
Abstract
Aims: It is currently unclear if the location of coronary artery disease affects decision making with regard to dual antiplatelet therapy (DAPT). We investigated if the presence of at least 30% luminal narrowing in the left main (LM) and/or proximal left anterior descending (pLAD) coronary arteries on angiography is an outcome modifier with respect to DAPT duration.
Methods and results: In the Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia (PRODIGY) study, 953 (54.3%) patients with and 801 (45.7%) without LM/pLAD lumen narrowing at the qualifying coronary intervention were randomised to six or 24 months of DAPT. Twenty-four month as compared to six-month DAPT reduced the occurrence of definite, probable or possible stent thrombosis by 50% in patients with (2.8% vs. 5.6%; HR 0.45, 95% CI: 0.23-0.89; p=0.02) but not in those without LM/pLAD lumen narrowing, with a highly significant interaction testing (PINT= 0.002). This result remained consistent irrespective of whether stenting was (PINT: 0.01) or was not (PINT: 0.02) performed in the LM/pLAD.
Conclusions: Left main and/or proximal LAD lumen narrowing may be a treatment modifier with respect to the duration of DAPT. Patients fulfilling these angiographic characteristics seem to benefit from a prolonged dual antiplatelet treatment. Trial registration: ClinicalTrials.gov Identifier: NCT00611286
Abbreviations
ACS: acute coronary syndrome
BARC: Bleeding Academic Research Consortium
CVA: cerebrovascular accident
DES: drug-eluting stents
LM: left main coronary artery
MACE: major adverse cardiovascular events
MI: myocardial infarction
NSTEMI: non-ST-segment elevation myocardial infarction
pLAD: proximal left anterior descending coronary artery
PRODIGY: Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia
QCA: quantitative coronary angiography
ST: stent thrombosis
TCFA: thin-cap fibroatheroma
Introduction
Significant lumen narrowing of the left main coronary artery (LM) and/or of the proximal left anterior descending coronary artery (pLAD) carries worse prognostic implications as compared to coronary stenosis located elsewhere in the coronary tree. Previous studies have shown that successful revascularisation of lesions located in these critical coronary segments improves survival as compared to medical therapy only1.
The duration of dual antiplatelet therapy after coronary stenting, which maximises benefits over risks, remains debated. While clinical trials have shown an ischaemic benefit after a prolonged course of therapy beyond 12 months2, recent meta-analyses have raised concerns about a possible increase in mortality after such treatment3. Consequently, the need for novel data informing clinical practice has been clearly voiced by the community4. Many have advocated the need to modulate DAPT length based on the balance between ischaemic versus bleeding hazard. However, it remains unclear how this balance should be quantified and standardised in clinical practice5.
In particular, whether the presence of lumen narrowing of the LM and/or pLAD in patients undergoing stenting should affect the decision making with respect to the duration of DAPT remains unknown, as no previous studies investigating DAPT duration have specifically focused on this patient population or reported the outcomes for this high-risk patient subgroup.
We hypothesised that the presence of lumen narrowing in the LM and/or pLAD may help in identifying patients at higher ischaemic risk who may particularly benefit from a prolonged DAPT course. Thus, we retrospectively explored the differential outcomes of 24 or six-month DAPT in this high-risk population in the Prolonging Dual Antiplatelet Treatment After Grading Stent- Induced Intimal Hyperplasia (PRODIGY) trial.
Methods
The design and main findings for the PRODIGY trial were previously reported6,7. All-comer patients received a balanced mixture of first and second-generation drug-eluting stents (DES) and bare metal stents during index intervention at three Italian sites. Thirty days later, they were randomly allocated to receive either six or 24 months of dual antiplatelet treatment. The study was conducted in accordance with the principles of the Declaration of Helsinki. The ethics committees of the three participating centres independently approved the protocol, and all participants gave written informed consent.
TREATMENT PROTOCOL
All patients received aspirin (80 to 160 mg orally indefinitely) and clopidogrel (75 mg/d) according to the randomisation scheme as follows: for either six months in the short DAPT group or 24 months in the prolonged DAPT arm irrespective of the previously implanted stent type or indication for the coronary procedure. All randomised patients returned for study visits at 30 days, and then every six months up to two years.
DEFINITION OF THE STUDY GROUPS
A lumen stenosis of at least 30% on angiography, appraised by visual estimation, was used to define the LM/pLAD lumen-narrowing group as this information was prospectively collected in the case report form for each coronary segment, irrespective of the final revascularisation strategy. The decision to treat or not to treat these and other coronary segments was left to the operators’ discretion.
STUDY ENDPOINTS
The primary efficacy endpoint of the PRODIGY trial was the incidence of major adverse cardiovascular events (MACE), a composite of death, myocardial infarction (MI), or cerebrovascular accident (CVA), whereas the key safety endpoint was Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding. Other endpoints included each component of the primary efficacy endpoint, cardiovascular death and stent thrombosis (ST), defined on the basis of the Academic Research Consortium criteria8. All study endpoint definitions were previously reported6.
Additionally, in order to increase the specificity of the possible stent thrombosis definition (i.e., any cardiovascular death occurring at least 30 days after stent implantation), we also applied a “modified” possible stent thrombosis definition9, restricting the adjudication of these events only to those who died suddenly or who experienced symptoms of myocardial ischaemia before death, occurring at least 30 days after stent implantation.
A blinded clinical events committee adjudicated all endpoints centrally. The time frame of interest was from 30 days (i.e., after pharmacological randomisation) to 24 months.
STATISTICAL ANALYSIS
In this retrospective analysis of the PRODIGY trial7, categorical variables were expressed as frequency (percentage), whereas continuous variables were expressed as median (interquartile range). Continuous variables were compared between randomised groups using the Wilcoxon rank-sum test, whereas for binary variables the chi-square test was used.
To test the cumulative incidence of events in the two subgroups presenting with or without LM/pLAD lumen narrowing, we performed a Cox regression analysis with interaction testing. Hazard ratios with 95% confidence intervals (CIs) were calculated for short-term clopidogrel versus long-term clopidogrel (i.e., values >1 indicated increased hazard in the long-term group).
Adjustment for NSTEMI presentation was performed due to the imbalance of this characteristic between the two treatment arms. Interaction tests were performed with likelihood ratio tests of the null hypothesis that the interaction coefficient was zero. A two-sided probability value <0.05 was considered significant. All analyses, performed on the basis of the intention-to-treat principle, were performed with SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
In total, the PRODIGY trial recruited 2,013 all-comer patients, 1,970 of whom were randomly allocated to receive DAPT for a duration of six or 24 months. For the current analysis, patients previously treated were eventually considered (Figure 1).
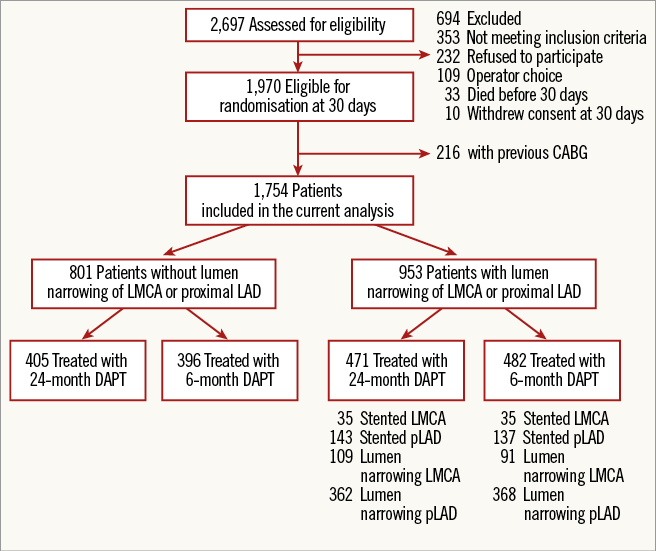
Figure 1. Study profile. Description of the study population.
The total number of patients with LM/pLAD lumen narrowing was 953 (54.3%), of whom 471 were randomised to 24-month DAPT and 482 to six-month DAPT, including 336 (35.2%) patients who received coronary stenting in the LM (7.3%) and/or in the pLAD (29.4%).
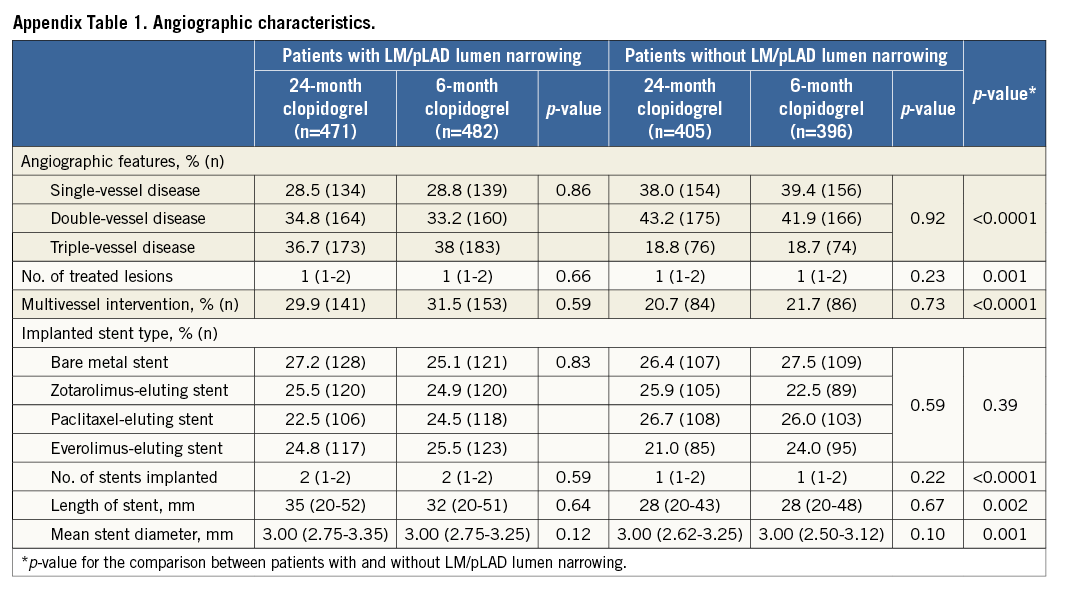
Patients with angiographic evidence of LM/pLAD lumen narrowing were older, more frequently had kidney function impairment, were less frequently smokers but more frequently had a history of PCI (Table 1). Their angiographic features were consistent with a more extensive CAD, requiring a more complex interventional procedure as compared to patients without LM/pLAD lumen narrowing (Appendix Table 1).
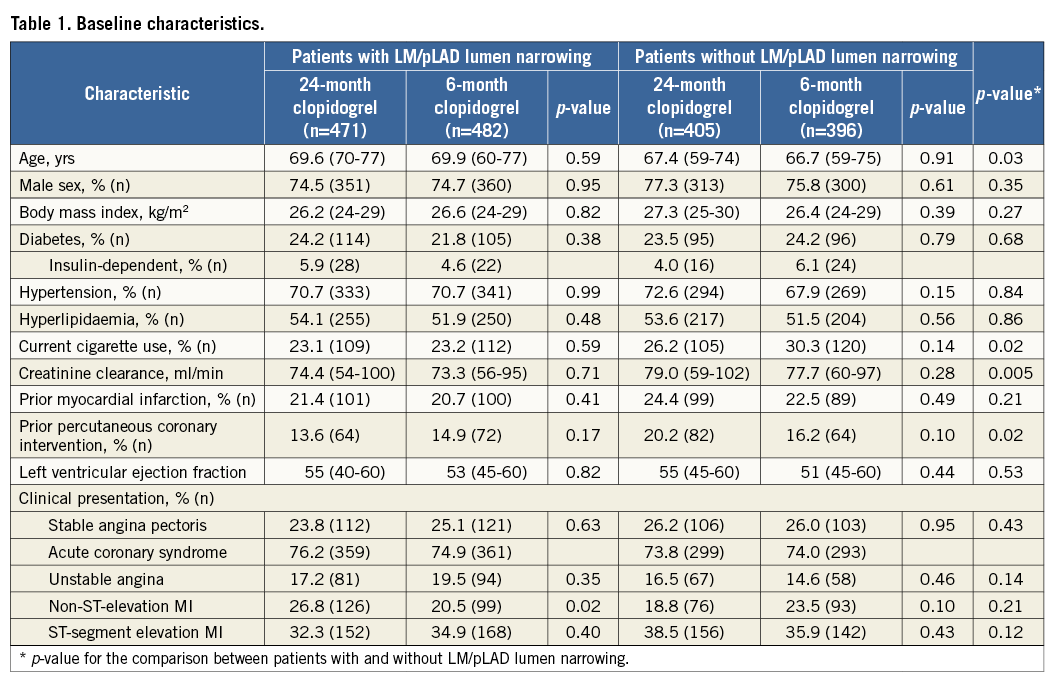
Amongst patients with or without LM/pLAD lumen narrowing, clinical and angiographic characteristics were well matched between the two DAPT duration groups, with the sole exception of a higher prevalence of non-ST-segment elevation myocardial infarction (NSTEMI) in patients with LM/pLAD lumen narrowing allocated to the 24 as compared to the six-month DAPT group (Table 1). Clinical follow-up at two years was complete for 99.1% of patients.
PATIENT-ORIENTED OUTCOMES
The composite of death, myocardial infarction or CVA at 24 months occurred in 105 (11%) patients with, as compared to 65 (8.1%) without LM/pLAD lumen narrowing (HR 1.37, 95% CI: 1.00-1.87; p=0.04). This difference was driven by a trend towards an increase in myocardial infarction (4.5% vs. 2.7%; p=0.053). When patients who received a coronary stent in the LM and/or pLAD were excluded from the analysis, the composite of death, myocardial infarction or CVA at 24 months remained consistently higher in individuals with (11.3%) as compared to those without (8.1%) LM/pLAD lumen narrowing (HR 1.42, 95% CI: 1.01-1.98; p=0.04), due to a significant increase in myocardial infarction (4.9% vs. 2.7%; p=0.036). Conversely, patients stented in the LM/pLAD did not suffer a significant increase of the primary endpoint rate. After adjustment for clinical and angiographic imbalances between the two groups, the composite endpoint of death, MI or CVA was not increased in patients with as compared to those without LM/pLAD lumen narrowing (adjusted HR 1.22, 95% CI: 0.89-1.68; p=0.21) or in patients who underwent stenting in these coronary segments (adjusted HR 1.37, 95% CI: 0.97-1.94; p=0.07).
The composite of death, MI or CVA did not differ with respect to DAPT duration in patients with (11.0% in the 24-month vs. 11.0% in the six-month DAPT arm; HR 0.96, 95% CI: 0.65-1.41; p=0.84) or those without LM/pLAD lumen narrowing (9.4% in the 24-month vs. 6.8% in the six-month DAPT arm; HR 1.45, 95% CI: 0.88-2.38; p=0.14) (Table 2, Figure 2). Similar findings were observed when each component of the primary endpoint was appraised separately. There was, however, a trend towards interaction (PINT=0.056) with respect to DAPT duration and the presence of LM/pLAD lumen narrowing, suggesting greater ischaemic protection towards the composite endpoint of cardiovascular death or myocardial infarction (MI) in patients treated with 24 as compared to six-month DAPT (Table 2, Figure 2).
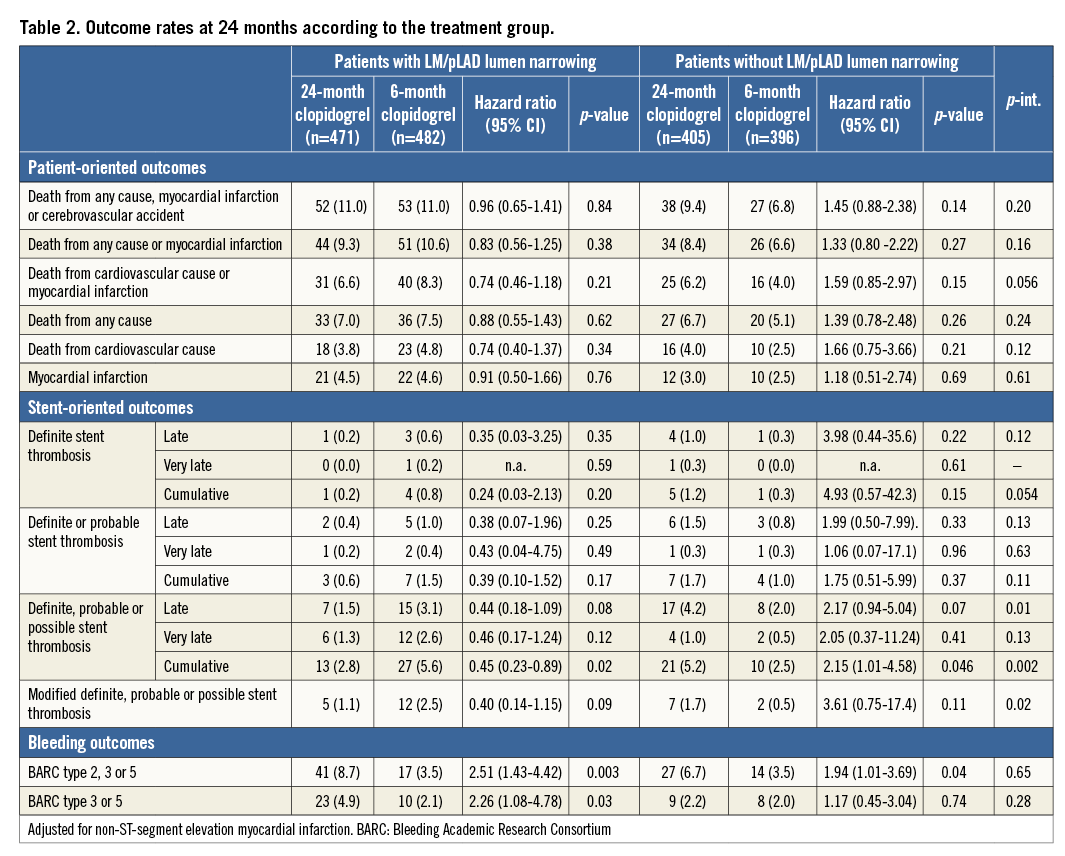

Figure 2. Patient-oriented outcomes. The subgroups with and without LM/pLAD lumen narrowing are shown for the patient-oriented ischaemic endpoints among patients randomised to six-month or 24-month DAPT.
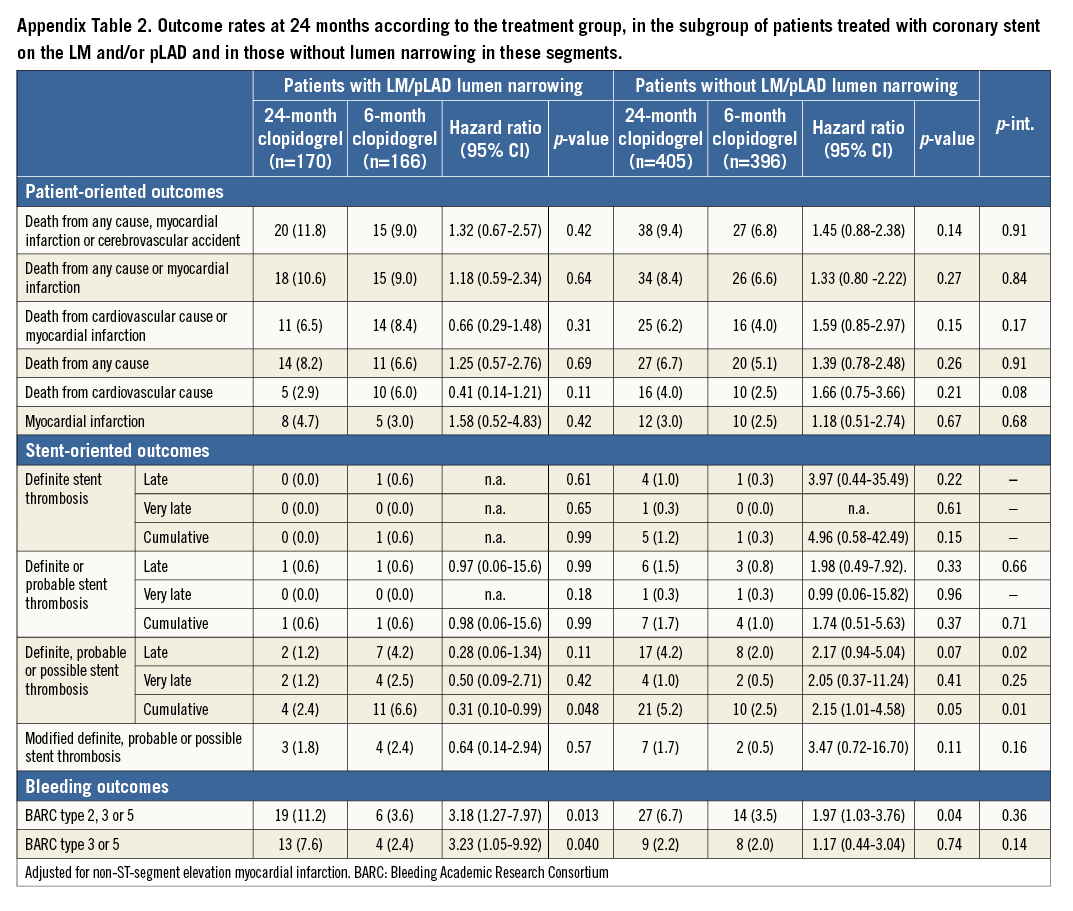
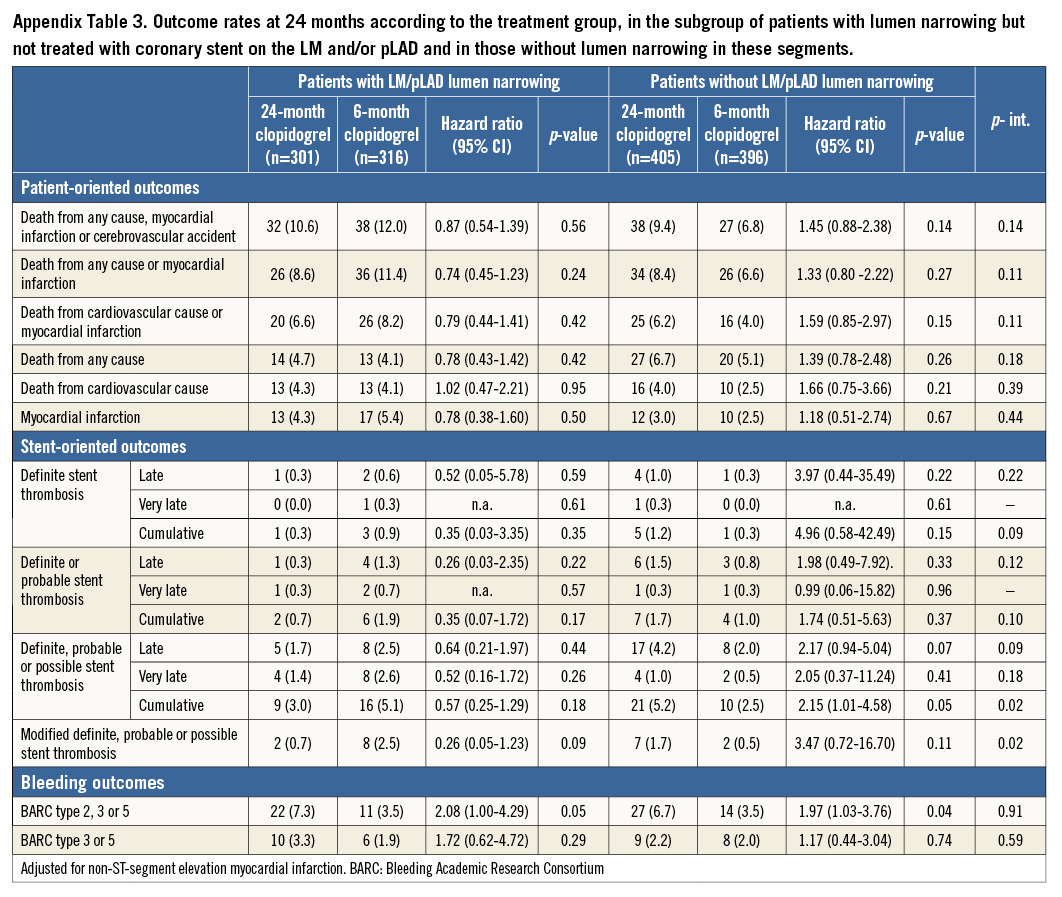
Results remained consistent regardless of whether stenting was (Appendix Table 2) or was not (Appendix Table 3) accomplished in the LM and/or pLAD. No signal of heterogeneity in the subgroups of patients undergoing stenting versus those who did not receive stent implantation in the LM/pLAD was observed for patient-oriented outcomes with respect to DAPT duration (Appendix Figure 1).
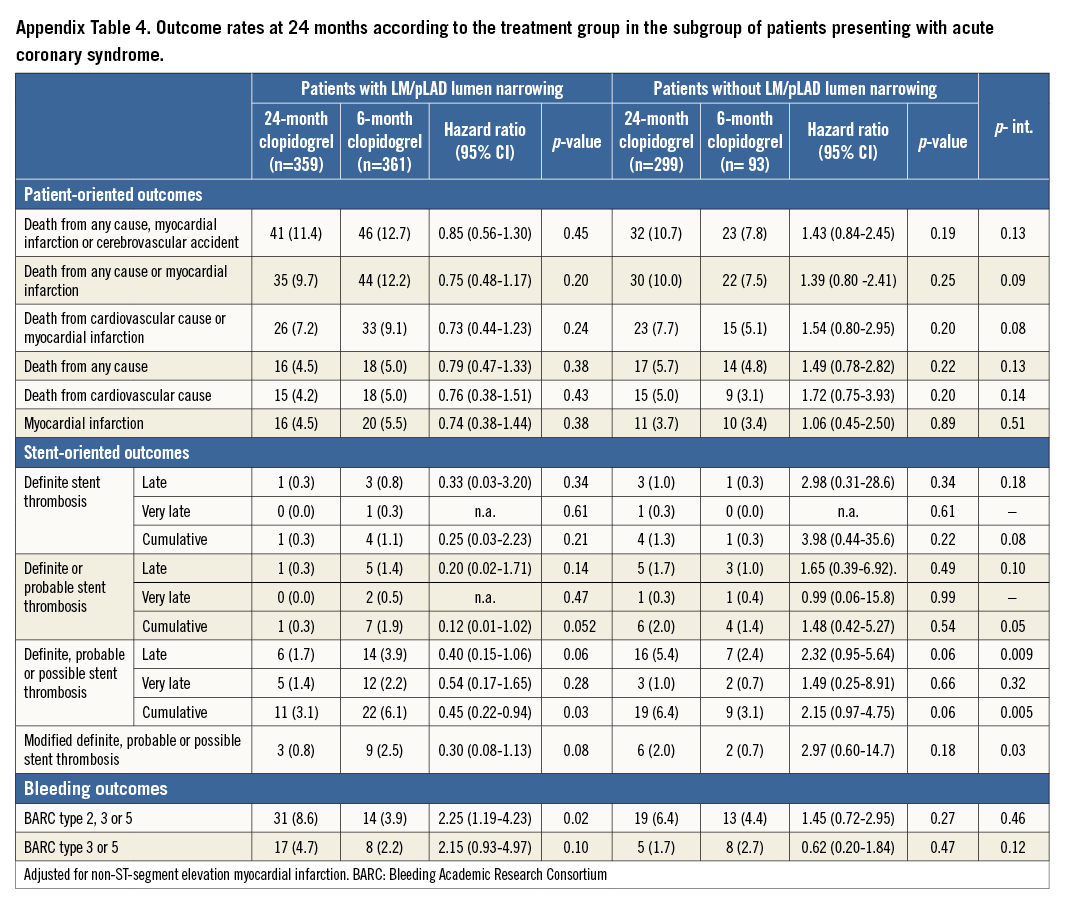
When the group of patients presenting with acute coronary syndrome (ACS) was appraised separately, the results were in line with those observed in the overall patient population (Appendix Table 4).
STENT-ORIENTED OUTCOMES
The risk of definite, definite or probable and definite, probable or possible ST was not increased in patients with as compared to those without LM/pLAD lumen narrowing. Similarly, the cumulative risk of definite stent thrombosis did not differ with respect to DAPT duration in patients with (0.2% in the 24-month vs. 0.8% in the six-month DAPT arm; HR 0.24, 95% CI: 0.03-2.13; p=0.20) or those without LM/pLAD lumen narrowing (1.2% in the 24-month vs. 0.3% in the six-month DAPT arm; HR 4.93, 95% CI: 0.57-42.3; p=0.15), with a trend towards interaction between the presence of LM/pLAD lumen narrowing and the duration of treatment (PINT=0.054) (Figure 3). Consistent findings were noted for the composite of definite or probable ST (Figure 3, Figure 4).
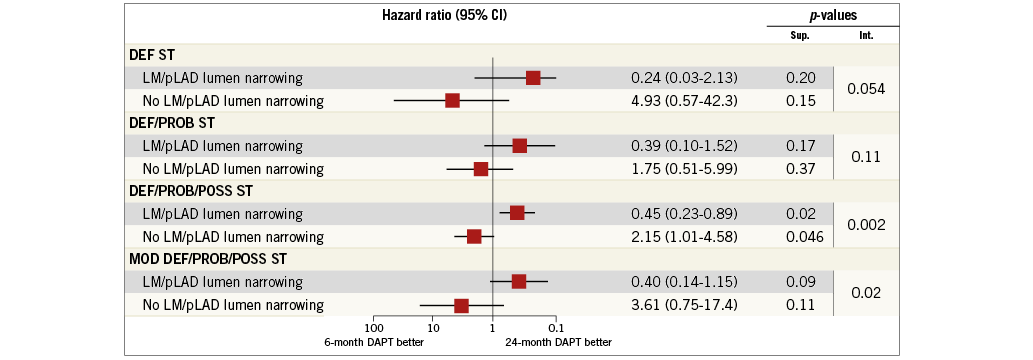
Figure 3. Stent-oriented outcomes. The subgroups with and without LM/pLAD lumen narrowing are shown for the stent-oriented endpoints of definite (DEF ST), definite or probable (DEF/PROB ST) and definite, probable or possible stent thrombosis (DEF/PROB/POSS ST) as well as the “modified” definite, probable or possible stent thrombosis (MOD DEF/PROB/POSS ST) definition among patients randomised to six-month or 24-month DAPT.
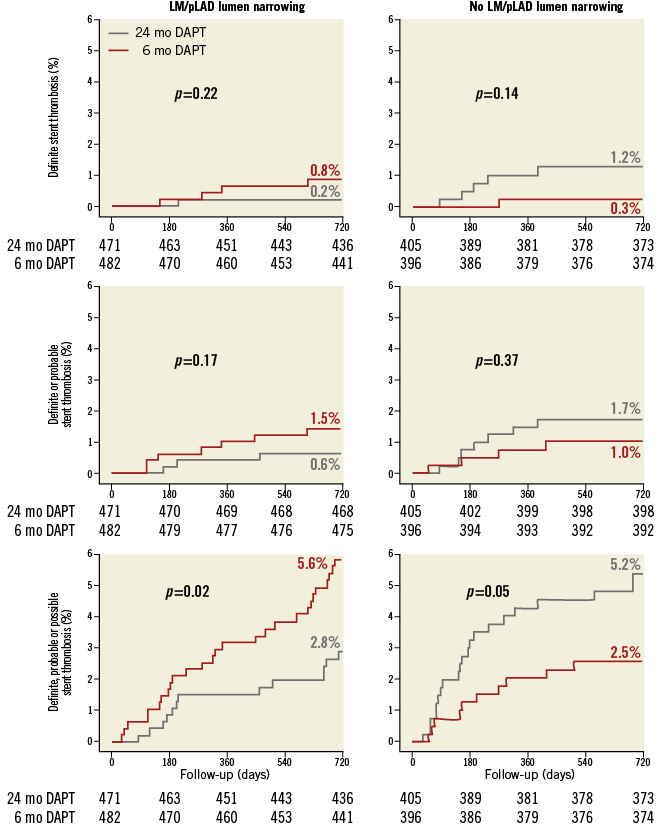
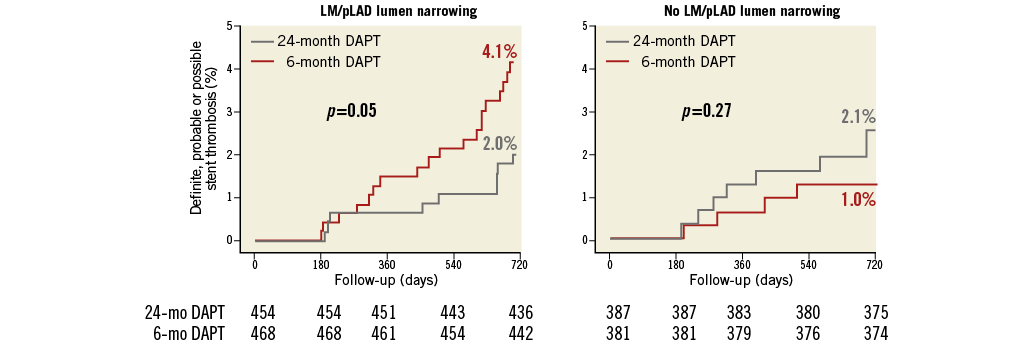
Figure 4. Cumulative incidence of stent-oriented outcomes. Cumulative incidence curves for the subgroups with (left) or without (right) LM/pLAD lumen narrowing and for the endpoints of definite (upper panel), definite or probable (middle panel) or definite, probable or possible stent thrombosis (lower panel).
Definite, probable or possible ST was significantly reduced by 24-month, as compared to six-month treatment among patients with LM/pLAD lumen narrowing (2.8% in the 24-month vs. 5.6% in the six-month DAPT arm; HR 0.45, 95% CI: 0.23-0.89; p=0.02) whereas it was increased in patients without LM/pLAD lumen narrowing (5.2% in the 24-month vs. 2.5% in the six-month DAPT arm; HR 2.15, 95% CI: 1.01-4.58; p=0.046), with highly significant interaction testing (PINT=0.002) (Figure 3, Figure 4). Results remained consistent when the “modified” stent thrombosis endpoint was appraised in conjunction with definite or probable events (Figure 3, Figure 5). At landmark analysis, after censoring all events occurring before six months, the time at which the two pharmacological arms started diverging, we still observed a reduction of definite, probable or possible stent thrombosis with 24 vs. six-month DAPT in patients with LM/pLAD lumen narrowing (HR 0.45, 95% CI: 0.20-0.99; p=0.05), whereas its increase in patients without LM/pLAD lumen narrowing was no longer present (HR 1.96, 95% CI: 0.59-6.53; p=0.27). A consistent, significant interaction effect between CAD location and duration of dual antiplatelet therapy was also observed at landmark analysis (PINT=0.044) (Appendix Figure 2). Consistently, when patients treated with first-generation DES were excluded, again similar findings were observed.
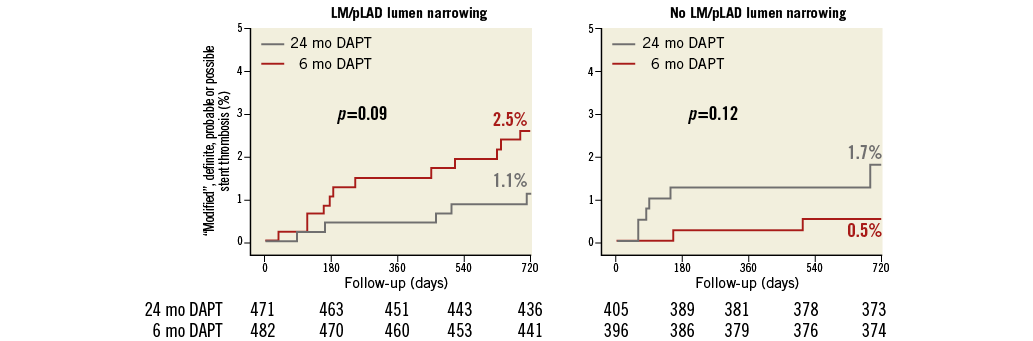
Figure 5. Cumulative incidence of “modified” definite, probable or possible stent thrombosis. Cumulative incidence curves for the subgroups with (left) and without (right) LM/pLAD lumen narrowing with respect to “modified” definite, probable or possible stent thrombosis.
Results were also consistent regardless of whether stenting was performed or not performed in the LM and/or pLAD (Appendix Table 2, Appendix Table 3). Again, no signal of heterogeneity was observed for stent-oriented endpoints between the subgroups treated or not treated with a coronary stent in the LM and/or pLAD with respect to DAPT duration (Appendix Figure 3).
In the subgroup of patients presenting with ACS, stent-oriented outcomes remained consistent with the results of the overall patient population (Appendix Table 4).
BLEEDING OUTCOMES
BARC 3 or 5 and 2, 3 or 5 bleeding occurred more frequently in the 24-month DAPT arm irrespective of CAD location within the coronary tree (Table 2). No statistical interaction was noted between the presence of LM/pLAD lumen narrowing and the duration of antiplatelet treatment.
Discussion
Our findings could be summarised as follows:
1) In an all-comer population undergoing coronary stenting, patients presenting with angiographic evidence of lumen narrowing on the LM and/or pLAD had higher clinical and angiographic risk characteristics at baseline, and underwent a more complex percutaneous treatment.
2) After 24 months, the rate of death for all causes, myocardial infarction or CVA was significantly higher in patients with LM/pLAD lumen narrowing, regardless of whether stenting was finally accomplished in these coronary segments. When adjusted for baseline imbalances, MACE rates did not differ between patients with or without LM/pLAD lumen narrowing, suggesting that, at least in our data set, luminal CAD in these segments may qualify more as a marker than a driver of adverse outcomes.
3) Twenty-four as compared to six-month DAPT significantly reduced the rate of definite, probable or possible stent thrombosis in patients with, but not in those without, LM/pLAD lumen narrowing, suggesting that this angiographic finding could be a treatment modifier with respect to DAPT duration. A consistent trend towards interaction between CAD location and DAPT duration was also noted for the composite of cardiovascular death and MI.
4) The 24-month DAPT regimen remained associated with possible benefits in patients with LM/pLAD lumen narrowing irrespective of whether a stent was or was not implanted in these segments or whether patients presented with acute coronary syndrome.
5) Minor and major bleeding occurred more often in patients receiving prolonged dual antiplatelet therapy, independently from the presence or absence of LM/pLAD lumen narrowing.
The proximal LAD and left main coronary artery supply 45 to 55% and 84 to 100% of blood flow to the left ventricle, respectively10. Accordingly, ischaemic events occurring in these segments are more often fatal. Coronary revascularisation of significant LM/pLAD stenosis demonstrated a long-term survival benefit compared with medical therapy alone, whereas the clinical implication of non-significant lesions in these segments, especially in stable coronary artery disease (SCAD) patients, is unclear1.
In the current analysis, we noted a higher incidence of ischaemic recurrences as well as a greater benefit from prolonged DAPT duration in patients with lumen narrowing of the LM/pLAD. A putative explanation for this finding could be the higher clinical and angiographic risk characteristics noted in these patients at baseline. This is consistent with previous studies which observed an additional benefit of a more potent antiplatelet therapy in patients at higher ischaemic risk11.
As an alternative, not mutually exclusive, hypothesis, LM/pLAD location of the atherosclerotic plaques per se may be a driver for the greater ischaemic risk. In support of this possibility, invasive imaging studies showed that atherosclerotic plaques located on the LM or the pLAD present a significantly higher percentage of lipidic and necrotic cores, entailing a higher vulnerability and increased risk of plaque rupture in this location12,13. In addition, thin-cap fibroatheroma (TCFA), a specific predictor of plaque vulnerability, was observed more frequently in the proximal segments of the coronary tree and in mild or moderate stenosis (i.e., stenosis <70% estimated visually during angiography)14-16.
It is tempting to speculate that the excess of ischaemic events, as well as the increased protection by a prolonged antiplatelet treatment, observed in patients with LM/pLAD lumen narrowing could be partly related to the progression and destabilisation of atherosclerotic plaques deemed not significant at baseline angiography and left untreated. Lending support to this suggestion, the PROSPECT trial17, which prospectively followed patients with acute coronary syndrome at presentation, showed that almost 50% of the recurrent ischaemic events observed during the three-year follow-up were not related to the coronary stent implanted, but rather to atherosclerotic lesions non-significant at baseline. In this context, an extended duration of DAPT could be beneficial to prevent, or at least mitigate, the effects of plaque rupture, as demonstrated by the recent DAPT trial2, showing a reduction of MACE and stent thrombosis after a 30-month rather than 12-month dual antiplatelet therapy with thienopyridines. Interestingly, in this trial 55% of the benefit observed in the prolonged therapy group was due to events not related to the stent implanted at baseline.
Our analysis included 336 patients who underwent coronary stenting on LM and/or pLAD, in whom prolonged DAPT provided a consistent benefit as compared to six-month therapy. Stent thrombosis occurring in these segments is often lethal, though the reduction of stent-related events with a prolonged or more potent antiplatelet therapy is particularly appealing2. However, this population has frequently been excluded from clinical trials evaluating the optimal DAPT duration, and there is currently poor and inconsistent evidence guiding this decision2.
To corroborate further the robustness of our findings, we evaluated the subgroup of patients with and without LM/pLAD lumen narrowing presenting with acute coronary syndrome. The current analysis showed a consistent benefit of a prolonged DAPT in patients with LM/pLAD lumen narrowing presenting with ACS, which is in keeping with previous findings from our study18.
An increased bleeding risk was observed in patients undergoing a prolonged DAPT regimen irrespective of the presence of LM/pLAD lumen narrowing, which may be perceived as clinically acceptable in the light of the clear signals of benefit observed in this high-risk population.
Limitations
Our study suffers from the following limitations:
1) The main finding of our analysis refers to stent-related events that did not constitute the primary endpoint of the PRODIGY trial. As such, the findings reported in the current retrospective analysis should not be interpreted as conclusive but as hypothesis-generating. Adequately powered, prospective studies are needed to confirm or refute our findings.
2) The definition of the lumen narrowing status was based on the visual estimation by the interventional cardiologist. This method suffers from inter- and intra-individual variability that could have been prevented by implementing quantitative coronary angiography (QCA) analysis. However, this would have limited the applicability of our results in practice, where routine QCA analysis is rarely performed.
3) The effect of DAPT on ischaemic events is related to the characteristics of the implanted stent. Evaluating the role of CAD location and duration of DAPT for each stent used could have given further insights, but was not performed given the high dispersion of events into subgroups. However, after excluding first-generation DES, the results were consistent with those observed in the overall patient population.
4) Stratification of the lumen-narrowing group according to different stenosis severity categories could have provided further insights into the link between lumen narrowing and ischaemic risk. Unfortunately, this information was not prospectively collected during the study.
Conclusions
A prolonged treatment with dual antiplatelet therapy reduced stent thrombosis in patients with lumen narrowing of the left main and/or proximal left anterior descending coronary artery, but not in those without these angiographic characteristics. The presence of coronary artery disease in these segments may be a treatment modifier impacting on the duration of dual antiplatelet therapy.
| Impact on daily practice Left main and/or proximal LAD lumen narrowing may be a treatment modifier with respect to the duration of DAPT. The presence of coronary artery disease in these segments carries a higher risk of ischaemic events, and patients fulfilling these angiographic characteristics seem to benefit from a prolonged duration of dual antiplatelet treatment. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
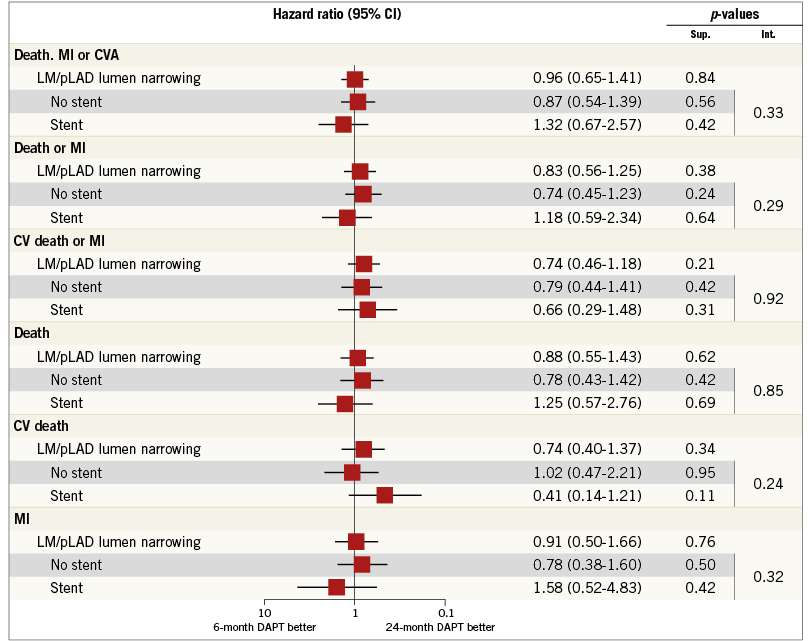
Appendix Figure 1. Patient-oriented outcomes. Subgroup analysis among patients with lumen narrowing of the LM and/or pLAD. The subgroups of patients treated or not treated with coronary stenting on LM and/or pLAD are shown with hazard ratios and 95% confidence interval among patients randomly assigned to either the 6-month or the 24-month dual antiplatelet therapy.
Appendix Figure 2. Landmark analysis at six months for the cumulative incidence of definite, probable or possible stent thrombosis. The landmark analysis censoring events occurring before six months is presented with cumulative incidence curves for the subgroups with (left) and without (right) LM/pLAD lumen narrowing with respect to definite, probable or possible stent thrombosis.
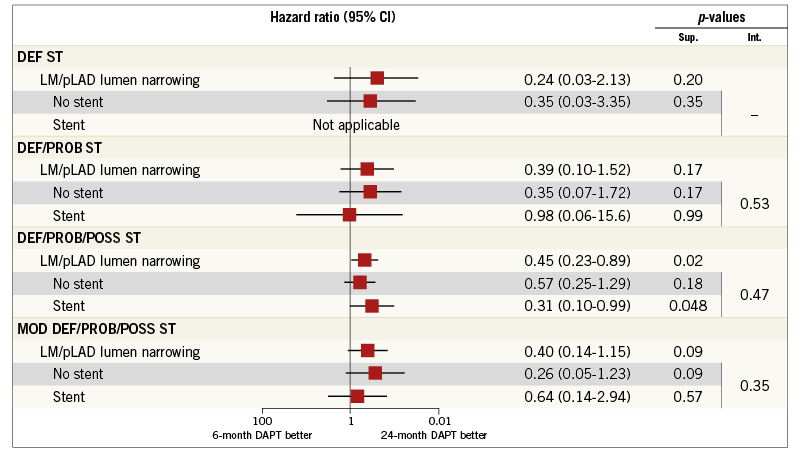
Appendix Figure 3. Subgroup analysis among patients with lumen narrowing of the LM and/or pLAD. The subgroups of patients treated or not treated with coronary stenting on LM and/or pLAD are shown with hazard ratios and 95% confidence interval for the stent-oriented endpoints, defined according to the Academic Research Consortium definition into definite (DEF ST), definite or probable (DEF/PROB ST) or definite, probable or possible stent thrombosis (DEF/PROB/POSS ST) as well as the “modified” definite, probable or possible stent thrombosis definition (MOD DEF/PROB/POSS ST) among patients randomly assigned to either the six-month or the 24-month dual antiplatelet therapy.

