
Abstract
Aims: The current study aimed to assess the difference in lumen dimension measurements between optical coherence tomography (OCT) and quantitative coronary angiography (QCA) in the polymeric bioresorbable scaffold and metallic stent.
Methods and results: In the randomised ABSORB Japan trial, 87 lesions in the Absorb arm and 44 lesions in the XIENCE arm were analysed. Post-procedural OCT-QCA lumen dimensions were assessed in matched proximal/distal non-stented/non-scaffolded reference (n=199), scaffolded (n=145) and stented (n=75) cross-sections at the two device edges using the Bland-Altman method. In the non-stented/non-scaffolded reference segments, QCA systematically underestimated lumen diameter (LD) compared with OCT (accuracy, –0.26 mm; precision, 0.47 mm; 95% limits of agreement as a mean bias±1.96 standard deviation, –1.18-0.66 mm). When compared to OCT, QCA of the Absorb led to a more severe underestimation of the LD (–0.30 mm; 0.39 mm; –1.06-0.46 mm) than with the XIENCE (–0.14 mm; 0.31 mm; –0.75-0.46 mm). QCA underestimated LD by 9.1%, 4.9%, and 9.8% in the reference, stented, and scaffolded segments, respectively. The protrusion distance of struts was larger in the Absorb arm than in the XIENCE arm (135±27 µm vs. 18±26 µm, p<0.001), and may have contributed to the observed differences.
Conclusions: In-device QCA measurement was differently affected by the presence of a metallic or polymeric scaffold, a fact that had a significant impact on the QCA assessment of acute gain and post-procedural minimum LD. (ClinicalTrials.gov, Identifier: NCT01844284)
Introduction
In contrast to metallic stents, the Absorb™ bioresorbable poly-L-lactide (PLLA) scaffolds (Abbott Vascular, Santa Clara, CA, USA) are partially translucent and radiolucent to gamma radiation, with the exception of the radiopaque platinum markers at the edges. Therefore, imaging interpretation of Absorb scaffolds with optical coherence tomography (OCT) or quantitative coronary angiography (QCA) has to be critically appraised.
OCT is widely recognised as a gold standard for the measurement of luminal dimensions for both metallic stents and polymeric scaffolds due to its resolution (<20 μm) and accuracy1. The comparative methodologies of lumen measurement by OCT in polymeric scaffolds and metallic stents have been introduced and applied for the current clinical trials2-4.
QCA is known to underestimate the lumen dimension systematically compared to OCT5. However, in the assessment of metallic stents, this difference between QCA and OCT might also be influenced by the radiopacity of the material, since the radiopacity of metallic stents could theoretically impact on the densitometric and edge software analysis of QCA6. In the assessment of polymeric scaffolds, their radiolucency theoretically does not impact on the QCA analysis, whereas their increased strut protrusion into the lumen could hinder the intracoronary laminar flow, which might result in underestimation of the lumen dimension due to altered contact of the contrast medium with the vessel wall7,8. Therefore, polymeric scaffolds and metallic stents –because of their inherent material properties– could introduce an incremental element of discrepancy between measurements by OCT and QCA. However, this hypothesis has not been investigated so far.
The purpose of the current study was to assess the difference between OCT and QCA measurements in polymeric scaffolds and metallic stents, and to investigate the mechanisms of discrepancy, if it occurs.
Methods
STUDY DESIGN
ABSORB Japan was a prospective, multicentre, randomised, single-blind, active-controlled clinical trial in which 400 patients undergoing coronary stent implantation in Japan were randomised in a 2:1 ratio to treatment with the Absorb everolimus-eluting BVS or the XIENCE Prime®/Xpedition® cobalt-chromium everolimus-eluting stent (both Abbott Vascular)3. The details of the trial have been described elsewhere3. A total of 38 investigational sites in Japan participated in the study. The study was conducted according to the Declaration of Helsinki. Prior to initiating the study, the institutional review board at each investigational site approved the clinical trial protocol. All patients provided written informed consent before enrolment.
Patients were randomised in a 2:1 ratio to Absorb vs. XIENCE using a central randomisation service. Randomisation was stratified by the presence of diabetes mellitus and the number of lesions to be treated. Patients were allocated randomly to one of the three intravascular imaging subgroups: intravascular ultrasound (IVUS) group (150 patients), OCT group 1 (125 patients), or OCT group 2 (125 patients), based on the schedules of intravascular imaging. In the present investigation, we analysed OCT data and QCA data from OCT group 13. The study flow chart is shown in Figure 1.
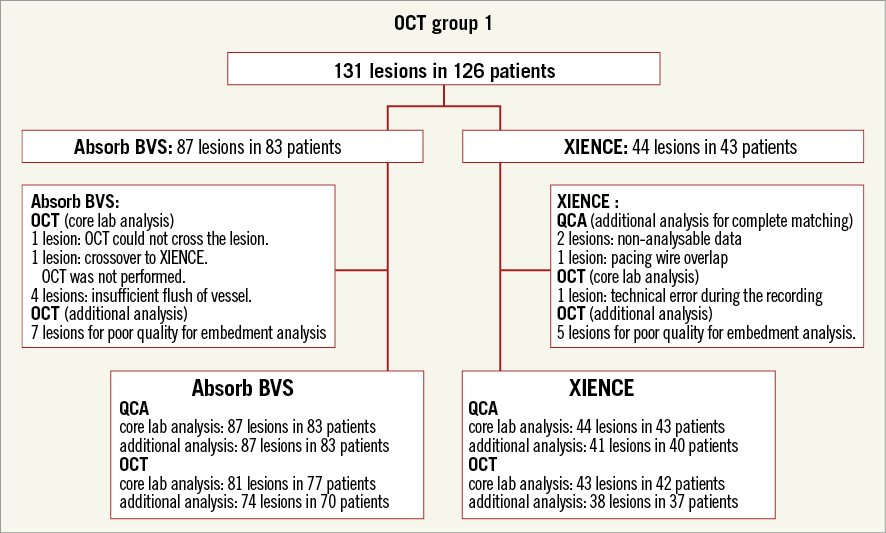
Figure 1. Study flow chart.
QUANTITATIVE CORONARY ANGIOGRAPHY
An angiographic core laboratory performed QCA analysis (QAngio® XA 7.3; Medis medical imaging systems bv, Leiden, The Netherlands). The following parameters were analysed by the core lab: mean lumen diameter (LD), minimum lumen diameter (MLD), interpolated reference vessel diameter (RVD), percentage diameter stenosis (%DS), minimum lumen area (MLA) based on the edge detection method9. MLA assessed by QCA was calculated from the MLD, which was provided by the software, using the formula: MLA=3.14x(MLD/2)2,6 (Appendix Figure 1). In addition to the core lab analysis of QCA, we performed QCA by the edge detection method at the co-localised position with the OCT cross-sections. This QCA analysis was performed according to standard procedures, using single-plane orthogonal projections of the target lesion with the CAAS system version 5.11 (Pie Medical Imaging BV, Maastricht, The Netherlands). For each lesion, the LDs at both device edges and a single LD at the extremities of the 5 mm proximal and distal to the device edges were analysed. The small radiopaque markers at the ends of the polymeric scaffolds and the radiopaque struts of metallic stents helped us to localise the in-device segment. Lumen area (LA) at each cross-section was calculated by the above-mentioned formula.
OPTICAL COHERENCE TOMOGRAPHY
OCT pullbacks were obtained at baseline after the stent or scaffold implantation by a frequency-domain C7 system using a Dragonfly™ catheter (St. Jude Medical, St. Paul, MN, USA) at a rotation speed of 100 frames/s and constant pullback speed of 20 mm/s, a frequency-domain ILUMIEN™ OPTIS™ system using a Dragonfly™ Duo catheter (both St. Jude Medical) at a rotation speed of 180 frames/s and constant pullback speed of 18 mm/s, or an optical frequency domain imaging (OFDI) Lunawave® console using a FastView® catheter (both Terumo Europe N.V., Leuven, Belgium) at a rotation speed of 160 frames/s and constant pullback speed of 20 or 40 mm/s with a non-occlusive technique, while contrast was infused through the guiding catheter at a continuous rate of 2-4 mL/s.
The OCT measurements were performed with the QIvus® software (Medis) by the core laboratory (Cardialysis, Rotterdam, The Netherlands). With adjustment for the pullback speed, the analysis of continuous cross-sections was performed at each 1 mm longitudinal interval within the treated segment. The following parameters were evaluated: mean and (projected) MLD and area, mean and minimum (abluminal) scaffold/stent diameter and area2. The LD of the matched cross-section analysis was calculated using a circular model10. The details are shown in the Appendix.
The additional OCT analysis (strut protrusion analysis) was performed with a newly developed specific software, QCU-CMS software version 4.69 (Leiden University Medical Center, Leiden, The Netherlands)11. The protrusion distance was measured by the software (Appendix Figure 1). The details of the analysis are described elsewhere11. The protrusion analysis by OCT was performed every 200 μm cross-section in case of OCT and every 250 μm in case of OFDI in the stent/scaffold segments. The cases with complete pullback and good image quality as defined by >70% of analysable frames were included in this specific analysis12. Mean strut protrusion distance was calculated as the average of protrusion distance in a lesion level and a cross-section level.
COMPARISON OF QCA AND OCT
The discrepancies between QCA and OCT were compared among scaffolded segment, stented segment, and proximal/distal reference segment. As described above, in each lesion, the treated segment and the peri-treated regions (defined by a length of 5 mm proximal and distal to the device edge) were analysed.
For matching of an OCT cross-section with a corresponding QCA cross-section, the following criteria were used in this study. Case examples for matched cross-sections in XIENCE and Absorb cases are shown in Figure 2. For the scaffolded segment, OCT cross-sections with proximal and distal metallic markers were matched with corresponding QCA cross-sections which were recognised using the radiopaque metallic markers of the polymeric device. For the stented segment, OCT cross-sections at both stent edges were matched with the corresponding QCA cross-sections which were recognised by radiopaque strut edges. The identification of the stent edges on OCT was defined as the point where the visualisation of the stent arc was circumferential, implying that the stent edge to some extent may include metallic struts. For proximal/distal reference segments, 5 mm proximal and 5 mm distal cross-sections in OCT and QCA analyses were analysed as matched cross-sections. Bifurcation segments in which the side branch occupied more than 45° of the cross-section were excluded in order to avoid tracing interpolation when quantifying the lumen12. In case the metallic marker of the Absorb could not be identified due to the wire shadow artefact or insufficient flush of blood, the cross-section and the associated proximal or distal edge cross-sections were not included in the analysis.
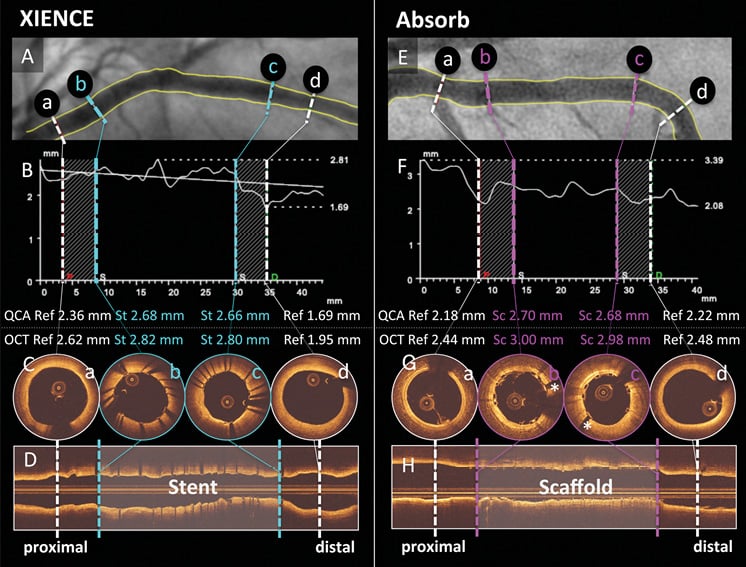
Figure 2. Case examples for matched cross-sections. Case examples of XIENCE (A-D) and Absorb (E-H) are shown. Cross-sections at reference (white) vessel (a, d) and stented (light blue)/scaffolded (pink) vessel (b, c) on QCA (A, B, E, & F) were matched with those on OCT (C, D, G, & H). *Metallic marker of Absorb. OCT: optical coherence tomography; QCA: quantitative coronary angiography; Ref: reference cross-section; Sc: scaffolded cross-section; St: stented cross-section
STATISTICAL ANALYSIS
Data are expressed as mean±standard deviation or median and interquartile range with differences (95% confidence interval). Group means for continuous variables with normal and non-normal distributions were compared using Student’s t-tests and Mann-Whitney U tests, respectively. Categorical variables were compared using the Pearson’s chi-square test or Fisher’s exact test, as appropriate. Generalised estimating equations modelling was performed to take into account the clustered nature of >1 stent/scaffold analysed from the same patients, which might result in unknown correlations among measurements within these scaffold clusters. Measurement agreements in LD at cross-section level and mean LD, MLD, MLA at lesion level by QCA and those by OCT were determined by comparing measurements of each analysis using the Bland-Altman method. Data are given as plots showing the absolute difference between corresponding measurements of both methods (y-axis) against the average of both methods (x-axis). Assuming OCT as a gold standard, the accuracy between OCT and QCA measurements and its precision were calculated. The 95% limits of agreement were calculated as mean bias±1.96 standard deviation. Simple linear regression analysis was used to evaluate the relationship between the strut protrusion distance and the OCT-QCA discrepancy. The Pearson correlation coefficient was used to evaluate the strength and direction of the linear relationship between the OCT-QCA discrepancy and strut protrusion. Statistical significance was assumed at a probability (p) value of <0.05. All statistical analyses were performed with SPSS, Version 23.0.0 (IBM Corp., Armonk, NY, USA) or MedCalc statistical software, version 14.12.0 (MedCalc Software bvba, Ostend, Belgium).
Results
PATIENT CHARACTERISTICS
From a total of 126 patients in the OCT group 1, 87 lesions from 83 patients in the Absorb arm and 44 lesions from 43 patients in the XIENCE arm were analysed. Baseline clinical characteristics are shown in Table 1. Lesion and procedural characteristics are summarised in Table 2. All baseline characteristics and the procedural variables were well balanced between both arms.
Results of pre- and post-procedural QCA and post-procedural OCT are shown in Table 3. Post-procedural in-device MLD by QCA was significantly smaller in the Absorb arm than in the XIENCE arm (2.42±0.38 mm vs. 2.58±0.43 mm, p=0.031). In-device acute gain by QCA was smaller in the Absorb arm than in the XIENCE arm (1.47±0.43 mm vs. 1.60±0.42 mm, p=0.086). In post-procedural OCT analysis, projected MLD was similar in both arms (Absorb 2.75±0.42 mm vs. XIENCE 2.72±0.54 mm, p=0.71). Both arms had similar mean lumen area and minimum lumen area.
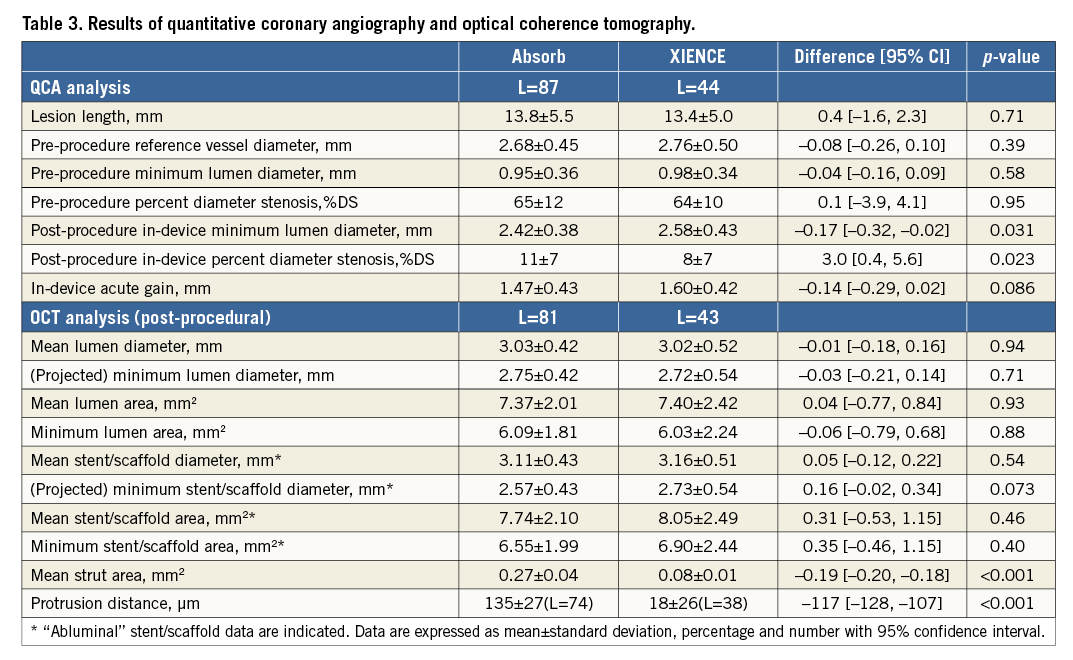
COMPARISON OF QCA WITH OCT PARAMETERS IN ABSORB AND XIENCE
Measurement agreements between QCA and OCT for mean LD, MLD, and MLA in a lesion level analysis are shown in Figure 3. When OCT was used as a gold standard, the Absorb arm had an accuracy of –0.36 mm between QCA and OCT, which was 0.20 mm less than the XIENCE arm. MLD and MLA were also more severely underestimated by QCA in the Absorb arm than in the XIENCE arm.
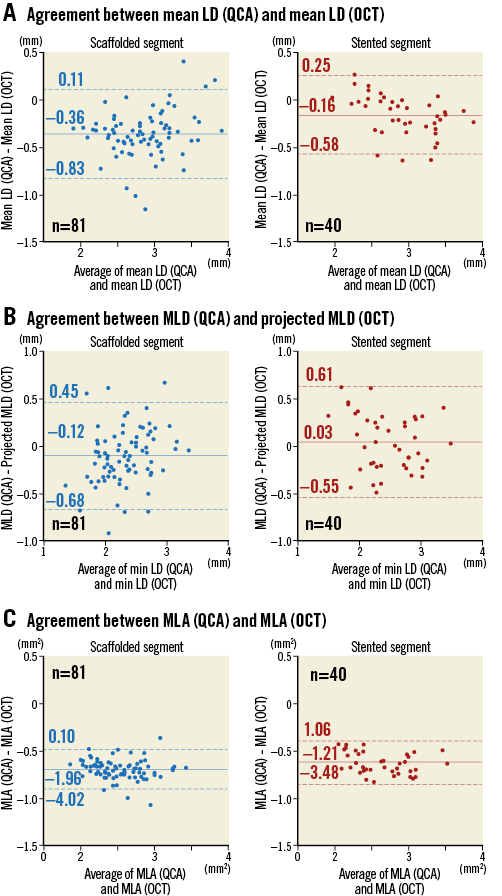
Figure 3. Measurement agreement between QCA and OCT. In the Absorb arm, QCA underestimated mean LD 0.20 mm more than in the XIENCE arm (A). MLD (B) and MLA (C) were also more severely underestimated by QCA in the Absorb arm than in the XIENCE arm. LD: lumen diameter; MLA: minimum lumen area; MLD: minimum lumen diameter; OCT: optical coherence tomography; QCA: quantitative coronary angiography
AGREEMENT BETWEEN QCA AND OCT PARAMETERS IN THE MATCHED CROSS-SECTION ANALYSIS
A total of 199 cross-sections at proximal/distal reference segments, 75 cross-sections at stented segments, and 145 cross-sections at scaffolded segments were evaluated in the matched cross-section analysis. Agreement between QCA and OCT parameters is shown in Figure 4. In proximal/distal reference segments without stents/scaffolds, QCA underestimated LD by 0.26 mm on average compared with OCT. When compared to OCT, QCA of the Absorb polymeric scaffolds led to a more severe underestimation of the LD (accuracy –0.30 mm; precision 0.39 mm) than with the XIENCE metallic stents (accuracy –0.14 mm; precision 0.31 mm). The same trend was observed in LA. Delta accuracy compared to the accuracy of reference segments (–0.26 mm in LD, –1.13 mm2 in LA) was significantly different between the XIENCE and the Absorb arms (LD: XIENCE +0.12±0.31 mm vs. Absorb –0.04±0.39 mm, p=0.002; LA: +0.37±1.55 mm2 vs. –0.26±1.90 mm2, p=0.014).
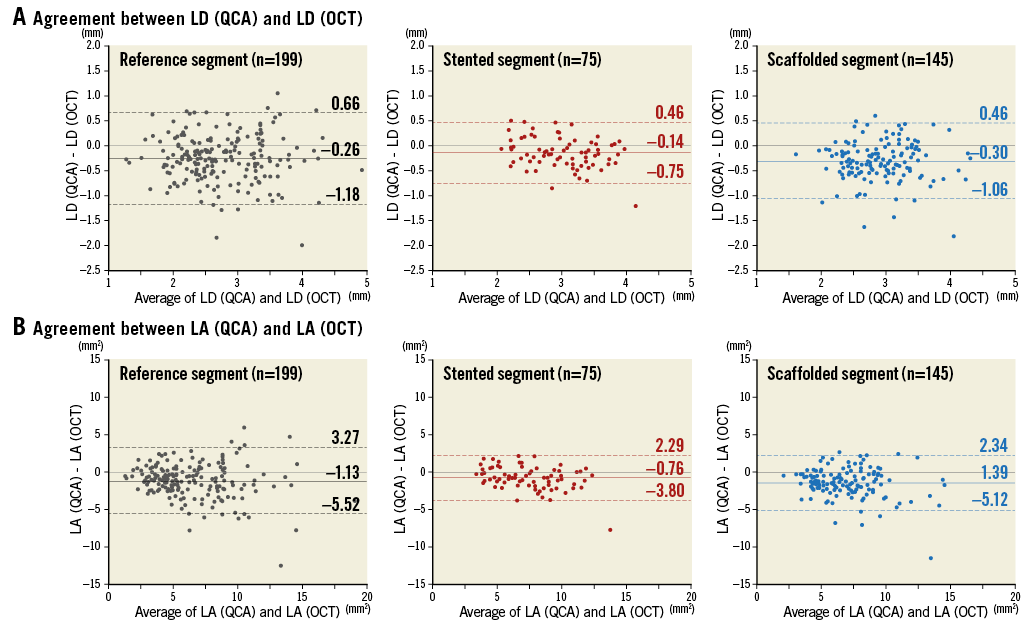
Figure 4. Agreement between OCT and QCA in matched cross-section analysis. In a matched cross-section analysis, lumen diameter (A) and lumen area (B) in 199, 75, and 145 cross-sections were evaluated at proximal/distal reference (black), stented (red), and scaffolded (blue) segments, respectively. Mean bias (solid line) and 95% limits of agreement (mean bias±1.96 standard deviation) (dotted line) are indicated. LA: lumen area; LD: lumen diameter; OCT: optical coherence tomography; QCA: quantitative coronary angiography
CORRELATION BETWEEN THE OCT-QCA DISCREPANCY AND STENT/SCAFFOLD PARAMETERS
In a lesion level analysis (Table 3), the protrusion distance of struts into the lumen was larger in the Absorb arm than in the XIENCE arm (135±27 µm vs. 18±26 µm, p<0.001). In a cross-section level analysis, mean protrusion distance had a moderate correlation with the OCT-QCA discrepancy of LD both in XIENCE and Absorb (correlation coefficient –0.418 for XIENCE, –0.440 for Absorb, both p<0.001) (Figure 5). Lumen eccentricity had very weak correlation with the discrepancy in both arms (correlation coefficient 0.221 for XIENCE, p=0.057; –0.184 for Absorb, p=0.027).
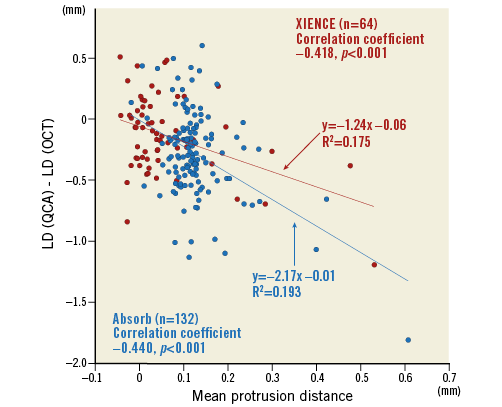
Figure 5. Correlation between the OCT-QCA LD discrepancy and stent/scaffold protrusion. Correlation between the OCT-QCA LD discrepancy and mean protrusion distance in XIENCE (red circle) and Absorb (blue circle) is shown in scatter plots. Mean protrusion distance had a moderate correlation with the OCT-QCA discrepancy of LD in both XIENCE and Absorb. LD: lumen diameter; OCT: optical coherence tomography; QCA: quantitative coronary angiography
Discussion
The main findings of the present study are summarised as follows. 1) In proximal/distal reference segments without stents/scaffolds, QCA underestimated LD by 0.26 mm on average compared with OCT. When compared to OCT, QCA of the Absorb polymeric scaffolds led to a more severe underestimation of the luminal dimension (accuracy –0.30 mm) than with the XIENCE metallic stents (accuracy –0.14 mm). 2) Strut protrusion into the lumen had a moderate correlation with the underestimation of QCA compared to OCT in both XIENCE and Absorb.
OCT is widely recognised as a gold standard for the measurement of luminal dimensions for both metallic stents and polymeric scaffolds due to its resolution and accuracy1. Detection of the vessel wall by OCT is the result of the backscattering reflection of the light from the most superficial (20 μm) endoluminal layer of the vessel wall, while the detection of luminal dimension by angiography is the result of a more or less laminar contact of contrast medium with the vessel wall which is influenced by the velocity of the most outer layer of laminar flow along the vessel wall. Therefore, the QCA measurement, by nature, underestimates the true dimension which is almost perfectly defined by OCT1,5,13.
The results of the present study are in line with previous reports1,5,13. Figure 6 summarises the relative difference of QCA-LD versus OCT-LD. In the reference segments, QCA underestimated LD by 9.1% compared to OCT. In the stented segments, QCA underestimated LD less (4.9%), whereas in the scaffolded segments QCA more severely underestimated LD (9.8%) compared to OCT. Figure 7 illustrates computational flow dynamics demonstrating the possible causes of discrepancy between luminal dimensions as determined by OCT or QCA in the native (reference), stented, and scaffolded vessels. In the stented vessel, laminar flow of contrast is disturbed by the protruded struts and cannot get into close contact with the vessel wall compared to the native unstented/unscaffolded vessels. However, high radiopacity of metallic struts could cause an artefactual outward enlargement of the lumen contours (blooming artefact of metal), resulting in less underestimation in the stented vessels than in the scaffolded vessels. A radiolucent polymeric strut does not cause any inherent X-ray artefact in the brightness function and analysis by QCA. In the scaffolded vessels, the laminar flow disturbance is larger than in the stented vessels due to more strut protrusions, resulting in less close contact of the contrast medium with the vessel wall. Thereby, scaffolded vessels generate possibly a more severe underestimation of the lumen dimension on QCA than the one observed in the native vessels.
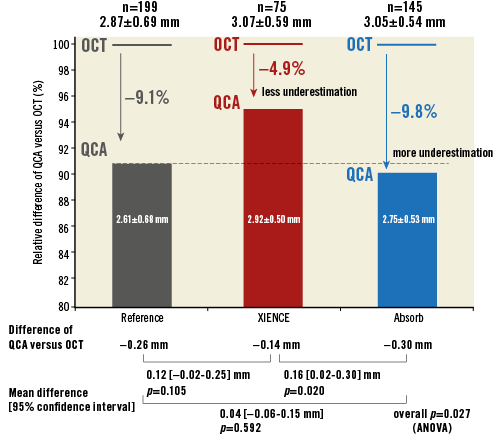
Figure 6. Relative difference of QCA-LD versus OCT-LD. In the reference segments (black), QCA underestimated LD by 9.1% compared to OCT; in the stented segments (red), QCA underestimated LD less (4.9%), whereas, in the scaffolded segments (blue), QCA underestimated LD more severely (9.8%) compared to OCT. OCT: optical coherence tomography; QCA: quantitative coronary angiography
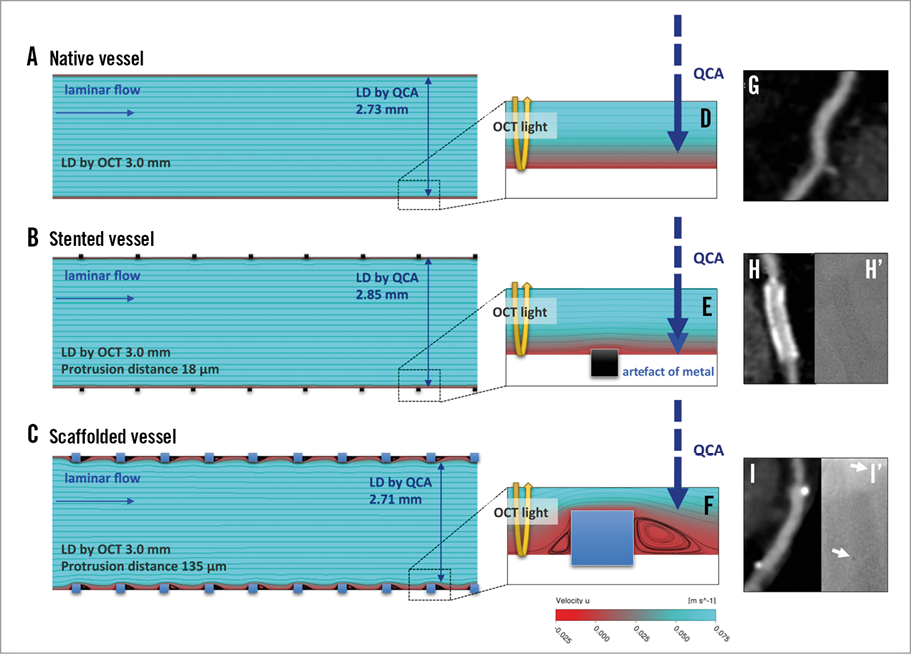
Figure 7. Possible causes of discrepancy between luminal dimensions as determined by OCT or QCA in the native, stented, and scaffolded vessels. Computational flow dynamics in native (A & D), stented (B & E), and scaffolded (C & F) vessels demonstrated the difference of contact of contrast with the vessel wall. The differences of radiopacity are demonstrated in MSCT (G, H, & I) and angiographic images (H’ & I’). White arrows in panel I’: metallic markers of Absorb. LD: lumen diameter; OCT: optical coherence tomography; QCA: quantitative coronary angiography
MATERIAL PROPERTY
The more radiopaque the metal is, the larger the luminal dimensions become on QCA. The basic algorithm of edge detection relies on the average weight of the first and second derivative of the brightness function, and presence of metal in the vessel wall will interfere with the edge detection6, a fact that has been repeatedly demonstrated for highly radiopaque metals such as tantalum, nitinol, and platinum6,14. Fortunately, cobalt-chromium is a metal with low radiopacity and thus there is less interference with edge detection, although a small but detectable effect can be demonstrated compared with the reference segment14. In other words, radiopacity has a tendency to enlarge artefactually the contour of the lumen, whereas the lumen contour might artefactually be reduced by the degree of strut protrusion. In contrast to metallic stents, the Absorb PLLA scaffolds are totally radiolucent. The material (PLLA) itself therefore does not interfere with the densitometric assessment by QCA but causes underestimation of the lumen dimension due to the physical hindrance for the contrast medium to contact the vessel wall as described above. Overall, the overestimation of the lumen with metallic stents and underestimation with polymeric scaffolds could generate lower acute gain and post-procedural MLD for the polymeric scaffold when compared to the metallic stent by QCA.
LAMINAR FLOW DISTURBANCE
When the struts protrude into the lumen of coronary arteries, the lumen physically loses some space for contrast medium, which could result in underestimation of size by QCA analysis. The Absorb scaffold has 157 μm of strut thickness and 27% of vessel wall area coverage while the XIENCE has 89 μm and 13%, respectively. Contrast medium cannot be in direct contact with the vessel, at least not in this stent/scaffold occupied surface area. In addition, the flow dynamics of a coronary artery are pulsatile, laminar, and non-Newtonian. When a stent is deployed, the individual struts promote blood flow separation, creating upstream and downstream recirculation zones15. In the recirculation zones, contrast medium cannot be physically in contact with the vessel wall16. When the strut shape is the same (e.g., both Absorb and XIENCE have rectangular shapes), the larger strut thickness creates reversal of flow upstream and downstream to the strut more frequently7,15. In the present study, a moderate correlation between OCT-QCA discrepancy of LD and protrusion distance would suggest that the laminar flow disturbance could be at least one of the mechanisms of OCT-QCA discrepancy.
ASSESSMENT OF ACUTE GAIN
In current clinical trials, “acute gain”, defined as the difference between pre- and post-procedural MLD, is assessed by QCA as a parameter of device performance3,4. The present study would imply the unfairness of this assessment for Absorb compared to XIENCE. For the assessment of MLD, the lower degree of underestimation in metallic stents and the more severe underestimation in polymeric scaffolds would logically generate a large discrepancy and unfairness for the comparison of the device performance. In the present study, in-device acute gain by QCA was smaller in the Absorb arm than in the XIENCE arm (Absorb 1.47±0.43 mm vs. XIENCE 1.60±0.42 mm, p=0.086), while post-procedural MLD by OCT as a substitute parameter of acute gain by OCT in both arms was similar (Absorb 2.75±0.42 mm vs. XIENCE 2.72±0.54 mm, p=0.71). Since pre-procedural OCT was not performed in this study population, the acute gain by OCT is not available. However, we could assume the similarity of pre-procedural MLD in both arms due to the randomised approach. Currently, we do not have an appropriate quantitative angiographic method to assess the impact of a slight change in radiopacity on the lumen dimension measurement.
The difference in QCA analysis and the absence of difference in OCT analysis could raise the question whether the commonly used acute gain analysis by QCA for the comparison of Absorb with XIENCE is appropriate and accurate. The QCA acute gain data from previous trials comparing polymeric scaffolds and metallic stents might be critically reconsidered3,4,17.
Limitations
First, the coronary sites matched by OCT and QCA may not have been exactly identical, especially at proximal/distal reference segments. Although the matching was performed with the radiopaque struts of XIENCE and the metallic markers of the Absorb at stented/scaffolded segments, the proximal/distal reference segments were determined as 5 mm proximal and distal from these matched cross-sections. The length on QCA and on OCT could not be identical due to the well-known unavoidable foreshortening effect of conventional angiography. Second, in the correlation analysis, cross-sections with malapposed struts were also included in the protrusion distance analysis. The impact of malapposed struts on laminar flow varies according to the malapposed distance18. The precise haemodynamics in the vessel with malapposed struts still remain to be elucidated. Therefore, the correlation between the OCT-QCA discrepancy and the protrusion distance could be just a hypothesis-generating finding, and further investigations are still warranted.
Conclusions
Using OCT and an untreated segment as a method and vessel of reference, it has been demonstrated that QCA is differently affected by the presence of a metallic stent or a polymeric scaffold, a fact that has a significant impact on the QCA assessment of acute gain and post-procedural MLD.
| Impact on daily practice The difference in radiopacity of polymer and metal theoretically influences the edge detection method of QCA for the assessment of Absorb polymeric scaffolds and XIENCE metallic stents. In the ABSORB Japan randomised trial, when compared to OCT, QCA underestimated LD by 9.1%, 4.9%, and 9.8% in the reference, stented, and scaffolded segments, respectively, a fact that had a significant impact on the QCA assessment of acute gain and post-procedural minimum LD. The present study would imply the unfairness of the assessment for Absorb compared to XIENCE, which could raise the question of whether the commonly used acute gain analysis by QCA for the comparison of Absorb with XIENCE is appropriate and accurate. The QCA acute gain data from previous trials comparing Absorb and XIENCE might be critically reconsidered. |
Guest Editor
This paper was guest edited by Johan H. C. Reiber, MSc, PhD; Leiden University Medical Center, Leiden, The Netherlands.
Acknowledgements
We would like to thank the investigators of the ABSORB Japan trial, as well as Susan Veldhof, Wai-Fung Cheong and Richard Rapoza from Abbott Vascular for their invaluable technical expertise.
Funding
The ABSORB Japan study was funded by Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
Y. Sotomi is a consultant of Goodman and has received a grant from Fukuda Memorial Foundation for Medical Research and SUNRISE lab. Y. Onuma and P.W. Serruys are members of the Advisory Board for Abbott Vascular. T. Kimura is a member of the Advisory Board for and receives a research grant from Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor is the CEO of Medis medical imaging systems bv, and has a part-time appointment at LUMC as Professor of Medical Imaging.
SUPPLEMENTARY DATA
Appendix. Optical coherence tomography methodology
We analysed lumen area, flow area, abluminal and endoluminal stent/scaffold area according to the previous publication2. Lumen area was measured using the continuous interface between a blood and non-blood structure. The flow area concept was introduced in 2010 to describe the vessel lumen filled by circulating blood, which reflects the blood supply conductance to the myocardium19. Abluminal and endoluminal stent/scaffold contours were delineated by a curvilinear interpolation connecting the midpoints of the abluminal and endoluminal leading edges of the reflective borders, respectively. In the present study, we reported lumen area, and abluminal stent/scaffold area as a stent/scaffold area. Lumen diameter of matched cross-section analysis was calculated using a circular model10.
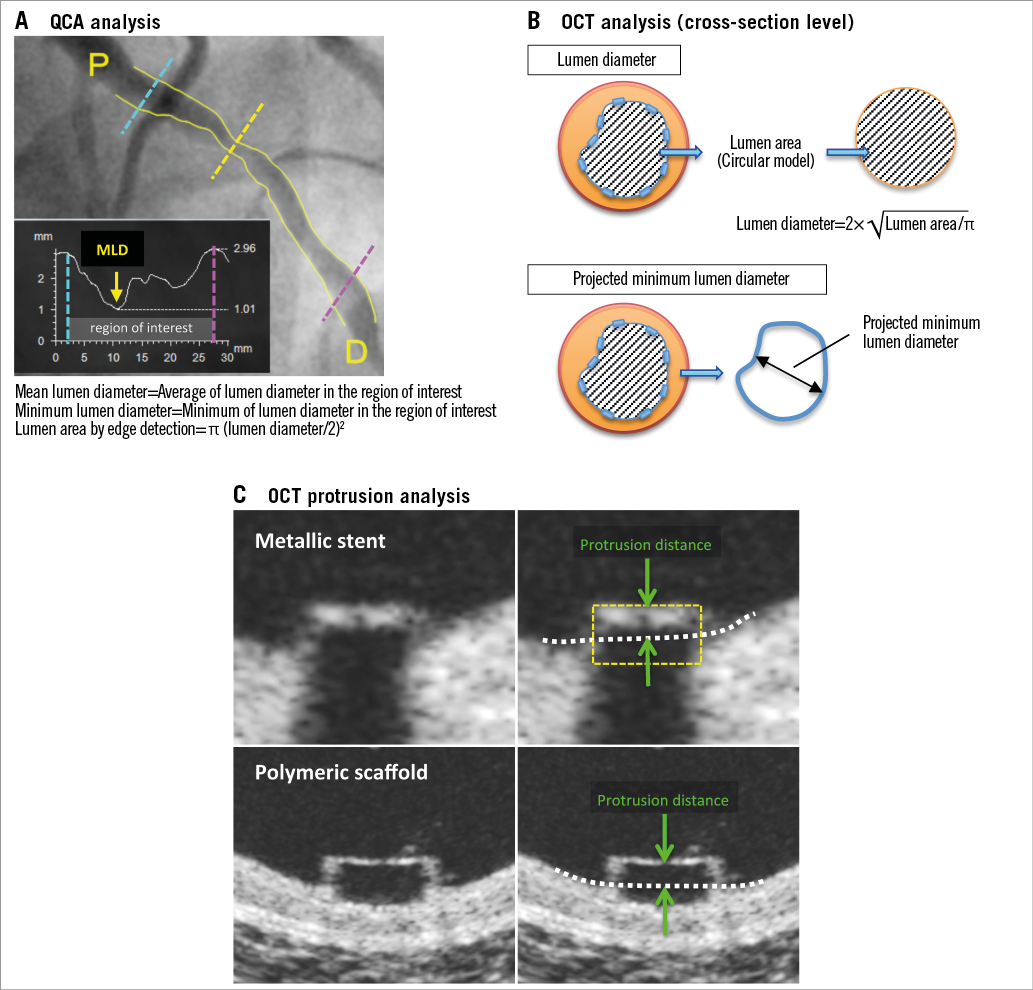
Appendix Figure 1. Methodology for QCA and OCT analysis. Methods used to measure parameters with QCA (A), OCT (B), and OCT protrusion analysis (C) are shown. Standard methodology for the assessment of QCA and OCT was applied in this study (A & B)9. In OCT protrusion analysis, protrusion distance (green) was automatically computed by the software using the interpolated lumen contour (white dotted line) and virtual metallic struts (yellow dashed box)11. OCT: optical coherence tomography; QCA: quantitative coronary angiography

