
Abstract
Aims: Immediately after stent/scaffold implantation, quantitative coronary angiography (QCA) in comparison to optical coherence tomography (OCT) more severely underestimates the lumen diameter (LD) in Absorb than in XIENCE. This OCT-QCA discrepancy has not been evaluated at long-term follow-up. The present study aimed to assess the accuracy of QCA with reference to OCT in Absorb as compared to XIENCE.
Methods and results: We assessed two-year QCA and OCT in the ABSORB Japan randomised trial (Absorb n=87, XIENCE n=44). The accuracy of QCA parameters was assessed with reference to OCT measurements. OCT-QCA luminal dimensions were compared in matched cross-sections at both edges of the scaffolds (n=127) and stents (n=78). OCT-QCA late lumen loss (LLL) was also assessed using the Bland-Altman method. The systematic error of LD on QCA in Absorb was –0.092 mm (relative difference –3.3%) with a random error of 0.473 mm, whereas in XIENCE the systematic error was –0.018 mm (–0.5%) with a random error of 0.477 mm. These OCT-QCA discrepancies did not differ between Absorb and XIENCE (p=0.275) at two-year follow-up. QCA tended to underestimate LLL more in Absorb than in XIENCE (QCA-LLL minus OCT-LLL: –0.180±0.308 mm vs. –0.058±0.322 mm, p=0.058) at two-year follow-up, although this comparison was not statistically powered.
Conclusions: The two-year dimensional measurements on QCA had minor and insignificant systematic errors between both devices. A discrepancy between QCA-LLL and OCT-LLL would raise a question as to whether this parameter is appropriate for the comparative assessment of device performance. ClinicalTrials.gov number: NCT01844284
Abbreviations
LD: lumen diameter
LLL: late lumen loss
MLD: minimum lumen diameter
OCT: optical coherence tomography
QCA: quantitative coronary angiography
Introduction
The difference in radiopacity of polymer and metal theoretically influences the edge detection method of quantitative coronary angiography (QCA) for the assessment of luminal dimensions of vessels treated with either radiolucent polymeric bioresorbable scaffolds or radiopaque metallic stents1. In the ABSORB Japan randomised trial, when compared to optical coherence tomography (OCT) as the gold standard for the measurement of luminal dimensions2, QCA underestimated lumen diameter (LD) by 9.1%, 4.9%, and 9.8% in the non-stented/non-scaffolded, stented, and scaffolded segments, respectively3. This fact would have a significant impact on the QCA assessment of post-procedural LD and acute gain. The study would imply the unfairness of the assessment for the Absorb™ bioresorbable scaffold compared to the XIENCE Prime™/Xpedition cobalt-chromium everolimus-eluting stent (both Abbott Vascular, Santa Clara, CA, USA), which raised the question as to whether the commonly used acute gain and late loss analysis by QCA for the comparison of Absorb with XIENCE is appropriate and accurate.
Angiographic late lumen loss (LLL), a reduction of minimum lumen diameter (MLD) from baseline to follow-up, would also theoretically be influenced by the differential accuracy of QCA analysis in Absorb and XIENCE. At baseline, not only the radiopacity of the struts but also the strut protrusion distance had an impact on the accuracy of the QCA assessments3. More protruded struts hinder laminar blood flow, resulting in a larger underestimation of LD by QCA4-7. However, at longer follow-up, protruded struts are fully covered and embedded into the vessel wall the surface of which becomes smooth8. Therefore, the accuracy of QCA with reference to OCT could also theoretically change at follow-up assessments. However, this has not yet been assessed.
The present study aimed to assess the accuracy of QCA with reference to OCT in Absorb and XIENCE at two-year follow-up in the ABSORB Japan randomised controlled trial and to evaluate the applicability of angiographic LLL as a parameter of device performance at long-term follow-up.
Methods
STUDY DESIGN
ABSORB Japan was a prospective, multicentre, randomised, single-blind, active-controlled clinical trial in which 400 patients undergoing coronary stent implantation in Japan were randomised in a 2:1 ratio to treatment with the Absorb everolimus-eluting bioresorbable scaffold or the XIENCE Prime/Xpedition cobalt-chromium everolimus-eluting stent9. The details of the trial have been described elsewhere9. A total of 38 investigational sites in Japan participated in the study. The study was conducted according to the Declaration of Helsinki. Prior to initiating the study, the institutional review board at each investigational site approved the clinical trial protocol. All patients provided written informed consent before enrolment.
Patients were randomised in a 2:1 ratio to Absorb vs. XIENCE using a central randomisation service. Randomisation was stratified by the presence of diabetes mellitus and the number of lesions to be treated. Patients were allocated randomly to one of the three intravascular imaging subgroups: intravascular ultrasound group (150 patients), optical coherence tomography (OCT) group 1 (125 patients), or OCT group 2 (125 patients), based on the schedules of intravascular imaging. In the present investigation, we analysed baseline and two-year follow-up data of OCT and QCA from OCT group 19.
QUANTITATIVE CORONARY ANGIOGRAPHY
An angiographic core laboratory (Beth Israel Deaconess Medical Center, Boston, MA, USA) performed QCA analysis (QAngio XA 7.3; Medis medical imaging systems, Leiden, the Netherlands) at baseline and two-year follow-up. It was not possible to blind the analysts to the device type based on the characteristic appearance of Absorb and XIENCE stent struts. The core lab computed reference vessel diameter, MLD, and diameter stenosis10. Angiographic LLL was computed as a change of MLD from baseline to follow-up (conventional angiographic LLL in Figure 1)8,9. In addition to the core lab analysis of QCA, we performed QCA by the edge detection method to evaluate the accuracy of luminal dimension assessment on QCA with reference to OCT at a co-localised position. The QCA analysis was performed according to standard procedures, using single projections with minimum foreshortening of the target lesion by the CAAS system, version 5.11 (Pie Medical BV, Maastricht, the Netherlands). We analysed the matched projections for baseline and two-year follow-up analyses. For each stented/scaffolded lesion, LDs at both device edges were analysed. The small radiopaque markers at the ends of the polymeric scaffolds and the radiopaque struts of the metallic stents helped us to localise the in-device segment. Details are described in the following paragraph. We employed this methodology in the previous study3, and the same methodology was applied in the two-year follow-up QCA analysis.
OPTICAL COHERENCE TOMOGRAPHY
OCT pullbacks were obtained at baseline after the stent or scaffold implantation and at two-year follow-up coronary imaging by a frequency-domain C7 system using a Dragonfly™ catheter (St. Jude Medical, St. Paul, MN, USA), a frequency-domain ILUMIEN™ OPTIS™ system using a Dragonfly™ Duo catheter (St. Jude Medical), or an optical frequency-domain imaging (OFDI) Lunawave® console using a FastView® catheter (Terumo Europe, Leuven, Belgium). The OCT measurements were performed with the QIvus software (Medis) by the core laboratory (Cardialysis, Rotterdam, the Netherlands). With adjustment for the pullback speed, the analysis of continuous cross-sections was performed at each 1 mm longitudinal interval within the treated segment. MLD was computed based on a circular model at post-device implantation and two-year follow-up8. By analogy with QCA, OCT-LLL was defined as a change of MLD from post-device implantation to two-year follow-up (conventional OCT-LLL in Figure 1). In the present study, we additionally evaluated the cross-sections with metallic markers in Absorb scaffolds and both edge cross-sections in XIENCE stents for the co-localising analysis with QCA3. The following parameters were evaluated: MLD, mean and minimum flow area, mean and minimum (abluminal) scaffold/stent area, and neointimal area11. In matched cross-section analysis at the edges of both devices, LD based on a circular model and mean neointimal thickness were computed12,13.
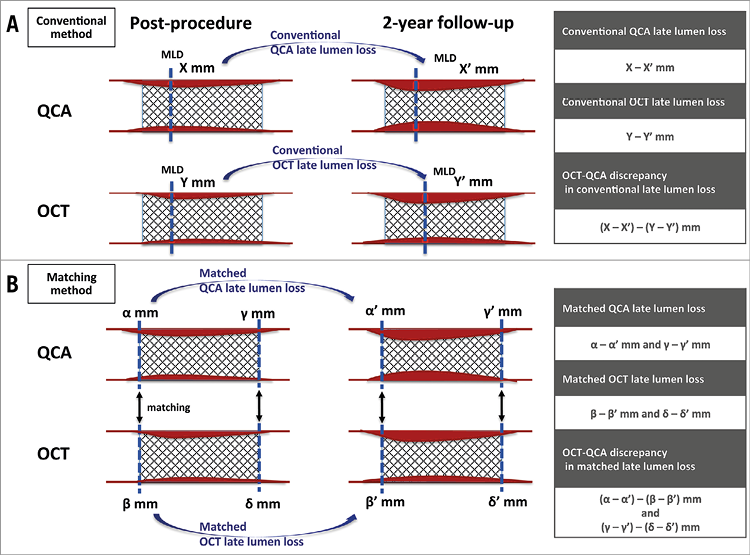
Figure 1. Late lumen loss assessment. A) Conventional late lumen loss assessment. B) Late lumen loss at the edges of both devices (matched late lumen loss). MLD: minimum lumen diameter; OCT: optical coherence tomography; QCA: quantitative coronary angiography
COMPARISON OF QCA AND OCT
In-device MLD and LLL on QCA and OCT at two-year follow-up (conventional LLL) were compared using Bland-Altman analysis. In addition, the LD discrepancies between QCA and OCT were compared in matched cross-sections at the edges of both devices (Figure 2). For matching of an OCT cross-section with a corresponding QCA cross-section, the following criteria were used in this study. For a scaffolded segment, OCT cross-sections with proximal and distal metallic markers were matched with corresponding QCA diameters which were measured at the sites of the radiopaque metallic markers of the polymeric device. For a stented segment, OCT cross-sections at both stent edges were matched with the corresponding QCA diameters which were measured at the site of radiopaque strut edges. The identification of the stent edges on OCT was defined as the cross-section in which stent struts were visualised circumferentially for the first and last time (first distal edge and last proximal edge). Bifurcation segments in which the side branch occupied more than 45° of the cross-section were excluded in order to avoid contour interpolation when quantifying the lumen14. Whenever the metallic marker of Absorb could not be identified due to the wire shadow artefact or insufficient flush of blood, cross-sections were not included in the analysis.
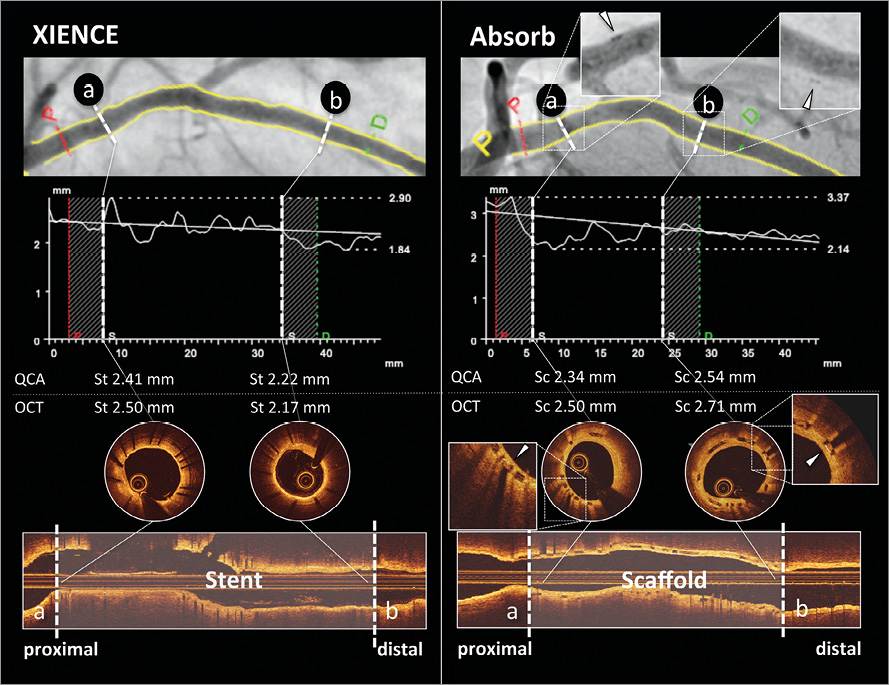
Figure 2. Case examples for matched cross-sections of the edges of both devices. Case examples of XIENCE (left panel) and Absorb (right panel) are presented. Proximal (a) and distal (b) edge cross-sections of the stented/scaffolded vessel on QCA (upper panel) were matched with those on OCT (lower panel). Absorb scaffold edges were identified by the metallic markers. White arrowhead in each magnified view in the right panel indicates a metallic marker of Absorb. OCT: optical coherence tomography; QCA: quantitative coronary angiography; Sc: scaffolded cross-section; St: stented cross-section
Based on the LD in matched cross-sections of the edges of both devices post implantation and at two-year follow-up, we computed “matched” LLL, as shown in Figure 1. The discrepancy between angiographically matched LLL and OCT matched LLL was also assessed for each device type using Bland-Altman analysis.
STATISTICAL ANALYSIS
Data are expressed as mean±standard deviation (95% confidence interval [CI]) or number (percentage). Categorical variables were compared using the Pearson’s chi-square test or Fisher’s exact test, as appropriate. Group means for continuous variables with normal and non-normal distributions were compared using a generalised linear mixed model. The model was employed to take into account the clustered nature of >1 stent/scaffold and >1 cross-section analysed from the same patients, which might result in unknown correlations among measurements within these stent/scaffold or cross-section clusters. The accuracy of QCA measurements was evaluated with reference to the OCT measurements as gold standard using the Bland-Altman method. Data are given as plots showing the absolute difference between corresponding measurements of both methods (y-axis) against the average of both methods (x-axis). The systematic error (mean difference) and random error (standard deviation) of QCA measurements were computed. The 95% limits of agreement were calculated as mean bias±1.96 standard deviation. The influence of mean neointimal thickness on the LD discrepancy between QCA and OCT was assessed with a generalised linear mixed model to adjust for the device type, clustered nature of >1 cross-section in the same lesion and >1 lesion in the same patient. Statistical significance was assumed at a probability (p) value of <0.05. All statistical analyses were performed with SPSS, Version 24.0.0 (IBM Corp., Armonk, NY, USA).
Results
STUDY POPULATION
The present study evaluated the OCT-1 subgroup of ABSORB Japan comprising 87 lesions from 83 patients in the Absorb arm and 44 lesions from 43 patients in the XIENCE arm. Details of baseline demographics, procedural characteristics, and the study flow chart have been previously reported3,8,15. Both arms were well balanced in terms of risk factors, lesion characteristics, and procedure.
COMPARISON OF TWO-YEAR MLD AND CONVENTIONAL LATE LUMEN LOSS ON QCA AND OCT IN ABSORB AND XIENCE
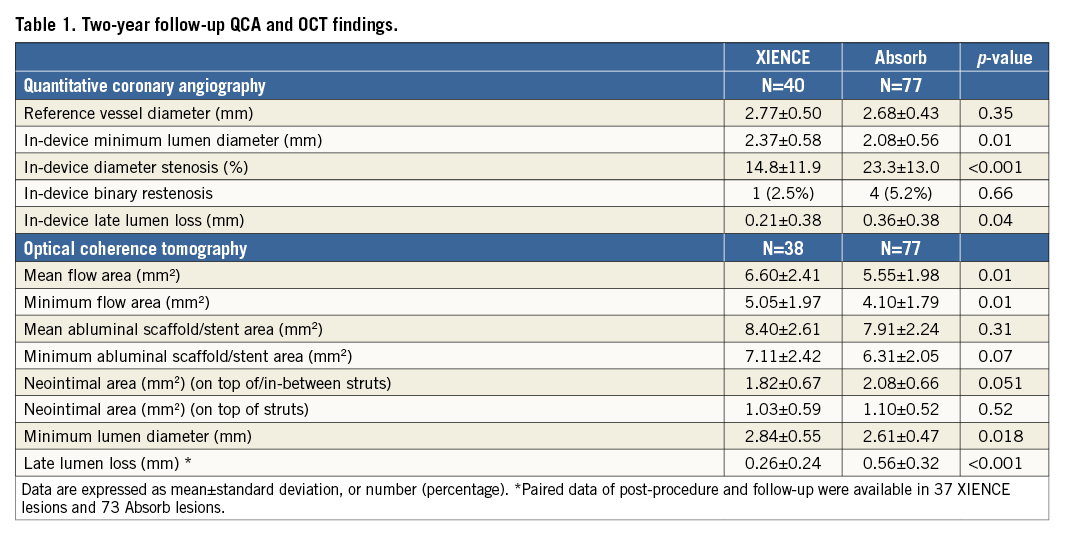
QCA and OCT results are summarised in Table 1. At two-year follow-up, MLD on QCA was 2.08±0.56 mm (n=77) vs. 2.37±0.58 mm (n=40) in Absorb and XIENCE, respectively (p=0.01). Angiographic in-device LLL was significantly higher in the Absorb arm than in the XIENCE arm (0.36±0.38 mm [n=77] vs. 0.21±0.38 mm [n=40], p=0.039). MLD on OCT was 2.61±0.47 mm (n=77) vs. 2.84±0.55 mm (n=38) in Absorb and XIENCE, respectively (p=0.02). OCT-LLL was also higher in the Absorb arm than in the XIENCE arm (0.56±0.32 mm [n=73] vs. 0.26±0.24 mm [n=37], p<0.001).
Paired two-year QCA and OCT MLD data were available in 75 lesions in the Absorb arm and 38 lesions in the XIENCE arm. Measurement agreement between QCA and OCT for MLD is presented as Bland-Altman plots in Figure 3. The systematic error of MLD on QCA with reference to OCT was –0.169 mm (95% CI: –0.226, –0.113) with a random error of 0.251 mm vs. –0.057 mm (95% CI: –0.143, 0.028) with a random error of 0.265 mm in Absorb vs. XIENCE, respectively (p=0.032). At two-year follow-up, QCA underestimated MLD with reference to OCT more severely in the Absorb arm than in the XIENCE arm.
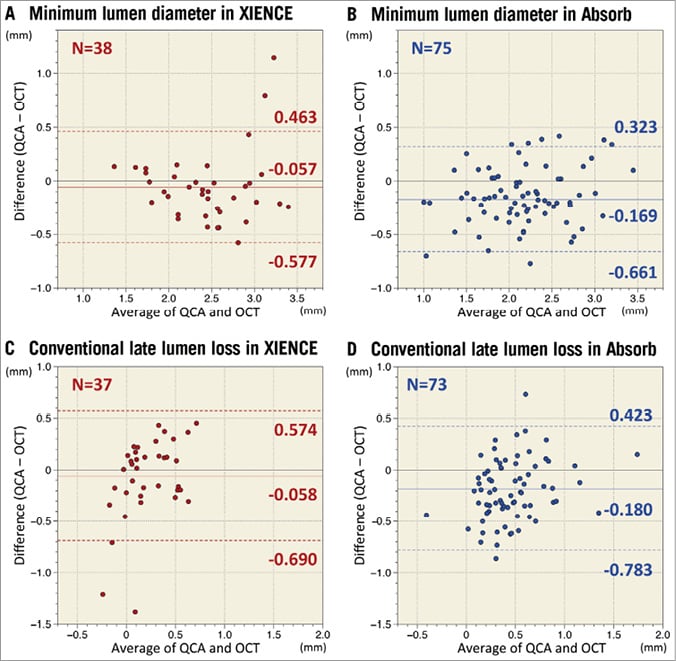
Figure 3. Agreement between QCA and OCT in minimum lumen diameter and conventional late lumen loss assessments. Measurement agreements between QCA and OCT for minimum lumen diameter and conventional late lumen loss are presented as Bland-Altman plots. Solid lines indicate mean difference and dotted lines present limits of agreement (mean±1.96 standard deviation). The systematic error of MLD on QCA with reference to OCT was –0.057 mm (95% CI: –0.143, 0.028) with a random error of 0.265 mm vs. –0.169 mm (95% CI: –0.226, –0.113) with a random error of 0.251 mm in XIENCE (A) vs. Absorb (B), respectively (p=0.032). QCA more severely underestimated MLD than OCT at follow-up. QCA tended to underestimate the late lumen loss more in the Absorb arm (D) than in the XIENCE arm (C) (late lumen loss on QCA minus late lumen loss on OCT: –0.180±0.308 mm [95% CI: –0.251, –0.109] vs. –0.058±0.322 mm [95% CI: –0.163, 0.047], p=0.058). OCT: optical coherence tomography; QCA: quantitative coronary angiography
Paired QCA and OCT conventional LLL data were available in 73 lesions in the Absorb arm and 37 lesions in the XIENCE arm. When compared to the OCT-LLL (Figure 3), QCA tended to underestimate the LLL more in the Absorb arm than in the XIENCE arm (LLL on QCA minus LLL on OCT: –0.180±0.308 mm [95% CI: –0.251, –0.109] vs. –0.058±0.322 mm [95% CI: –0.163, 0.047], p=0.058).
MATCHED CROSS-SECTION ANALYSIS AT THE EDGES OF BOTH DEVICES
A total of 78 cross-sections at a stented segment and 127 cross-sections at a scaffolded segment were evaluated in the matched cross-section analysis. Measurement agreement between LDs on QCA and OCT is indicated by Bland-Altman plots in Figure 4. When compared with OCT, the systematic error of QCA in scaffolded vessels was –0.092 mm (95% CI: –0.174, –0.010) with a random error of 0.473 mm, whereas in the stented vessels the systematic error of QCA was –0.018 mm (95% CI: –0.124, 0.089) with a random error of 0.477 mm. Figure 5 presents the relative difference of LD assessed by QCA as compared to OCT. In the stented segments, QCA presented almost identical LD as compared to OCT (–0.5%), whereas in the scaffolded segments QCA underestimated LD by 3.3%. However, this difference between devices did not reach statistical significance (p=0.275). Mean neointimal thickness presented no significant correlation with LD discrepancy between QCA and OCT (coefficient 0.128 [95% CI: –0.373, 0.629], p=0.615).
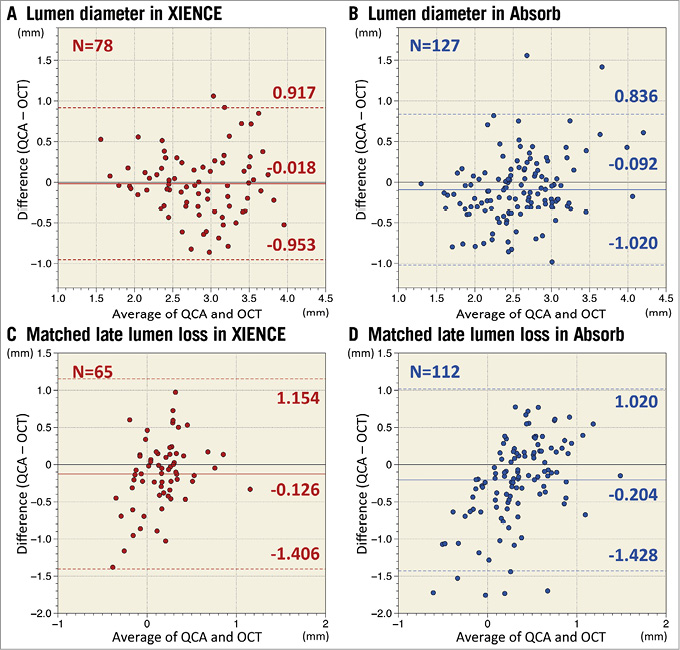
Figure 4. Agreement between QCA and OCT in lumen diameter and late lumen loss at matched edge cross-sections. Measurement agreements between QCA and OCT for lumen diameter and late lumen loss at matched cross-sections of the edges of both devices are presented as Bland-Altman plots. Solid lines indicate mean difference and dotted lines present limits of agreement (mean±1.96 standard deviation). When compared with OCT, the systematic error of QCA in stented vessels was –0.018 mm (95% CI: –0.124, 0.089) with a random error of 0.477 mm (A), whereas in the scaffolded vessels the systematic error was –0.092 mm (95% CI: –0.174, –0.010) with a random error of 0.473 mm (B). The systematic error of matched angiographic late lumen loss in the Absorb (D) was –0.204 mm (95% CI: –0.321, –0.088) with a random error of 0.624 mm, whereas in the XIENCE (C) the systematic error of matched angiographic late lumen loss was –0.126 mm (95% CI: –0.285, 0.034) with a random error of 0.653 mm. These systematic errors did not differ between Absorb and XIENCE (p=0.431). OCT: optical coherence tomography; QCA: quantitative coronary angiography
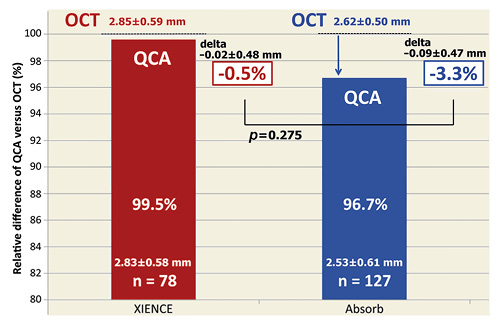
Figure 5. Relative difference of LD assessed by QCA as compared to OCT. In the stented segments, QCA presented almost identical LD as compared to OCT (–0.5%), whereas in the scaffolded segments QCA underestimated LD by 3.3%. However, this difference between devices did not reach statistical significance (p=0.275). LD: lumen diameter; OCT: optical coherence tomography; QCA: quantitative coronary angiography
Measurement agreement of matched LLL at the edges of both devices is presented in Figure 4. The systematic error of matched angiographic LLL with reference to OCT-LLL in the Absorb was –0.204 mm (95% CI: –0.321, –0.088) with a random error of 0.624 mm, whereas in the XIENCE the systematic error was –0.126 mm (95% CI: –0.285, 0.034) with a random error of 0.653 mm. These systematic errors did not differ statistically between Absorb and XIENCE (p=0.431).
Discussion
The main findings of the present study can be summarised as follows. 1) Conventional angiographic in-device LLL at two-year follow-up was significantly higher in the Absorb arm than in the XIENCE arm. 2) Conventional OCT-LLL was larger than conventional angiographic LLL in both arms. 3) At follow-up, QCA presented, in the stented vessels, an almost identical lumen diameter as compared to OCT measurements (–0.5%), while in the scaffolded vessels QCA underestimated the OCT luminal dimension by 3.3% on average, although this difference did not reach statistical significance. 4) QCA tended to underestimate the two-year conventional LLL more severely in the Absorb arm than in the XIENCE arm.
ACCURACY OF QCA MEASUREMENT AT FOLLOW-UP IN STENTED AND SCAFFOLDED VESSELS
QCA is known to underestimate the lumen dimension systematically compared to OCT in non-stented/non-scaffolded vessels16. Excellent accuracy of OCT measurements was previously reported in a phantom study. The systematic error was –0.03 mm, while the random error was 0.02 mm, allowing us to take OCT measurements as the gold standard17. Our previous study demonstrated that, immediately after stent/scaffold implantation, QCA underestimated LD by 4.9% and 9.8% in the stented and scaffolded vessels (p=0.020), respectively3. There was a positive correlation between the OCT-QCA LD discrepancy and stent/scaffold protrusion distance. A flow dynamics simulation model demonstrated more disturbed laminar blood flow due to more protruded struts in the scaffolded vessel than in the stented vessel3,18. At two-year follow-up, this flow disturbance would theoretically have disappeared due to the smooth surface of the stented/scaffolded lumen as a result of complete neointimal coverage. Therefore, we expected that the difference at baseline would have diminished at follow-up.
In the present study, MLD was more severely underestimated by QCA in the Absorb arm than in the XIENCE arm. However, the location of the MLD in QCA and OCT could not coincide perfectly due to foreshortening of the vessel in QCA. Therefore, we performed the matched cross-section analysis at the edges of each device. In the matched cross-section analysis, two-year QCA presented almost identical measurements to OCT in stented vessels, while in the scaffolded vessels it underestimated LD by 3.3%. However, this difference did not reach statistical significance, suggesting that QCA would measure the LD comparably in both stented and scaffolded vessels at follow-up as we expected. A blooming artefact of radiopaque metallic struts could play a role in the almost identical measurement of QCA as compared to OCT19, although the influence of the radiopacity of cobalt-chromium might theoretically be limited.
LATE LUMEN LOSS ASSESSMENT
Previously in the same population, we demonstrated that the angiographic acute gain as a common parameter of acute device performance would be unfair for Absorb polymeric scaffolds as compared to XIENCE metallic stents, since QCA more severely underestimated lumen dimension immediately after device implantation in the Absorb arm than in the XIENCE arm3. The present study also demonstrated the unfairness in the assessment of LLL, not for Absorb but for XIENCE. Immediately after device implantation, QCA underestimated LD more severely in the Absorb arm than in the XIENCE arm (p=0.020), whereas at two-year follow-up the difference between Absorb and XIENCE diminished (p=0.275). As a result, angiographic LLL of Absorb would be incorrectly underestimated when compared to XIENCE. In the present study, conventional angiographic LLL of Absorb tended to be more severely underestimated than that of XIENCE with reference to conventional OCT-LLL. When we evaluated the matched LLL, the difference between the devices did not reach statistical significance. This is presumably due to inadequate statistical power. Higher standard deviation in matched LLL (approximately 0.6 mm) than that of conventional LLL (approximately 0.3 mm) requires a larger sample size to have adequate power. The unpowered analysis cannot allow any robust conclusions to be drawn. However, the current results raise the question as to whether we should use this parameter for the evaluation of device performance, especially when comparing polymeric and metallic devices.
The clinical value of conventional angiographic LLL measurements has been broadly reported and remains unquestionable. However, as we demonstrated in the current study, accuracy of the conventional LLL remains a matter of debate20. In the ABSORB II (36 months), ABSORB Japan (24 months), ABSORB China (12 months), and TROFI II (six months) trials, angiographic LLL was statistically higher in the Absorb arm than in the XIENCE arm, whereas the EVERBIO II and ABSORB Japan (13 months) trials presented a non-significant difference in angiographic LLL between Absorb and XIENCE8,9,21-24. These non-significant data especially should be interpreted with caution. In future trials comparing bioresorbable scaffolds and metallic stents, another imaging parameter with more accurate methods should be recommended for comparable evaluation of device performance.
Study strengths and limitations
The present study is the largest randomised population evaluated so far with combined OCT and angiographic assessments at two-year follow-up. However, some limitations should be acknowledged. First, although we took OCT as the gold standard, OCT artefacts (obliquity and eccentricity) could influence the LD measurements25. When the catheter is not parallel to the longitudinal axis of the vessel wall or the optical beam is not at a 90° angle with respect to the artery wall, the image may undergo elliptical distortion, making measurements less accurate. Metallic shadow could also influence the luminal measurement, especially in cases with malapposed struts. Second, the extent of biodegradation could also have influenced the current results. We assessed baseline data in the previous study and two-year data in the present study3. However, a different time point could possibly have a different result. Lastly, although this was the largest randomised population with OCT to date, the study could still be underpowered to detect the OCT-QCA discrepancy between Absorb and XIENCE. Therefore, the results should be considered hypothesis-generating and should be interpreted with caution.
Conclusions
The two-year dimensional measurements on QCA had minor and insignificant systematic errors between the devices. A discrepancy between QCA-LLL and OCT-LLL raises the question as to whether the parameter is appropriate for the comparative assessment of device performance. QCA-LLL in previous and future trials comparing Absorb and XIENCE should be interpreted with caution.
| Impact on daily practice In the ABSORB Japan trial, the two-year dimensional measurements on QCA with reference to OCT had minor and insignificant systematic errors between scaffolded (Absorb bioresorbable scaffold) and stented (XIENCE metallic stent) vessels, whereas immediately after device implantation QCA underestimated lumen diameter by 9.8% and 4.9% in the scaffolded and stented vessels, respectively. A discrepancy between angiographic late lumen loss and OCT late lumen loss reported in the present study raises the question as to whether the conventional parameter, angiographic late lumen loss, is appropriate for the comparative assessment of device performance. Angiographic late lumen loss in previous and future trials comparing Absorb and XIENCE should be interpreted with caution. |
Guest Editor
This paper was guest edited by Johan (Hans) H.C. Reiber, PhD, FESC, FACC; Division of Image Processing, Leiden University Medical Center, Leiden, the Netherlands.
Funding
The ABSORB Japan study was funded by Abbott Vascular.
Conflict of interest statement
Y. Sotomi has received speaker honoraria from Abbott Vascular Japan and grants from Fukuda Memorial Foundation for Medical Research and SUNRISE lab. Y. Onuma and P.W. Serruys are members of the Advisory Board for Abbott Vascular. G. Stone reports Columbia University receiving royalties from Abbott Vascular for the sale of the MitraClip. J.J. Popma receives institutional grants from Abbott Vascular. K. Kozuma and K. Tanabe are members of the Advisory Board of Abbott Vascular Japan, and receive honoraria for lectures from Abbott Vascular Japan. T. Kimura is a member of the Advisory Board for Abbott Vascular and receives a research grant. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

