Abstract
Background: Meta-analyses of randomised trials of paclitaxel-coated peripheral devices found an association with worse long-term survival.
Aims: We aimed to assess long-term mortality in patients treated with drug-coated versus non-drug-coated devices who are insured by Medicare Advantage (MA), an alternative to traditional Medicare that represents >30% of the Medicare eligible population. We analysed data from an MA administrative claims data source that includes both inpatient and outpatient femoropopliteal artery revascularisation procedures.
Methods: Patients treated with or without drug-coated devices for femoropopliteal artery revascularisation from 4/2015-12/2017 were studied using Optum’s De-identified Clinformatics Datamart Database. Mortality was assessed up to December 2019 using Kaplan-Meier cumulative mortality curves and Cox proportional hazard models. Inverse probability of treatment weighting was used to adjust for differences between groups.
Results: Of 16,796 patients revascularised, 4,427 (26.4%) were treated with drug-coated devices: 3,600 (81.3%) balloons and 827 (18.7%) stents. The median follow-up was 2.66 years (IQR 2.02-3.52). Treatment with drug-coated devices was associated with similar long-term mortality to non-drug-coated devices (adjusted HR 1.03, 95% CI: 0.96-1.10; p=0.39). Results were comparable for patients treated with balloons alone (adjusted HR 1.00, 95% CI: 0.92-1.08; p=0.96) or stents (adjusted HR 1.02, 95% CI: 0.88-1.18; p=0.78). These findings did not differ based on treatment setting, disease severity, age, sex or comorbidity burden (interaction p>0.05 for all).
Conclusions: In this large cohort, there was no evidence of increased long-term mortality following treatment with drug-coated devices.
Introduction
Drug-coated devices used for femoropopliteal artery revascularisation have been an integral component of contemporary clinical vascular practice, based on their durable improvement in long-term patency and reductions in clinically driven target lesion revascularisation as compared with uncoated devices1,2. Since approval, drug-coated devices have become the primary device used for peripheral endovascular intervention (PVI)3 and are recommended in society practice guidelines for most lesion types4.
Despite their established periprocedural and short-term safety, a meta-analysis published in December 2018 raised questions regarding risk of decreased long-term survival after exposure to paclitaxel-coated devices5. The impact of this meta-analysis has been widespread, including halting of randomised trials, restriction in use by the Food and Drug Administration to those deemed at highest risk, and a dramatic decline in daily utilisation6,7,8. Many have cautioned that the evidence is not definitive, due to the heterogeneity among the included devices and trials, substantial loss to follow-up, and lack of a putative mechanism of harm9,10. Moreover, none of the studies included in the meta-analysis was powered to assess mortality.
While a randomised trial powered to assess long-term survival following treatment with drug-coated devices would be the most definitive way to exonerate harm caused by these devices, the very large number of patients and long length of follow-up required reduce the feasibility of such a study11. As a result, the scientific community has turned to real-world data (RWD) to evaluate the safety of these devices. Numerous real-world studies have been published to date, among U.S., European and Asian patient cohorts12,13,14,15,16,17,18,19; none has reproduced the harm signal seen in the initial meta-analysis.
As the prevalence of peripheral artery disease (PAD) increases with age, Medicare claims data capture a critical U.S. PAD population and are an important source of RWD to examine long-term survival after PVI. To date, the longest-term published Medicare safety data come from the traditional Medicare fee-for-service population treated in an inpatient setting12,13. However, not all Medicare enrolees’ procedures are observed in traditional Medicare data. Medicare Advantage (MA) health plans are offered by private companies that contract with Medicare to provide hospital and medical benefits, and now enrol over one third of the Medicare eligible population20. Furthermore, patients insured by MA tend to be a healthier subset of Medicare-eligible patients21 and thus may have fewer competing risks of death.
As such, to provide a more complete evaluation of the long-term safety of drug-coated devices used for PVI among Medicare beneficiaries, we analysed data from an MA administrative claims data source that includes both inpatient and outpatient femoropopliteal artery revascularisation procedures.
Methods
DATA SOURCE AND STUDY SAMPLE
Using MA claims from Optum’s De-identified Clinformatics® Datamart Database, we conducted an observational retrospective cohort study of patients who underwent femoropopliteal artery revascularisation procedures with a drug-eluting stent (DES), drug-coated balloon (DCB), bare metal stent (BMS), or uncoated balloon (PTA) between April 2015 and December 2017. Optum® is a nationwide sample of administrative health insurance data including medical and prescription claims and enrolment records. The data include demographics, diagnosis codes, procedure codes, place of service codes, prescription drug codes as well as dates of service and death. Procedures occurring in the inpatient hospital setting were identified using International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) and procedures occurring in the outpatient hospital setting were identified using Current Procedural Terminology and Healthcare Common Procedure Coding System (HCPCS) C-codes (Supplementary Table 1). The study cohort was stratified by treatment with either a drug-coated device (DCB or DES) or a non-drug-coated device (BMS or PTA alone). For those who underwent multiple revascularisation procedures during the same index encounter, patients were included in the drug-coated group if they received any drug-coated device treatment, patients were included in the non-drug-coated group if they did not receive any drug-coated device treatment, and patients were included in the stent group if they received any stent. Patient index date was the date of the first revascularisation procedure observed in the data set. Patients were excluded if they were less than 18 years of age from the start of the lookback period or had an index procedure date occurring after death or disenrolment. They were also excluded if they had a PVI within 90 days of the start of the study period, as drug-coated device treatment could not be ascertained with fidelity prior to the establishment of the specific device codes (Supplementary Figure 1).
PATIENT CHARACTERISTICS
Baseline patient comorbidities were identified using diagnosis, procedure, and national drug codes that were present on any encounter during a 90-day lookback period prior to each individual’s index event. Comorbidities were determined using the Elixhauser comorbidity index definitions (Supplementary Table 2). Additional comorbidities identified using ICD-9-CM and ICD-10-CM/PCS codes included tobacco use, critical limb ischaemia (CLI), and adjunctive atherectomy procedures (Supplementary Table 3). Cardiovascular medication claims within the lookback period (exclusive of the index procedure date) were also reported and were categorised as statins, ACE inhibitors/ARBs, beta-blockers, P2Y12 inhibitors, and anticoagulants (Supplementary Table 4). Patient age, sex, U.S. region, insurance plan type (e.g., Health Maintenance Organisation [HMO] or Preferred Provider Organisation [PPO]), and procedure clinical setting (e.g., inpatient vs outpatient) were recorded from the index procedure date.
OUTCOMES
The primary outcome was all-cause mortality and was analysed up to 31 December 2019. Mortality data were ascertained using the CMS Master Beneficiary Summary File.
STATISTICAL ANALYSIS
Categorical variables were reported as counts and percentages, and continuous variables as means with standard deviations. The primary analysis compared drug-coated versus non-drug-coated devices. Kaplan-Meier methods were used to estimate the cumulative incidences of long-term mortality, and unadjusted Cox proportional hazards regression models were used to compare groups. Pre-specified subgroup analyses included: stent implantation (DES vs BMS); balloon angioplasty alone (DCB vs PTA); procedure setting (inpatient vs outpatient); age (median); sex; presence of CLI; and tercile of Elixhauser comorbidity score. A landmark analysis was also performed starting at one year, which corresponds to the last time point in the Katsanos meta-analysis where no mortality difference was observed. For the device sub-analyses, if a patient received both a DCB and a BMS, they were included in the DCB group. If a patient received both a DCB and a DES, they were included in the DES group. To account for differences in patient populations, we used propensity score-based inverse probability of treatment weighting (IPTW)22. Propensity scores were calculated by generating a logistic regression model that included over 44 patient characteristics and comorbidities to predict the probability of each patient receiving a particular treatment. Standardised mean differences (SMD) were used to assess the covariate balance before and after the IPTW adjustment. Variables with an SMD of <0.1 were considered balanced. A Cox proportional hazards regression model was then used to calculate hazard ratios. Derivation of propensity scores, propensity score-based weights, and hazard ratio estimation were performed separately for each subgroup analysis. Interaction tests for differences between categories within each subgroup were estimated using seemingly unrelated regression. A p-value <0.05 was considered significant.
Computation of propensity scores was performed using R Core Team, 2017 (R Foundation for Statistical Computing, Vienna, Austria). Remaining statistical analyses were performed using Stata, version 15.1 (StataCorp, College Station, TX, USA).
ETHICS APPROVAL
The study was approved by the Western Institutional Review Board with a waiver of informed consent for a retrospective data analysis.
Results
BASELINE CHARACTERISTICS
The study included 16,796 patients who received femoropopliteal artery revascularisation procedures during the study period. The average age of the patient population was 73.3±8.8 years, 44.4% were female, 50.8% had CLI, and 63.1% of procedures were performed in an outpatient location. DCB (either alone or with BMS) was used in 3,600 (21.4%), PTA (alone) in 5,840 (34.8%), DES (either alone or with DCB/PTA) in 827 (4.9%), and BMS (either alone or with PTA) in 6,529 (38.9%) (Table 1). Overall, sociodemographic characteristics and comorbidities between those treated with and without drug-coated devices were similar. However, patients treated with drug-coated devices received more atherectomy, more often carried diagnoses of claudication and diabetes, were less often insured by an HMO, and were more often treated in the East, North, Central and South Atlantic regions (Table 1, Figure 1). In addition, patients revascularised with a drug-coated device were more often being treated with a beta-blocker, P2Y12 inhibitor and a statin.
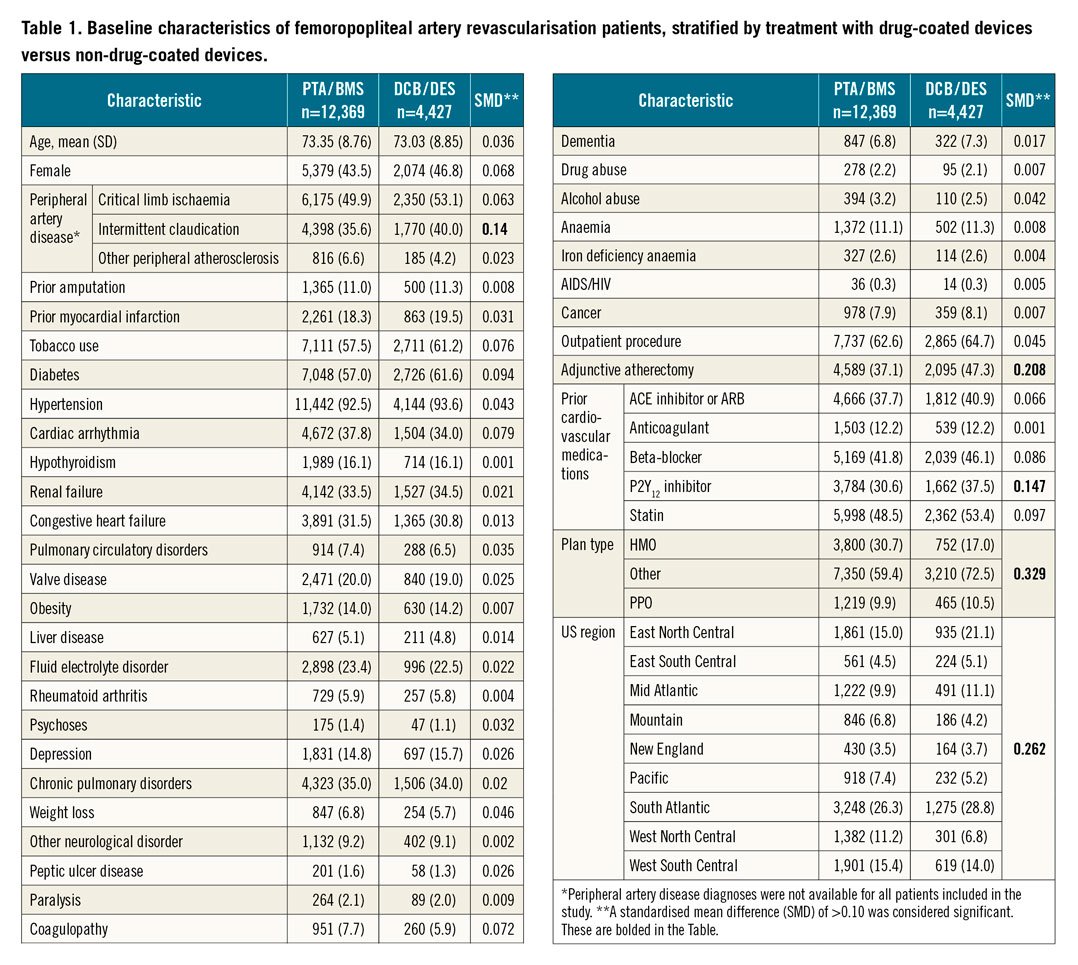
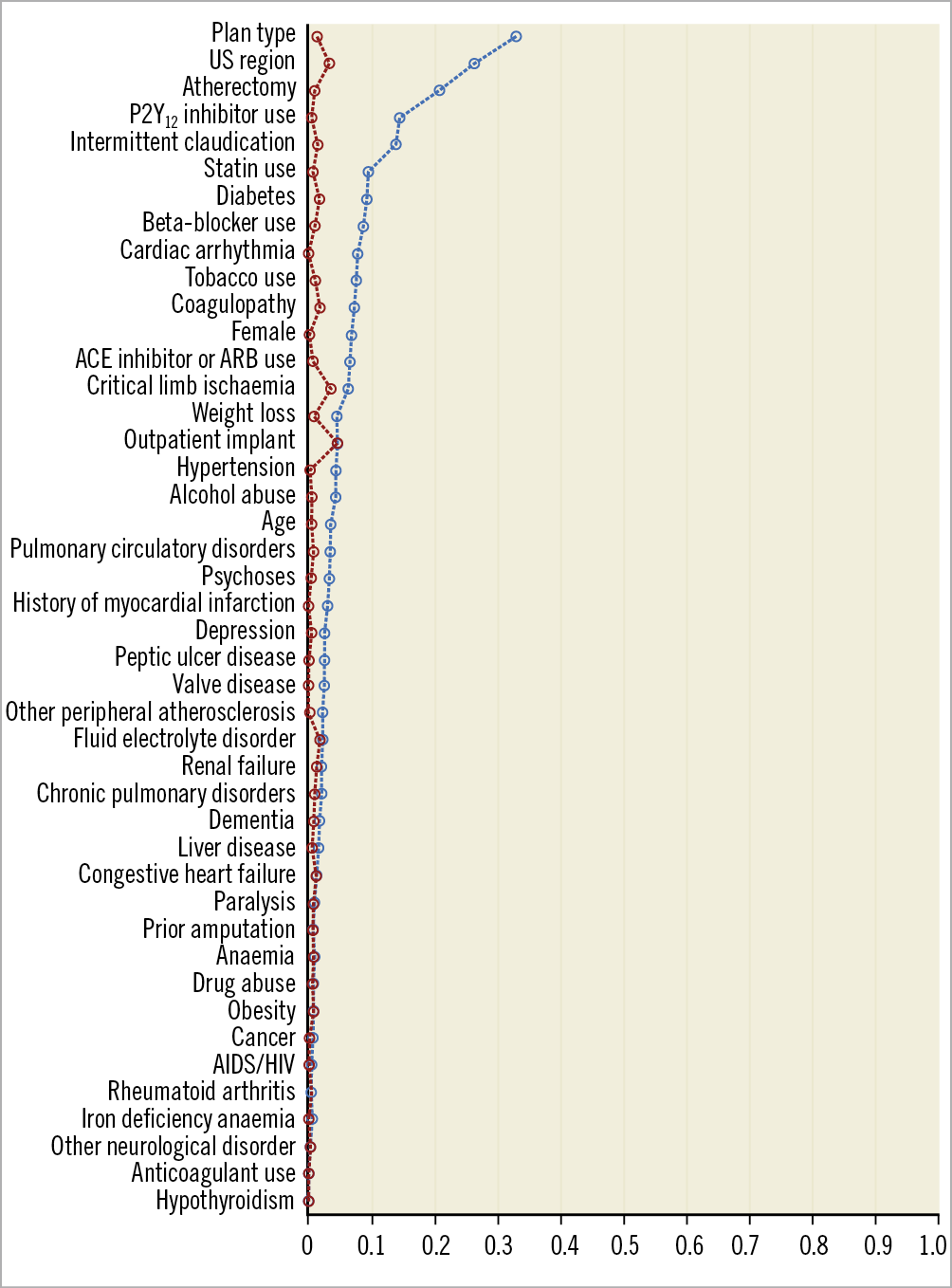
Figure 1. The standardised mean differences (SMDs) in characteristics between patients treated with drug-coated and non-drug-coated devices. Prior to inverse probability of treatment weighting, there were several imbalances in characteristics, as represented by SMDs ≥0.10. After weighting, no residual imbalances were noted, with all SMDs <0.10.
LONG-TERM SURVIVAL FOLLOWING PVI
Following femoropopliteal artery revascularisation, the median follow-up was 2.66 years (IQR 2.02-3.52). Prior to adjustment, treatment with drug-coated devices compared with non-drug-coated devices was associated with a similar cumulative risk of mortality at the end of the observation period (44.2% vs 43.3%), with a non-significant hazard ratio of 0.96 (95% CI: 0.90-1.02, p=0.19) (Supplementary Figure 2). The cumulative mortality risk was also comparable following DCB treatment compared with PTA treatment (42.8% vs 42.8%, respectively). However, there was a statistically significant difference in hazards between DCB and PTA in favour of DCB (HR 0.90, 95% CI: 0.84-0.97; p=0.01) (Supplementary Figure 3). DES use was associated with greater crude cumulative mortality compared with BMS use (51.0% vs 44.1%, respectively) at the end of the observation period, with a significant difference in the hazards prior to adjustment (HR 1.30, 95% CI: 1.16-1.46; p<0.001) (Supplementary Figure 4).
After application of the weights, all patient variables were balanced, with SMDs of <0.1 (Figure 1, Supplementary Table 5). In adjusted analysis, treatment with drug-coated devices was not associated with a difference in risk of all-cause mortality compared with non-drug-coated devices (adjusted HR 1.03, 95% CI: 0.96-1.10; p=0.39) (Figure 2). Moreover, no differences in hazards of mortality were observed after stratifying by device type (DCB vs PTA: adjusted HR 1.00; 95% CI: 0.92-1.08; p=0.96, and DES vs BMS: adjusted HR 1.02, 95% CI: 0.88-1.18; p=0.78) (Figure 3, Figure 4). There was no difference in the safety signal after landmarking at one year (Supplementary Figure 5).
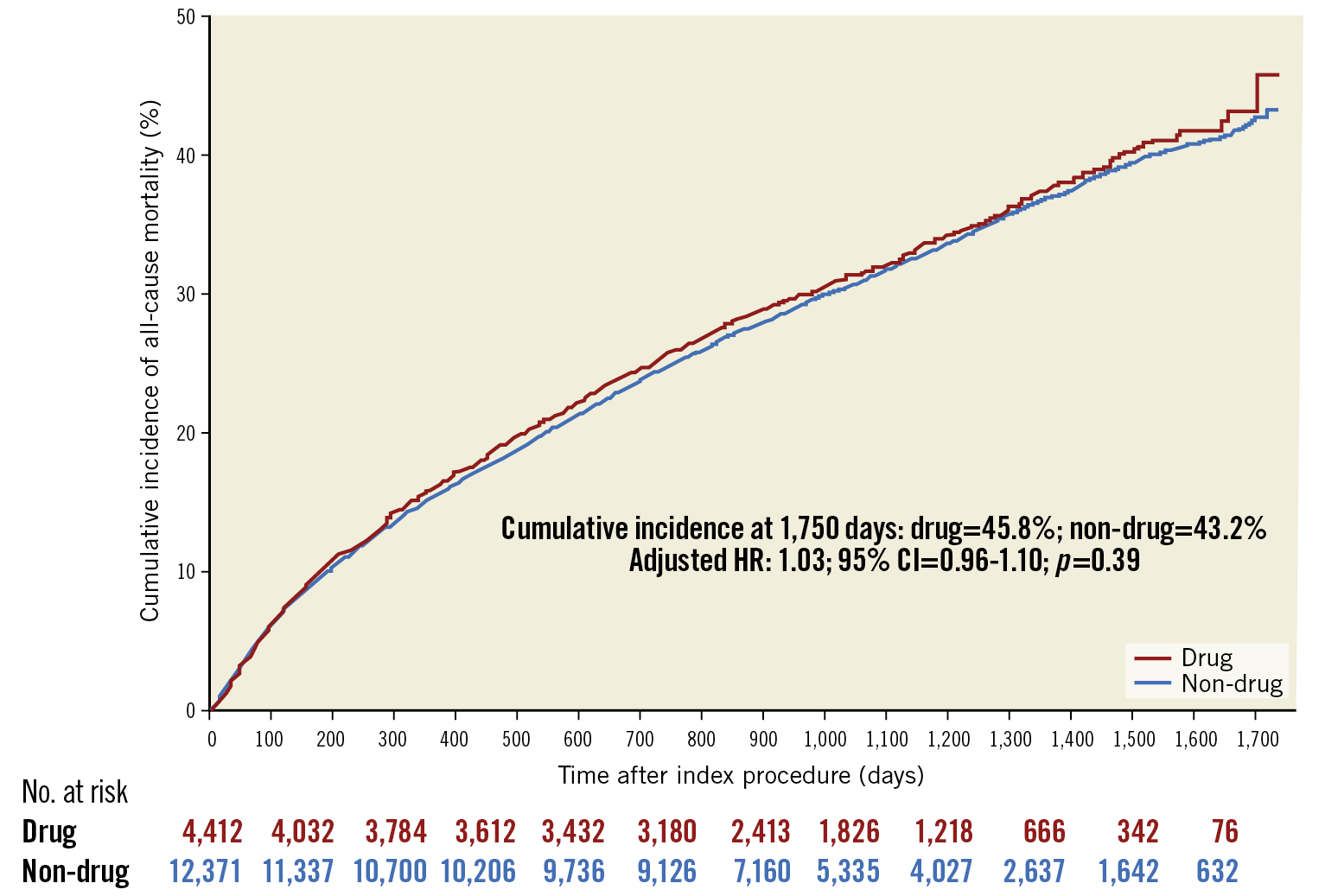
Figure 2. Adjusted cumulative incidence of mortality curves of patients treated with drug-coated and non-drug-coated devices. The number at risk is the inverse probability of treatment weighted population.
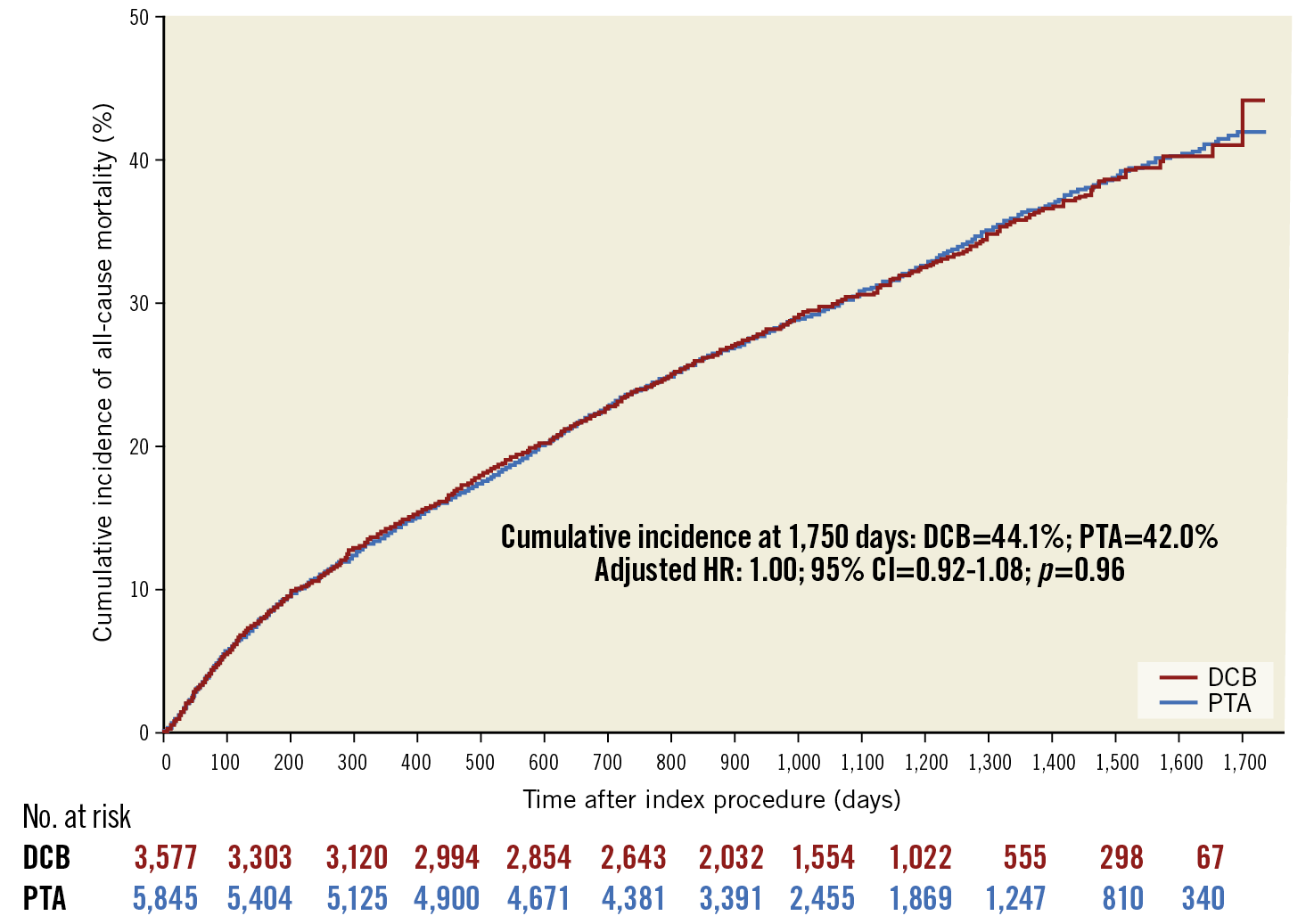
Figure 3. Adjusted cumulative incidence of mortality curves of patients treated with drug-coated and non-drug-coated balloons. The number at risk is the inverse probability of treatment weighted population.
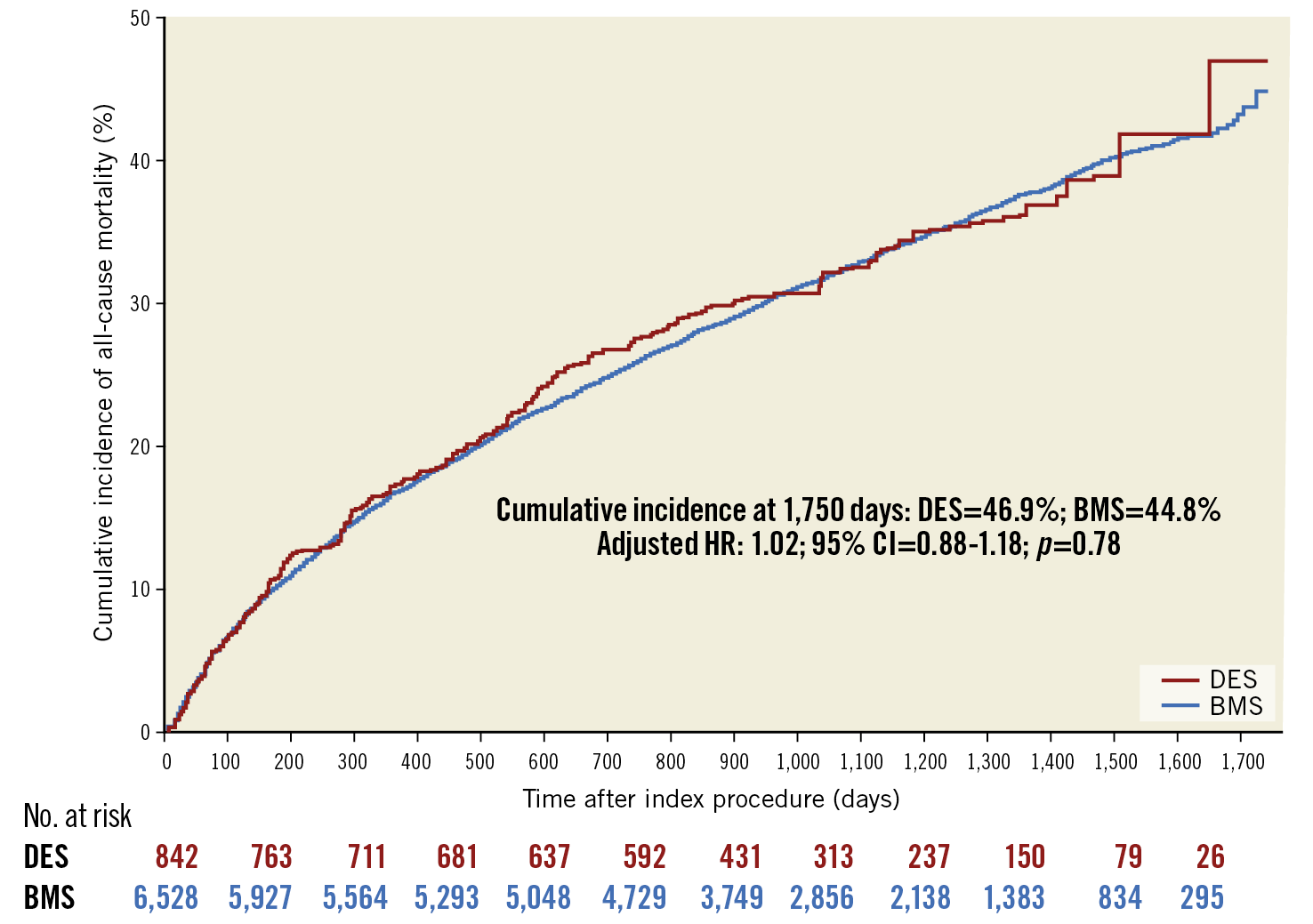
Figure 4. Adjusted cumulative incidence of mortality curves of patients treated with drug-eluting stents and bare metal stents. The number at risk is the inverse probability of treatment weighted population.
Of all patients, 33.2% had a subsequent intervention during follow-up (Supplementary Table 6). Of those who initially did not receive a drug-coated device (N=12,369), 5.8% (N=723) were successively treated with a drug-coated device. Among patients who initially received a drug-coated device (N=4,427), 14.7% (N=649) were successively treated with another drug-coated device.
SUBGROUP ANALYSES
Adjusted hazard ratios of all-cause mortality following treatment by drug-coated versus non-drug-coated device for the pre-specified subgroups are shown in Figure 5. Revascularisation with drug-coated devices was not associated with lower survival relative to those treated with non-drug-coated devices across different age categories, sex, PAD diagnosis (CLI vs other), procedure setting, or burden of comorbidities (interaction p>0.05 for all).
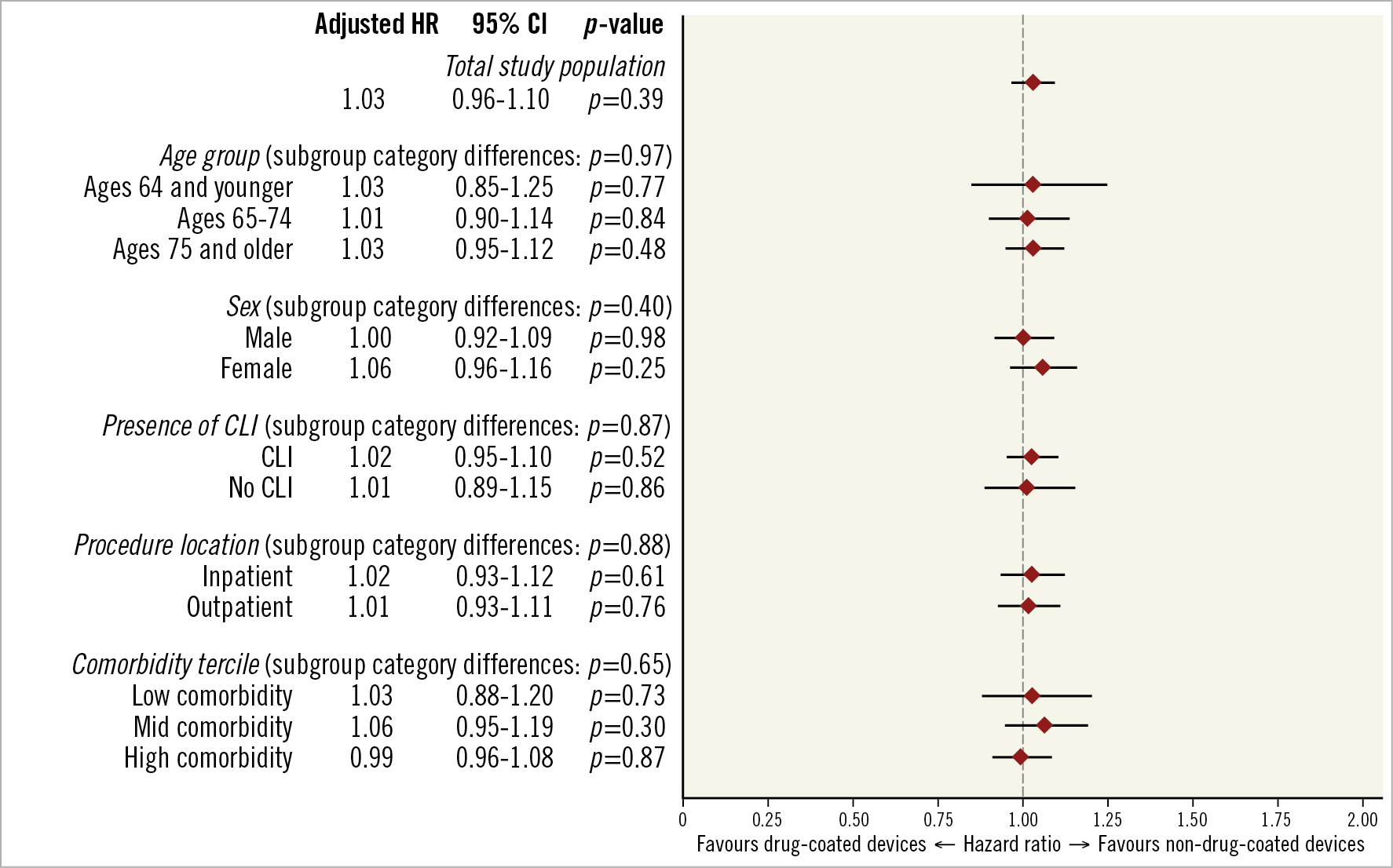
Figure 5. Adjusted subgroup analyses of drug-coated versus non-drug-coated devices and all-cause mortality. Each subgroup underwent separate inverse probability of treatment weighting. Interaction tests for differences between categories within each subgroup were estimated using seemingly unrelated regression.
Discussion
This large, nationwide analysis examined nearly 17,000 MA enrolees who underwent PVI and were followed for up to four years, with more than 25% treated with a drug-coated device. Although we used IPTW to ensure comparable groups for analyses, the majority of sociodemographic characteristics and comorbidities of those treated with and without drug-coated devices were similar (SMD <0.1) prior to adjustment. In both unadjusted and adjusted analyses, we found no evidence of reduced long-term survival among those treated with drug-coated devices compared with non-drug-coated devices. A similar finding was observed among the subgroup of patients treated with DCB compared with PTA. Although there was a difference in mortality for patients treated with DES relative to BMS prior to adjustment, there was not a statistically significant difference in the adjusted hazard ratio. Furthermore, the overall findings persisted irrespective of procedure setting, PAD severity, age, sex, or burden of comorbidities.
The initial meta-analysis that found evidence of long-term harm associated with drug-coated devices had an immediate impact on clinical care, with peripheral paclitaxel-coated device trials being halted and ongoing device use being limited6. However, as these data were further evaluated, multiple limitations have been identified. The included randomised trials were small and underpowered for important subgroup analyses, had a sizeable degree of heterogeneity in patient characteristics and device types, and had a large proportion of missing survival data23. These limitations were further highlighted by an individual patient-level data meta-analysis, which demonstrated substantial attenuation in mortality risk with the addition of more complete follow-up data24.
Nevertheless, as a result of the detection of this mortality signal, there has been a call for more data to provide evidence of long-term safety of drug-coated devices. Although randomised clinical trials are the standard approach to evaluating device safety, the impracticality of performing a de novo trial of sufficient size and duration to exonerate an association with death promptly shifted the focus to RWD as a primary source of continued device evaluation. The strength of RWD in this circumstance includes: the large number of procedures performed prior to the publication of the meta-analysis; the broad population of PAD patients that are captured; availability of baseline characteristics, comorbidities, and prescribed medications; and the ability to evaluate survival on an ongoing basis as mortality records continue to become available. This last feature is particularly salient, as patients treated after the paclitaxel mortality controversy are likely to differ substantially with regard to overall disease severity and adverse event risk compared with patients treated prior to publication.
This analysis builds upon prior studies by using RWD to evaluate the long-term safety of drug-coated devices in the following ways12,13,14,15,16,17,18. Unique to this data set is the inclusion of a large, representative U.S. patient population that has not yet been studied. Prior Medicare analyses have only focused on fee-for-service beneficiaries12,13,18, whereas the MA population typically represents a healthier elderly population for whom there may be less competing risk of death21. Furthermore, the analysis is strengthened by the use of both inpatient and outpatient procedural data, as well as adjustment for differences in medication use at the time of the procedure. This study also fills a necessary gap relative to the randomised trial data, as the ascertainment of mortality in Medicare data is near complete, whereas RCTs were lacking follow-up in up to 40% of patients, and with 10% still missing in the updated patient-level meta-analysis23,24.
With these considerations in mind, our analysis did not demonstrate any evidence of harm associated with drug-coated devices, both among all-comers and among key patient subgroups. The lack of any evidence of harm with these devices has now been replicated in a number of real-world studies and should further the conversation regarding the true safety of paclitaxel-coated devices used in peripheral arterial revascularisation.
Limitations
This analysis must be interpreted within the context of its design. First, this study is a retrospective, observational analysis, and the causal effect of treatment on all-cause mortality may be influenced by unobserved confounders. However, the strong similarities in measured patient characteristics between the two groups prior to IPTW adjustment as well as the similar mortality rates in both unadjusted and adjusted analyses suggest that treatment selection bias may be less influential in this comparison. Second, claims data lack clinical detail that may be relevant to treatment choice and mortality, such as lesion length and number of balloons or stents used. Although these variables may be important for device selection, they have not been found to predict long-term mortality25,26. Insurance claims data also lack the device details needed to calculate cumulative paclitaxel exposure. Third, although the data include a median follow-up time of approximately two years, with some patient follow-up up to four years, there were mortality effects found in the meta-analysis beyond the median follow-up time in our data. Lastly, the study population is not necessarily representative of all MA patients, as these data are only from a single MA vendor.
Conclusions
In this large cohort of MA beneficiaries, there was no evidence of increased long-term mortality following treatment with drug-coated devices. These findings persisted by device type, procedure setting, and patient characteristics. This analysis is the first to involve an MA patient population and contributes to a growing body of RWD finding no association between drug-eluting devices and harm in routine clinical practice.
|
Impact on daily practice In this large cohort, there was no evidence of increased long-term mortality following treatment with drug-coated devices. These findings persisted by device type, procedure setting, and patient characteristics. This analysis is the first to involve a Medicare Advantage patient population, which makes up more than one third of Medicare coverage, and contributes to a growing body of real-world studies finding no association between drug-eluting devices and harm in routine clinical practice. |
Funding
The present work was funded by a research grant from Medtronic to Beth Israel Deaconess Medical Center, and from the Smith Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center. A pre-specified statistical analysis plan was created collaboratively with statisticians from the Center for Devices and Radiologic Health of the U.S. FDA. Medtronic had full access to all the data and conducted the analysis with an outcome-blind approach using two analysts. Dr Secemsky wrote the first draft of the manuscript, and Drs Secemsky and Yeh reviewed all data and output and made the final decision to publish.
Conflict of interest statement
M. Bonaca reports grants/other from Amgen, AstraZeneca, Audentes, Bayer, BIDMC, BMS, Janssen, Lexicon, Medtronic, Merck, NovoNordisk, Pfizer, and Regeneron. C. Hess reports grants from Merck, Janssen, Bayer, and Amgen. E. Secemsky reports grants/personal fees from Medtronic, BD, Cook, CSI, Boston Scientific and Philips, personal fees from Abbott and Janssen, and grants from AstraZeneca. He is funded by NIH/NHLBI K23HL150290 and Harvard Medical School’s Shore Faculty Development Award. R. Yeh reports grants/personal fees from Medtronic, Abbott Vascular, and Boston Scientific, and grants from BD Bard, Cook Medical, and Philips Medical. B. Manda, L. Bockstedt, E. Barrette, J. Monteiro and T. Hanson report employment from Medtronic.
Supplementary data
To read the full content of this article, please download the PDF.

