
Abstract
Aims: The purpose of our study was to report our experience with minimally invasive segmental artery coil embolisation (MISACE) to prevent spinal cord ischaemia (SCI) after endovascular repair (ER) of thoracoabdominal aortic aneurysm (TAAA).
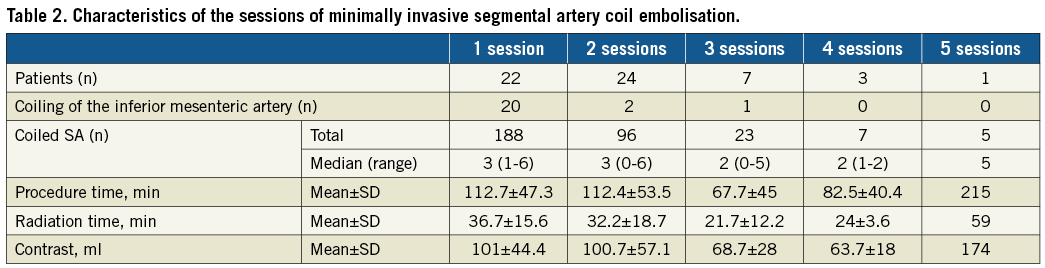
Methods and results: A cohort of 57 patients with TAAAs was treated by MISACE followed by ER between October 2014 and December 2017. The TAAA Crawford classification was: type I, n=5; type II, n=12; type III, n=27; type IV, n=13. The average maximum aortic diameter was 62.7±8.8 mm. Patients had a median of 5 coiled SAs (range: 1-19). MISACE was completed in one (n=22), two (n=24), three (n=7), four (n=3) or five (n=1) sessions. The maximum number of coiled SAs per session was six. After completion of MISACE, 77.7% of direct segmental arterial flow was occluded. After a mean of 83±62 days, 55 of the patients received total ER of their TAAA. At 30 days after ER, no patient developed SCI and three patients had died.
Conclusions: MISACE to precondition the paraspinous collateral network prior to endovascular repair of thoracoabdominal aortic aneurysm is clinically feasible. The safety profile is promising and there is good reason to explore this new staging strategy further.
Abbreviations
CTA: computed tomography angiography
ER: endovascular repair
L: lumbar
MISACE: minimally invasive segmental artery coil embolisation
SA: segmental artery
SCI: spinal cord ischaemia
TAAA: thoracoabdominal aortic aneurysm
Th: thoracic
Introduction
Total endovascular repair of thoracoabdominal aortic aneurysm (TAAA) has achieved remarkable success over the past decade, offering low perioperative morbidity and mortality1. However, ischaemic spinal cord injury (SCI) continues to be the most devastating complication, reducing postoperative survival2,3. Its frequency varies greatly in the literature, exceeding 20%, despite various intraoperative and postoperative neuroprotective strategies4-6. During the past decade, the collateral network concept of spinal cord perfusion has been developed. The network is capable of maintaining perfusion pressure when direct inflow is sacrificed, most likely by triggering arteriogenesis within the paraspinous arterial network7,8. Its most important implication is staging segmental artery (SA) sacrifice which can potentially eliminate permanent SCI. Selective, transfemoral minimally invasive SA coil embolisation (MISACE) provides the endovascular solution for therapeutic staged repair of extensive aortic pathologies, aiming for ischaemic arteriogenic preconditioning of the collateral network. It has the advantage that it is followed by only one definitive single procedure of aneurysm exclusion, and that it is applicable prior to open and endovascular repair. The goal of our study was to report on our initial clinical experience with MISACE in preventing ischaemic SCI after endovascular exclusion of TAAA.
Methods
STUDY DESIGN
In this single-centre study we evaluated the technical feasibility and early perioperative outcome using the concept of MISACE in patients with TAAA receiving endovascular repair between October 2014 and December 2017. This was a retrospective analysis of 83 patients who underwent endovascular repair of their thoracoabdominal aortic aneurysms. In this group, the incidence of SCI was 6% and the 30-day mortality rate was 8.4%. Thirteen patients were treated on an emergency basis and seventy patients underwent a planned procedure of endovascular repair. This analysis identified 57 patients in whom MISACE was utilised as the first step of a staged approach prior to endovascular exclusion of the TAAA. Thirteen patients were excluded from MISACE for various reasons (Figure 1). The Institutional Review Board approved the analysis of the retrospective data set.
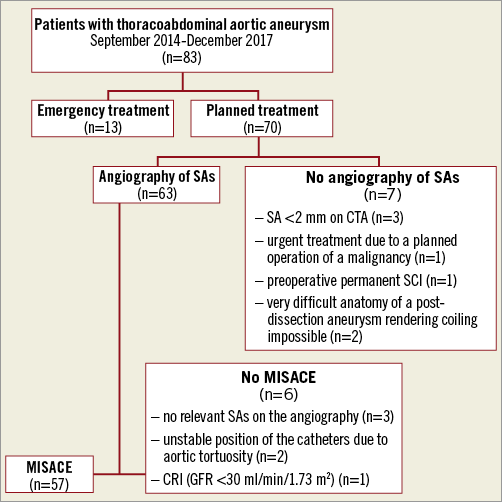
Figure 1. Flow chart showing the selection of patients for minimally invasive segmental artery coil embolisation. CRI: chronic renal insufficiency; CTA: computed tomography angiography; GFR: glomerular filtration rate; SA: segmental artery; SCI: spinal cord ischaemia
PATIENTS
Patients considered too frail for open surgery with a TAAA larger than 5.5 cm or rapid aneurysm progression of more than 5 mm within six months were included. Patients with symptomatic and ruptured aneurysms were excluded as well as those having contraindications for segmental artery coiling, as described later in this section. Every patient was discussed by the institutional vascular review board. Aneurysms were classified according to the Crawford classification (I-IV)3.
ANALYSIS OF PATENT SEGMENTAL ARTERIES
Based on a computed tomography angiography (CTA), patent segmental arteries were identified and counted at the point of the planned aortic repair. The assessment was performed twice in every patient by two different investigators.
MINIMALLY INVASIVE SEGMENTAL ARTERY COIL EMBOLISATION
The sessions of MISACE were scheduled during the manufacturing time of the custom-made stent graft. The procedure was performed under local anaesthesia by embolisation of the ostial segment of the SA at the thoracoabdominal level, without spinal fluid drainage and under continuous monitoring of the neurologic function 48-72 hours after the procedure. The administration of antihypertensive drugs was temporarily paused prior to the procedure to allow permissive hypertension. Through a common femoral artery access using a 5 Fr or a 6 Fr sheath, the SAs were embolised with stainless steel or platinum coils over a microcatheter using the coaxial technique, as previously described9 (Figure 2). Vascular plugs were used directly via the diagnostic catheters in case of larger SAs10. The use of chemo-embolising agents or embolisation particles was strictly prohibited as they could cause non-target ischaemic damage, as demonstrated in several case reports11. Post-embolisation angiography was performed to confirm coil/plug position and arterial occlusion or arterial slow-flow anticipating impending occlusion. The goal was to occlude all open SAs at the aortic level to be covered by the stent graft. A maximum of six SAs were planned to be occluded in one session, in order to avoid iatrogenic SCI. Planned SA embolisation could not be achieved in all attempts for technical reasons, such as the inability to catheterise specific segmental arteries originating from the sac due to vessel tortuosity or because of the dimension of the aneurysm. Another limitation was the contrast agent load as well as the time needed for the complete embolisation procedure. In this scenario, multiple sessions of coiling of SAs were performed to achieve the occlusion of all the planned SAs. Patients with a glomerular filtration rate <30 mL/min/1.73 m2, patients with pre-existing neurological deficit as well as those with no relevant or small (<2 mm) segmental arteries at the aortic area planned to be covered by the stent graft were excluded from the embolisation of the segmental arteries.
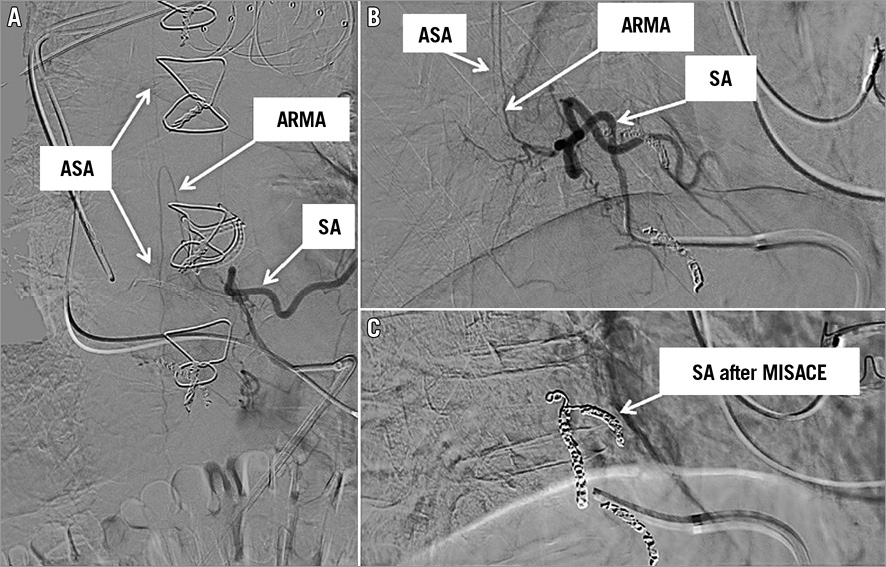
Figure 2. Angiogram of the segmental artery at the level of the 8th thoracic vertebral body. The anterior radiculomedullary artery (ARMA) originating from the segmental artery (SA) and feeding the anterior spinal artery (ASA) (A & B). The complete occlusion of the SA after coil embolisation (C).
ENDOVASCULAR REPAIR OF THE ANEURYSM
Standard design or custom-made devices (Cook Medical Inc., Bloomington, IN, USA, or JOTEC GmbH, Hechingen, Germany) were used for the elective cases. The complete exclusion of the aneurysm was performed no sooner than seven days after the MISACE to allow the preconditioning of the collateral network. Endovascular aortic interventions were performed in a hybrid operating room (Allura Xper FD20; Philips Healthcare, Best, the Netherlands), under general anaesthesia. Classic perioperative neuroprotective strategies were applied to prevent ischaemic spinal cord injury as per institutional protocol. Since 2014, because of a number of previous neurologic complications, no routine preoperative placement of cerebrospinal fluid drainage (CSFD) was performed. The implantation technique of branched and fenestrated endografts has been described previously4. The fenestrations were connected to the target vessels through a transfemoral approach and the branches through a transaxillary approach. In the case of branched endografts, the femoral access was closed before connecting the branches through the axillary route to allow earlier reperfusion of the extremities and the hypogastric arteries. The mean arterial pressure was kept at >80 mmHg. Extubation of the patients to assess the neurologic status was attempted in the operating room.
PERIOPERATIVE MANAGEMENT AND POSTOPERATIVE EVALUATION
All patients underwent standardised postoperative management with at least 24-hour monitoring in the intensive care unit. The mean arterial blood pressure was kept at >80 mmHg and the administration of any antihypertensive drugs was temporarily paused. In addition, transfusion of blood products was indicated in the first 48 hours after the procedure to keep a target haemoglobin ≥10 mg/dL. Neurologic examination was performed prior to intervention, every six hours during the stay on the intensive care unit and daily on the normal ward. Before discharge, CTA was routinely performed for quality control.
CLINICAL OUTCOMES
We analysed technical feasibility, complications (including the onset of SCI at 30 days), perioperative mortality and the technical success of the final treatment, defined as successful placement of an aortic stent graft and target vessel stents.
DATA COLLECTION AND STATISTICAL ANALYSIS
Demographics, medical history and procedure-related data were extracted for analysis from a prospectively maintained electronic database. Complications were defined using the Society for Vascular Surgery’s reporting standards for endovascular aortic aneurysm repair12. Preoperative and postoperative imaging studies were analysed with multiplanar reconstruction and the technique of centreline of flow measurement on a workstation (3mensio Medical Imaging BV, Bilthoven, the Netherlands). Data were analysed using SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA). Categorical variables are presented as percentages, and continuous variables are presented as mean±standard deviation or median values.
Results
PATIENT DEMOGRAPHICS
This study included 57 consecutive eligible patients. The characteristics of patients and thoracoabdominal aneurysms treated are shown in Table 1. The majority of patients (n=54 [94.7%]) were treated for an atherosclerotic TAAA, whereas three patients (5.3%) were treated for a post-dissection TAAA. The mean maximal aortic diameter was 62.7±8.8 mm. Ten patients had a previous repair of the aorta at a median of 6.5 years (range, 0.5-18) before the diagnosis of the current aneurysm. For the treatment of the current aortic aneurysm, eight patients received an aortic procedure as part of a staged aortic coverage approach before MISACE. In this group of patients, the median time between the aortic procedure and the first session of MISACE was as long as 64 days (range, 24-298), partly due to the postoperative recovery after open aortic surgery, and partly due to the fact that one patient temporarily refused the continuation of treatment. An average of 10±4.4 SAs (median: 9; range: 2-26) were found patent on the CTA and were to be sacrificed during the final procedure of complete aneurysm exclusion. Assuming that the basic arterial vertebro-spinal vascular unit consists of two segmental vessels, left and right, arising from the dorsal surface of the aorta at each spinal level, an average of 39.1±22% (median: 36.6%; range: 0-81.2%) of the SAs were primarily occluded in the area of the aorta to be covered by the stent graft.
MISACE
Segmental artery embolisation was performed in one session in twenty-two patients, in two sessions in twenty-four patients, in three sessions in seven patients, in four sessions in three patients, and one patient received five sessions of coiling. A total of 319 SAs were occluded by MISACE with each patient having a median of five coiled SAs (range, 1-19), occluding 60% of direct open segmental arterial inflow (range, 14.3-100%) and a median of 77.7% of the total segmental arterial inflow (range, 38.8-100%) (Figure 3). The technical details of the coiling sessions can be found in Table 2. The statistical analysis using the Wilcoxon rank-sum test between the patients who underwent a repair with a branched device and patients with a non-branched device revealed no statistically significant difference between the coiling sessions, regarding the number of sessions, the number of total coiled SAs, the procedure time, the radiation time and the volume of contrast media. Furthermore, there was no statistical difference between the sessions of coiling in the patients with a patent hypogastric artery compared with the patients with a unilateral occluded hypogastric artery. In three patients, after a first successful session of coiling, the open segmental arteries which were planned to be coil-occluded during further sessions were technically impossible to occlude due to the unstable position of the catheters caused by the extreme kinking of the aorta and the large diameter of the aneurysm. In one patient, a lost pushable coil was successfully retrieved out of the patient using a snare without causing any embolic event. Back pain indicating skeletal muscle ischaemia developed in thirteen patients. This resolved with no neurological deficit, and no alteration of renal function occurred after coiling; adjunctive non-steroidal anti-inflammatory drugs were administered.
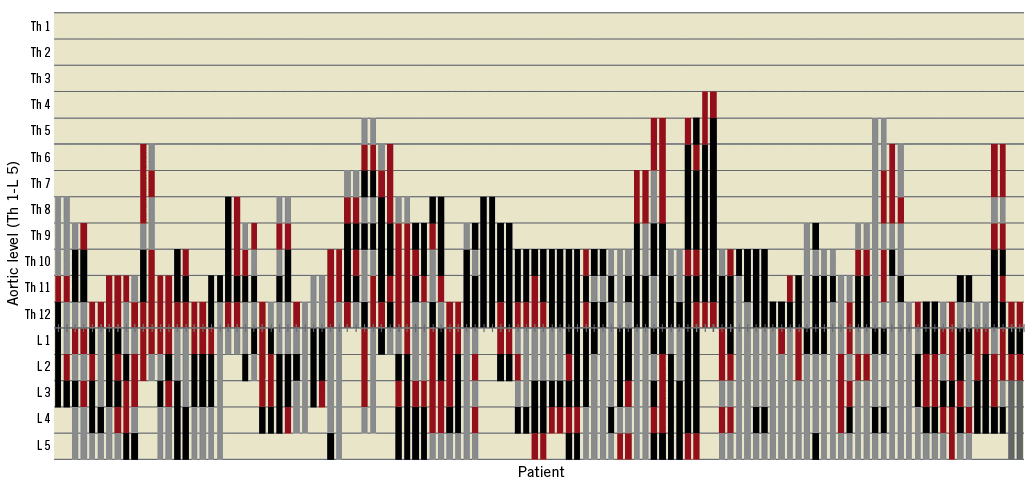
Figure 3. Distribution of segmental arteries after minimally invasive segmental artery coil embolisation at the level of the planned aortic coverage. Open SAs are shown in red, chronically occluded SAs are shown in grey and embolised SAs are shown in black. Each patient is represented by two parallel bars, indicating the right and the left side of the aorta. L: lumbar; SA: segmental artery; Th: thoracic
INTERVAL MANAGEMENT
The time interval between the last session of coiling and the complete exclusion of the aneurysm was a median of 65 days (mean 83±62 days) with a wide range (7-248). The minimal treatment interval was chosen in four cases; three patients had very large aortic aneurysms with a high risk of rupture (>80 mm), and one patient had a penetrating aortic ulcer. One 74-year-old male high-risk patient with post-dissection Crawford type II TAAA of a maximum aortic diameter of 84 mm died 35 days after completion of three sessions of MISACE, while waiting for the implantation of the custom-made stent graft. Unfortunately, the patient was not readmitted to the hospital and no autopsy was performed, so the definitive cause of death remains unknown. Another 83-year-old female patient died 181 days after successful coiling of all the planned segmental arteries and stenting of the proximal segment of the thoracic aorta due to infection of the vascular endo-conduit placed in the iliac arteries to facilitate the introduction of the thoracic stent graft.
ENDOVASCULAR REPAIR OF TAAA
Endovascular exclusion of the thoracoabdominal aneurysm was performed in 55 patients (Figure 4). Total endovascular operating time was 175±57 minutes and the radiation time was 60.5±23.4 minutes. Five patients were treated with a commercially available thoracic stent graft, thirty-one patients received a custom-made fenestrated stent graft, fourteen patients received a branched stent graft and five patients were treated with a combination of fenestration and branches. Technical success for visceral artery stenting was 99% (198/200), including stenting of 48 celiac axes, 50 superior mesenteric arteries, and 100 renal arteries, with a mean of 4.0±0.2 vessels per patient. In two patients the celiac trunk could not be connected to the stent graft due to ostial occlusion by the fabric of the stent graft. The subclavian artery was patent in all patients. Nine patients had a unilateral occlusion of the hypogastric artery. Length of aortic coverage averaged 270.3±89.4 mm (range 118-490), which corresponded to 62±18% (range 32-100%) of the estimated length from the subclavian artery to the aortic bifurcation.
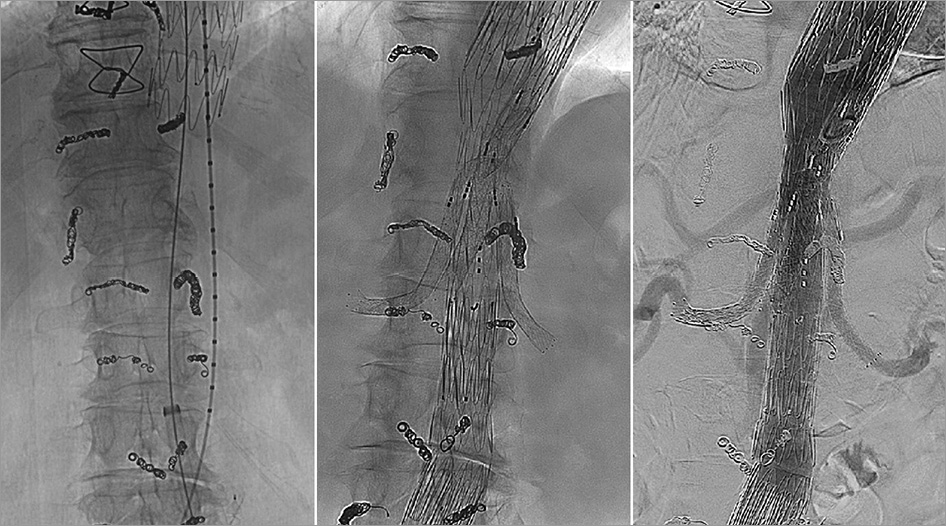
Figure 4. MISACE at the thoracoabdominal level followed by endovascular exclusion of the thoracoabdominal aneurysm and completion angiography.
PERIOPERATIVE/EARLY POSTOPERATIVE MORTALITY
After complete exclusion of the TAAA, there was one in-hospital death in a 71-year-old female patient with an asymptomatic type II TAAA with a maximum diameter of 65 mm who had received a replacement of the aortic arch using the frozen elephant trunk procedure six years previously. Fifty days after the last session of coiling she underwent a complete exclusion of her TAAA using a custom-made stent graft. Six hours postoperatively, a contained rupture of the aorta due to an endoleak type III was diagnosed and the patient died despite emergent endovascular treatment.
NEUROLOGICAL COMPLICATIONS
None of the fifty-four patients alive after the complete exclusion of their aneurysm developed spinal cord ischaemia. One patient developed dysarthria due to a post-interventional minor stroke. She recovered completely before discharge on the tenth postoperative day.
ENDOLEAK RATE
One endoleak type II was encountered in our cohort. One patient developed a type Ib endoleak at the end of the iliac limb which was treated with an iliac branched device in a subsequent session. Seven patients presented a small type III endoleak; these were left untreated at the end of the procedure.
Discussion
Spinal cord ischaemia still remains the Achilles’ heel of both open and endovascular TAAA repair, with significant impact on one-year survival2. Multiple factors (in endovascular repair primarily coverage of large aortic segments, while in open repair aortic cross-clamp time and body temperature) can lead to acute SCI, which frequently leads to permanent paraplegia4,13,14. The collateral network concept7 suggests the amelioration of the acute perfusion loss by selected staged segmental artery occlusion –with MISACE under controlled haemodynamic conditions in the awake patient– thus minimising risk of acute ischaemic cord injury, allowing regeneration and de novo generation of arterial collaterals fed by alternative inflow sources (i.e., subclavian and hypogastric arteries).
The feasibility of coil embolisation of segmental arteries was demonstrated in the animal model by Geisbusch et8. The first-in-man clinical application of MISACE for collateral preconditioning before open and totally endovascular TAAA repair to minimise ischaemic SCI has already been described by Etz et al15.
The criteria to embolise SAs are still based on sparse experimental data and therefore clinically rather arbitrary. Criteria such as a 2.5 mm or 2.0 mm vessel diameter without ostial stenosis were established because a large SA is easier to catheterise and embolise, and experience derives from endoleak prevention strategies9. In our study group, vessels of at least 2 mm were embolised and we were able to occlude 77.7% of direct segmental arterial flow to the spinal cord. In animal experiments, coiling of only two SAs was enough to reduce the damage after simulated TAAA repair dramatically, with little or no histologic damage to the areas of the spinal cord corresponding to the coiled segmental arteries as a result of the MISACE procedure8.
We present the first clinical series with the MISACE procedure prior to endovascular TAAA repair in a cohort of 57 highly selected patients. The patients had non-symptomatic large and rapidly growing Crawford type I to IV aortic aneurysms. To our knowledge, we are the first to describe the coiling of multiple SAs at the thoracic level in humans. MISACE was comparatively safe, with no neurological deficit after the coiling sessions. The back pain we observed in thirteen patients testifies to the need to monitor very closely and proceed with great caution, even in a selected high-risk population.
Our goal was to coil as many SAs as technically feasible in the aortic segment that was planned for coverage by the stent graft. As a practical safety measure, we performed the consecutive staged coil embolisation in multiple sessions with assessment of spinal cord viability between stages to avoid a critical acute reduction in spinal cord perfusion. Based on experimental studies, we chose a minimum interval of at least one week between two MISACE sessions to allow sufficient arterial preconditioning to occur. The risks of interval rupture and the potential for incomplete treatment through non-compliance or loss to follow-up are two major problems of staging. None of our patients was lost to follow-up or declined to return; however, two patients died while waiting for exclusion of the aneurysm with a custom-made stent graft.
After final exclusion of the TAAA we encountered no SCI. To our knowledge, these results are clearly below the average reported results with any other endovascular staging strategy for TAAA repair5,16,17.
Covering the segmental arteries by stenting the thoracic aorta in type II and type III aortic disease offers a feasible modality to stage the aortic repair besides MISACE. This repair leads to a type Ib endoleak, which is then excluded after insertion of the visceral segment device. O’Callaghan et al reported 11.1% temporary SCI in the staged group of type II TAAA in comparison to 37.5% of paraplegia in the non-staged group16. Another type of aortic staged repair is temporary aneurysm sac perfusion (TASP), e.g., by a visceral branch left intentionally open17. This intentional endoleak is thought to prevent immediate complete thrombosis of the aneurysmal sac, maintaining perfusion of some of the segmental arteries. In a second stage, the closure of this branch is executed in order to complete the total exclusion of the aneurysm. Kasprzak et al reported 3% paraplegia when maintaining a branch/fenestration open compared to 27% in patients treated in a single fully occlusive intervention17.
The regional differences in perfusion pressure across the collaterals caused by the permissive endoleak after the first stage may not suffice to stimulate the desired arteriogenic preconditioning of the spinal cord network. Furthermore, microembolisation has been increasingly recognised as a cause of spinal cord ischaemia13, the risk for which might be higher with TASP than with rather controlled MISACE.
Minimally invasive selective segmental artery coil or plug embolisation (MISACE) has the potential to solve these two issues by addressing both pathological mechanisms of ischaemic spinal cord injury: it promotes the regeneration or de novo generation of the arterial paraspinous collateral network and conceivably minimises embolisation to the small arteries of the spinal cord.
Limitations
This study was performed retrospectively and might have limitations regarding data collection. As is well known with retrospective and non-controlled studies, effect sizes tend to be overestimated. Furthermore, clinical experience is required to optimise the MISACE strategy, particularly with regard to the number of sessions required and the minimum acceptable interval between. The optimal balance between radiation time, utilised contrast dye and effective segmental artery occlusion still needs to be addressed, particularly with regard to radiation-induced increased cancer mortality18.
Conclusions
Minimally invasive segmental artery coil embolisation (MISACE) to precondition the paraspinous collateral network is clinically feasible and very encouraging regarding safety. MISACE followed by aneurysm exclusion may drastically reduce paraparesis and paraplegia after total endovascular repair of extensive TAAA.
| Impact on daily practice Clinically evident permanent SCI after the endovascular treatment of TAAA was not observed after performing MISACE in our cohort. The ultimate proof of MISACE’s success requires a randomised trial which is currently underway, namely Paraplegia Prevention in Aortic Aneurysm Repair by Thoracoabdominal Staging With “Minimally-Invasive Segmental Artery Coil-Embolization (MISACE)”: A Randomized Controlled Multicentre Trial – PAPA-ARTiS (NCT03434314). PAPA-ARTiS is jointly funded by the European Union’s Horizon 2020 research and innovation programme (733203) and the German Research Foundation (ET 127/2-1). |
Conflict of interest statement
D. Branzan declares modest travel grants from JOTEC GmbH and Cook Medical. D. Scheinert and A. Schmidt declare modest consulting grants from Cook Medical. The other authors have no conflicts of interest to declare.

