlarged LV cavity (Figure 1, Table 1, Moving image 2)13,16-19. Patients with this entity are more often men and frequently have concomitant coronary artery disease20. Paradoxical LF-LG AS occurs in 5 to 20% of the AS population and, as opposed to classic LF-LG, this entity is characterised by a preserved LVEF and a small LV cavity with pronounced concentric remodelling (Figure 1, Table 1, Moving image 3)4,8,9,21. This entity is more frequent in women and is often associated with concomitant arterial hypertension. In patients with classic LF-LG AS, the low stroke volume is mainly due to depressed LV systolic function, whereas in paradoxical LF-LG it is predominantly related to a restrictive physiology (Table 1). Furthermore, although patients with paradoxical LF-LG have a preserved LVEF, their LV systolic longitudinal is typically reduced, particularly in the basal segments, and this phenomenon further contributes to reducing stroke volume21,22. Besides LV systolic/diastolic dysfunction, other concomitant factors may also cause or worsen a low-flow state in AS patients, including mitral regurgitation, mitral stenosis, tricuspid regurgitation, atrial fibrillation, constrictive pericarditis, etc.
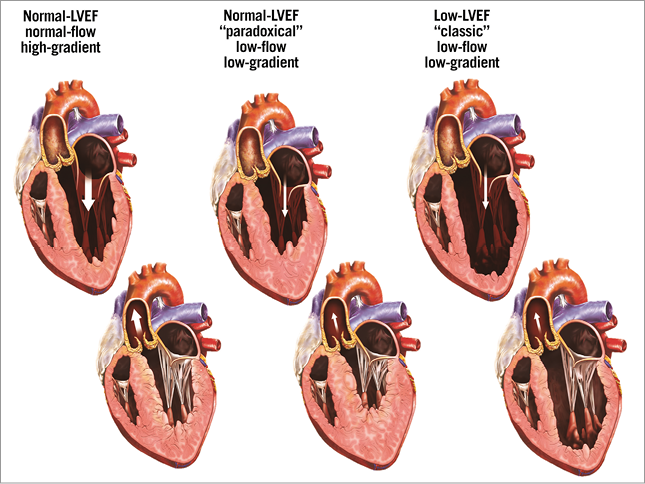
Figure 1. Different patterns of severe AS according to flow, gradient, and LV geometry. Top panels: diastole. Bottom panels: systole. Reproduced with permission19.
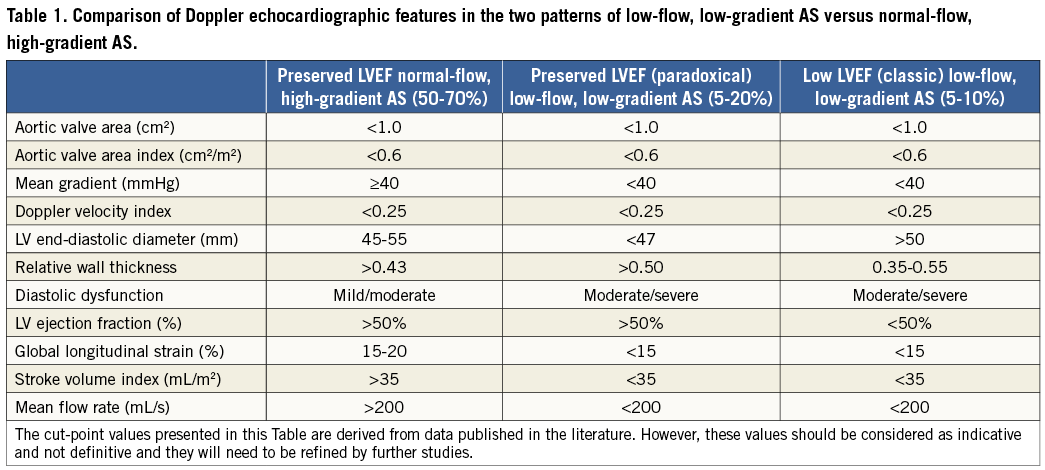
The first essential step in the evaluation of patients with LF-LG AS is to rule out measurement errors (Figure 2). Particular attention should be paid to the measurement of stroke volume in the LV outflow tract, since this parameter is used to identify the presence of low flow (i.e., stroke volume index <35 mL/m2) and also to calculate the AVA23. Hence, an underestimation of stroke volume could lead the echocardiographer to conclude falsely that the patient has a LF-LG severe AS, whereas in fact he or she has moderate AS with normal flow. Proper confirmation of the presence and type of LF-LG (classic versus paradoxical) also requires the identification of the Doppler echocardiographic features presented in Table 1 as well as the factor(s) responsible for the low-flow state. A comprehensive Doppler echocardiographic exam is thus essential for diagnosis and characterisation of LF-LG AS as well as for the identification of the causative factors.
Assessment of stenosis severity
In the setting of a low-flow state, it is often difficult to separate patients with true-severe AS (TS AS) from those with pseudo-severe AS (PS AS), i.e., with a reduced aortic opening due to limited flow in the setting of only mild to moderate aortic valve obstruction13,24-27. This distinction is essential since patients with TS AS are the ones who may benefit from AVR, whereas the patients with PS AS may not benefit from this intervention and should rather be treated medically27,28. Approximately one third of patients with LF-LG AS have PS AS and the proportion is similar in patients with classic versus paradoxical LF-LG18,27-29.
Dobutamine stress echocardiography (DSE) with low-dose (up to 20 µg/kg/min) protocol has been shown to be useful to differentiate TS from PS AS in patients with classic LF-LG (Figure 2)1-3,17,18,25,26. Different parameters and criteria have been proposed in the literature to identify TS AS including a peak stress MG ≥30 or 40 mmHg, a peak stress AVA <1.0 or 1.2 cm2 and/or an increase in AVA <0.2 or 0.3 cm217,25,26. In the ESC/EACTS guidelines3, TS AS is considered to be present when there are small changes in AVA (increase <0.2 cm2 and remaining <1.0 cm2) with increasing flow rate but a significant increase in gradients (MG >40 mmHg). In the 2014 ACC/AHA guidelines2, TS AS is defined by a MG >40 mmHg (or VPeak ≥4 m/s) with an AVA ≤1.0 cm2 at any DSE stage. However, given that all parameters of stenosis severity are flow-dependent, the magnitude of their augmentation during DSE may vary greatly depending on the extent of LV flow reserve (i.e., the increase in flow during DSE)30. Hence, in patients with minimal flow reserve, the AVA (small) - gradient (low) discordance may persist at the end of DSE and the stenosis severity therefore remains uncertain. In such patients with persisting discordance at DSE, it could be useful to calculate the projected AVA at normal flow rate (i.e., 250 mL/s) using the following equation29:
![]()
where AVARest and AVAPeak are AVA at rest and peak DSE and QRest and QPeak are transvalvular flow rate (stroke volume divided by ejection time) at rest and peak DSE. A projected AVA ≤1.0 cm2 suggests the presence of TS AS. This new parameter has the advantage of being standardised for flow rate, and it has been shown to predict actual stenosis severity and clinical outcomes better in patients with classic LF-LG AS18,27,29,30. In contrast, aortic valve resistance does not appear to offer any significant advantage in these patients given that it is, in fact, more flow-dependent than AVA31. Dobutamine stress catheterisation can also be used to differentiate TS from PS AS26 but it is more invasive than DSE and is also subject to technical pitfalls and measurement errors. DSE is thus the preferred approach to confirm stenosis severity in patients with classic LF-LG AS (Figure 2). A recent study also showed that DSE or exercise stress echocardiography can be used in patients with paradoxical LF-LG AS and the same parameters and criteria (including projected AVA) can be applied in these patients (Figure 2)27. However, DSE should not be used in patients with severe LV restrictive physiology, which is frequently found in patients with paradoxical LF-LG AS. Exercise stress echocardiography may be used in patients who are asymptomatic or have mild equivocal symptoms. The DSE parameters and criteria presented above to differentiate TS from PS AS can also be applied for exercise stress echocardiography.
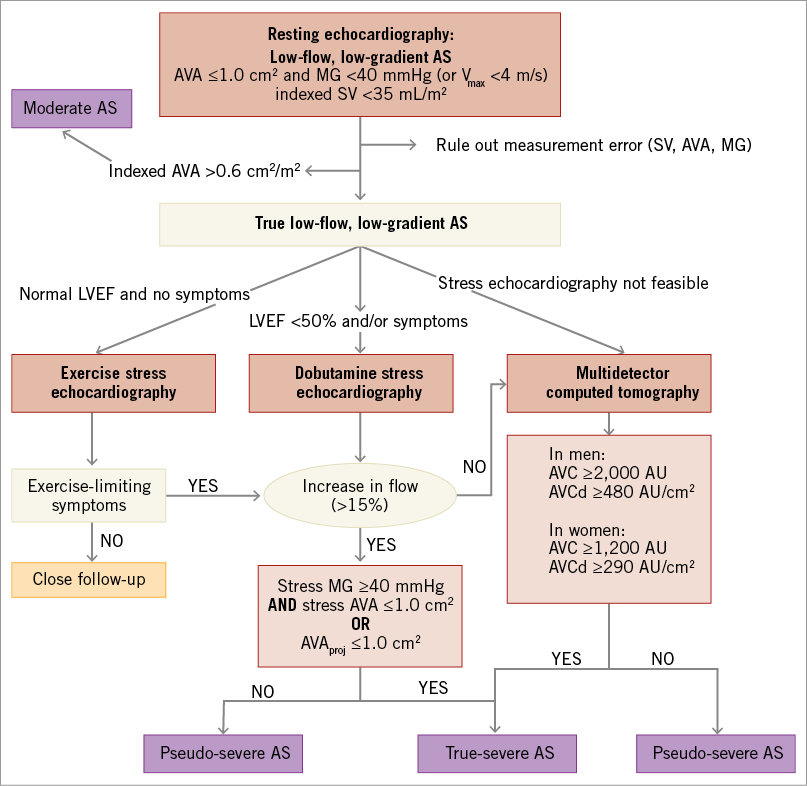
Figure 2. Algorithm for the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis. When the increase in mean flow rate (stroke volume/LV ejection time) is <15%, the results of DSE with regard to stenosis severity (AVA, MG, and projected AVA) are generally inconclusive. AS: aortic stenosis; AVA: aortic valve area; AVC: aortic valve calcification; AVCd: AVC density, i.e., AVC divided by aortic annulus cross-sectional area; LVEF: left ventricular ejection fraction; MG: mean gradient; SV: stroke volume In a significant proportion (~20 to 30%) of patients with classic or paradoxical LF-LG AS, DSE is not feasible due to contraindications (complex ventricular arrhythmia, rapid atrial arrhythmia, unstable angina, severe restrictive physiology, etc.) or it is inconclusive due to absence of flow reserve or poor image quality. In such patients, quantification of aortic valve calcification (AVC) by MDCT can be used to corroborate stenosis severity (Figure 2). Indeed, AVC has been shown to correlate well with AS haemodynamic severity6,32,33. However, this relationship is gender-dependent: women reach haemodynamically severe AS with a lower amount of AVC compared to men, even after accounting for body surface area and aortic annulus cross-sectional area6,34. It is thus important to use lower AVC cut-point values in women (AVC ≥1,200 AU) than in men (AVC ≥2,000 AU) to identify severe AS6. The AVC can also by indexed to the cross-sectional area of the aortic annulus to obtain the “AVC density” (AVCd), and the threshold values for severe stenosis are: AVCd ≥290 AU/cm2 in women and AVCd ≥480 AU/cm2 in men6. The measurement of AVC by MDCT has several advantages, including the fact that it is highly feasible, simple, reproducible, and accurate. More importantly, this parameter is not influenced by flow or haemodynamics and therefore, as opposed to all other modalities including Doppler echocardiography, cardiac magnetic resonance (CMR), and cardiac catheterisation, it does not require dobutamine (or other) stress agent to differentiate TS from PS AS in patients with classic or paradoxical LF-LG. Hence, AVC by MDC provides a valuable alternative to DSE in these patients when the latter is not feasible or inconclusive (Figure 2). MDCT also has some limitations including: i) the exposure to ionising radiation; ii) the variability of the AVC measurements depending on the image acquisition/analysis system, software, and protocol; and iii) the occurrence (even if low) of false negative (i.e., low AVC despite TS AS) or false positive (i.e., high AVC despite PS AS) cases. DSE and MDCT are thus complementary imaging modalities, which allow the confirmation of stenosis severity in patients with LF-LG AS (Figure 2).
Like echocardiography and CMR, MDCT allows the measurement of the anatomic valve orifice area by planimetry. However, due to the flow contraction phenomenon, the anatomic AVA often overestimates the Doppler-derived effective AVA, which is the main determinant of the transvalvular gradient and LV pressure overload. Nonetheless, if, in a patient with a small (<1.0 cm2) effective AVA, 2D echocardiography, CMR or MDCT reveals a large anatomic AVA (>1.5 cm2), one should suspect an error in the measurement of the effective AVA by Doppler echocardiography. As for the effective AVA, the anatomic AVA may be pseudo-severe, and thus dobutamine stress is required to confirm stenosis severity.
Risk stratification
It is well known that depressed LVEF is a major predictor of mortality in the general AS population, regardless of the type of treatment –conservative or AVR11,13,20. Recently, low flow (i.e., stroke volume index ≤35 mL/m2) has also been shown to be a powerful risk factor in patients with AS, which is possibly stronger than LVEF12,20,35,36. A low gradient (MG <40 mmHg) has been reported to be a predictor of post-procedural mortality in the subset of patients undergoing surgical or transcatheter AVR, but recent studies suggest that this risk apparently associated with low gradient is, in large part, due to the concomitant presence of low flow12,35-37.
In patients with classic LF-LG, very low resting gradient (MG <20 mmHg) is associated with an increased risk of mortality following surgical AVR. The presence of very low gradient is probably a composite marker of more severe low-flow state (i.e., more severely reduced stroke volume index) and/or of less severe stenosis. Furthermore, in these patients, the absence of LV flow reserve, as documented by a percent increase in stroke volume <20% during DSE, is a predictor of perioperative mortality following AVR. Indeed, previous studies have reported that patients with no flow reserve, who represent approximately 30-40% of patients with LF-LG AS, have an operative mortality of between 22 and 33% compared to 5-8% in patients with flow reserve14,17,38. Hence, the absence of flow reserve is useful for operative risk stratification in patients with LF-LG AS prior to surgical AVR but, on the other hand, this factor does predict long-term mortality or recovery of LVEF following AVR39. Other DSE parameters of valve or LV function, including a projected AVA <1.2 cm2 and peak stress LVEF <35% during DSE, have been shown to predict long-term mortality both in patients treated surgically and in those treated conservatively18,29. The peak stress LVEF is probaly a composite marker of resting LV function and contractile reserve with DSE. The fact that the best cut point of projected AVA to predict outcomes (i.e., 1.2 cm2) is higher than that to identify TS AS (i.e., 1.0 cm2) suggests that the increased load imposed by a moderate AS may be tolerated well by a normal ventricle but poorly by a failing ventricle.
Recent studies suggest that echocardiographic parameters of LV longitudinal systolic function, including mitral annulus displacement or global longitudinal strain and strain rate, are superior to LVEF to quantify the extent of myocardial impairment and to predict outcomes in patients with classic or paradoxical LF-LG21,40,41. These parameters are surrogate markers of the severity of subendocardial fibrosis and they appear to be among the most promising imaging biomarkers in LF-LG AS. Given that platforms from different ultrasound system vendors may provide somewhat different results for myocardial strain, there is a need for the realisation of multicentre studies using a common and vendor-independent platform in order to establish the best cut points of global longitudinal strain to predict outcome and response to therapy in patients with LF-LG AS.
The quantification of myocardial fibrosis by cardiac magnetic resonance (CMR) imaging also appears to be a very promising approach to enhance risk stratification in patients with classic or paradoxical LF-LG AS21,42. Patients with extensive myocardial fibrosis have poor outcomes under conservative therapy but high operative mortality and limited regression of symptoms following surgical AVR21,42. Besides focal fibrosis which can be assessed by late gadolinium enhancement, it may also be important and probably more relevant to assess diffuse fibrosis using contrast-enhanced T1-mapping techniques in these patients with LF-LG AS. The main limitation of CMR is the high cost and often limited availability of this modality as well as the lack of standardisation of the imaging sequence and criteria that should be used to quantify myocardial fibrosis. Additional studies are needed to establish further and refine the role of the quantification of LV longitudinal strain by speckle tracking and of myocardial fibrosis by CMR in patients with LF-LG AS.
Therapeutic management
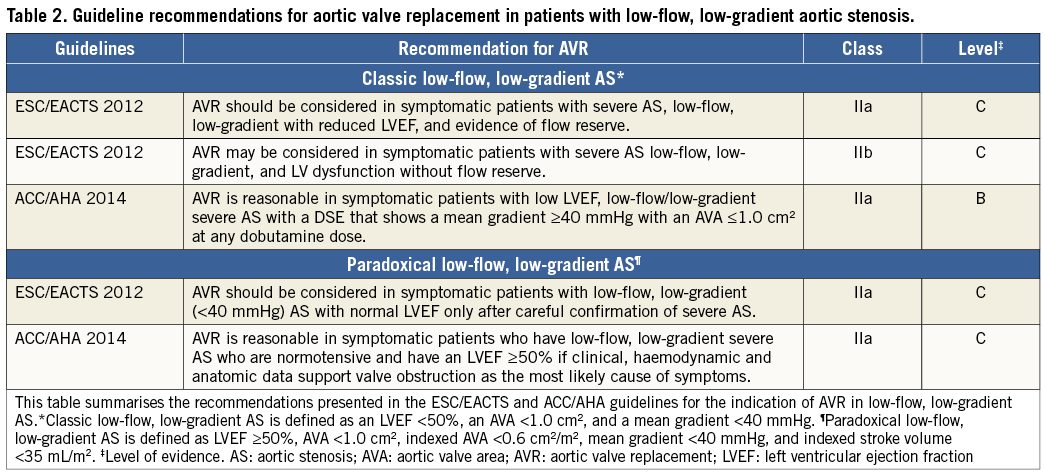
As underlined in the two previous sections, multimodality imaging is useful to determine stenosis severity and operative risk, which are key pieces of information to guide therapeutic management of LF-LG AS (Figure 3). Cardiac catheterisation and angiography do not provide any additional information on aortic valve or LV function besides that obtained by DSE, MDCT or CMR. However, cardiac catheterisation is useful to detect and document the presence of significant obstructive coronary disease, which may dictate the need for a revascularisation procedure. Table 2 presents the recommendations of the ESC/EACTS and ACC/AHA guidelines with regard to the indication for AVR in patients with classic and paradoxical LF-LG AS.
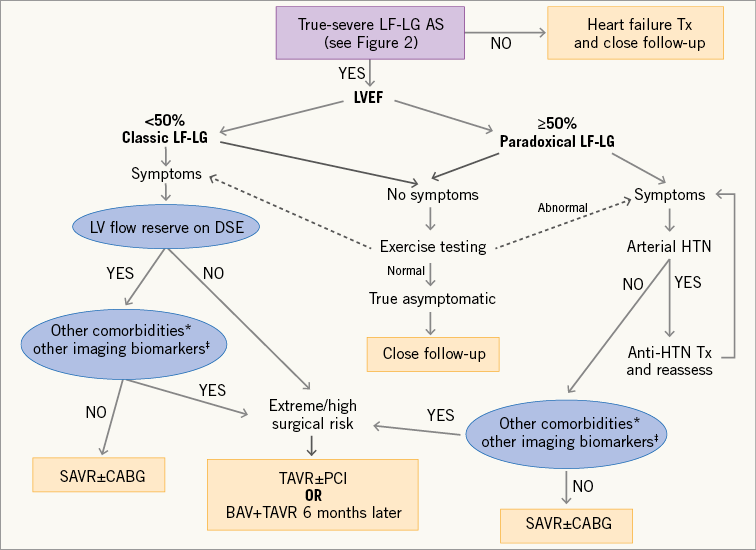
Figure 3. Algorithm for the therapeutic management of patients with low-flow, low-gradient aortic stenosis. *Comorbidities associated with high/extreme surgical risk. †Emerging biomarkers obtained by non-invasive multimodality imaging (DSE, MDCT and CMR), including severe myocardial fibrosis, severely reduced global longitudinal strain, severe LV restrictive diastolic pattern. These biomarkers also include the presence of a small aortic annulus (<21 mm) as this factor may increase the surgical risk as well as the risk of prosthesis-patient mismatch. BAV: balloon aortic valvuloplasty; CABG: coronary artery bypass graft surgery; HTN: hypertension; PCI: percutaneous coronary intervention; Tx: treatment; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement
Classic LF-LG AS
Patients with classic LF-LG AS who have evidence of TS AS and LV flow reserve should undergo surgical AVR (Figure 3, Table 2). Coronary artery bypass graft surgery should be performed concomitantly in patients with clinical indications for revascularisation. However, in patients with no flow reserve or with other comorbidities associated with high surgical risk, transcatheter AVR may provide a good alternative to surgery (Figure 3)35,36,43-45. In patients in whom recovery of LV function as well as the regression of symptoms after procedure are uncertain (e.g., patients with extensive myocardial fibrosis, severe frailty, and/or severe comorbidities, such as oxygen-dependent chronic obstructive pulmonary disease)21, a staged approach with balloon valvuloplasty first followed by surgical or transcatheter AVR six months later (if LV function/symptoms improve) may be the preferred strategy. In the PARTNER I trial cohort A (high risk), there was no significant difference between transcatheter and surgical AVR in the subset of patients with classic LF-LG AS36. However, patients with no flow reserve (i.e., patients with higher surgical risk) were excluded from this trial, therefore introducing a selection bias. In a non-randomised study including both LF-LG patients with flow reserve and those with no flow reserve, transcatheter AVR was associated with better and faster recovery of LVEF43.
Patients with classic LF-LG and evidence of PS AS (Figure 2) should be treated conservatively, but they require aggressive heart failure therapy and close follow-up (Figure 3)28. However, these patients may need AVR if the stenosis progresses to the severe stage during follow-up and/or if medical therapy fails to improve symptoms.
Paradoxical LF-LG AS
The ESC/EACTS and ACC/AHA guidelines2,3 recommend AVR (Class IIa) in patients with paradoxical LF-LG AS if they fulfil the following conditions (Table 2): i) presence of symptoms; ii) confirmation of TS AS; and iii) symptoms most likely related to AS. Moreover, given that arterial hypertension is frequent in patients with paradoxical LF-LG and that it may contribute to the low-flow state, the AVA gradient discordance and the symptoms, the ACC/AHA guidelines2 recommend first to optimise antihypertensive therapy and then to reassess stenosis severity and symptoms once blood pressure has been normalised before eventually considering AVR.
Paradoxical low-flow AS is often associated with several factors (i.e., pronounced concentric remodelling, advanced myocardial fibrosis, severe diastolic function, severely impaired LV systolic longitudinal strain, small aortic annulus, etc.) which may increase the risk of perioperative mortality as well as the risk of prosthesis-patient mismatch following surgical AVR4,8,21. Transcatheter AVR may help to reduce both the operative risk and the incidence of prosthesis-patient mismatch in these patients36,46. Hence, as in patients with classic LF-LG AS, transcatheter AVR may provide a valuable alternative to surgical AVR in patients with paradoxical LF-LG AS, particularly if they have the risk factors mentioned above (Figure 3). In this regard, the investigators of the PARTNER IA trial revealed that patients with paradoxical LF-LG AS appear to have better short-term survival with transcatheter AVR compared to surgical AVR36. However, these patients may poorly tolerate even a mild degree of acute aortic regurgitation due to their restrictive physiology. Hence, if transcatheter AVR is considered for the treatment of paradoxical LF-LG severe AS, it is essential to avoid any degree of paravalvular regurgitation. Additional studies are needed to determine which is the best procedure, i.e., surgical versus transcatheter AVR, in the different subsets of patients with classic or paradoxical LF-LG severe AS.
Conclusion
A multimodality imaging approach, including comprehensive resting echocardiography, DSE, and/or MDCT, is the key to successful management of patients with classic or paradoxical LF-LG AS, who represent a highly challenging subset from both a diagnostic and a therapeutic standpoint. This approach is indeed essential for confirmation of stenosis severity, risk stratification, and therapeutic decision making in these patients. DSE and AVC quantification by MDCT provide important complementary information that is crucial to differentiate TS from PS AS and therefore to select the most appropriate therapy (i.e., AVR vs. medical) for a given patient. The assessment of LV flow reserve by DSE is useful to stratify the operative risk and guide decision making between surgical and transcatheter AVR. Other imaging biomarkers, such as the global LV longitudinal strain measured at rest and during DSE or the amount of myocardial fibrosis assessed by CMR, may provide incremental information for risk stratification and therapeutic management in LF-LG AS, but additional studies are needed to validate and refine these emerging biomarkers further.
Conflict of interest statement
P. Pibarot received research grants from Edwards Lifesciences for Echocardiography Core Laboratory Analyses in transcatheter valve therapy studies. The other author has no conflicts of interest to declare.
Online data supplement
Moving image 1. Representative example of echocardiographic images of the LV cavity and aortic valve in the parasternal long-axis view for patients with normal flow, high gradient.
Moving image 2. Representative example of echocardiographic images of the LV cavity and aortic valve in the parasternal long-axis view for patients with classic low flow, low gradient.
Moving image 3. Representative example of echocardiographic images of the LV cavity and aortic valve in the parasternal long-axis view for patients with paradoxical low flow, low gradient.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Representative example of echocardiographic images of the LV cavity and aortic valve in the parasternal long-axis view for patients with normal flow, high gradient.
Moving image 2. Representative example of echocardiographic images of the LV cavity and aortic valve in the parasternal long-axis view for patients with classic low flow, low gradient.
Moving image 3. Representative example of echocardiographic images of the LV cavity and aortic valve in the parasternal long-axis view for patients with paradoxical low flow, low gradient.

