
Abstract
Aims: FANTOM II is a prospective multicentre trial assessing the safety and efficacy of the Fantom sirolimus-eluting bioresorbable coronary scaffold (BRS). The present substudy focuses on the six- and nine-month IVUS findings.
Methods and results: A total of 240 patients with de novo coronary artery lesions presenting with stable or unstable disease were included in two sequential cohorts (cohort A [n=117] and cohort B [n=123]) in which angiographic follow-up was performed at either six or nine months, respectively. Matched IVUS data were available for 35 paired cases in cohort A and 26 paired cases in cohort B. At six months, mean and minimum scaffold area (SA) decreased from 6.09±1.08 mm2 to 5.88±1.07 mm2, p=0.009, and 5.27±0.99 mm2 to 5.05±0.99 mm2, p=0.01, respectively. At nine months, no significant change in mean scaffold and minimum scaffold area was observed (6.46±1.11 mm2 to 6.38±0.96 mm2; p=0.35, and 5.45±1.00 mm2 to 5.36±0.86 mm2; p=0.32, respectively). Neointimal hyperplasia area was low at both six (0.11±0.12 mm2) and nine months (0.20±0.21 mm2), as was in-scaffold obstruction volume (1.94±2.25% at six months, and 3.40±4.11% at nine months).
Conclusions: The use of the Fantom BRS in stable coronary artery disease was associated with low rates of neointimal hyperplasia volume and in-scaffold volume obstruction at both six and nine months.
Abbreviations
BRS: bioresorbable scaffold
BVS: bioresorbable vascular scaffold
QCA: quantitative coronary analysis
DAT: desaminotyrosine
DES: drug-eluting metallic stents
EEM: external elastic membrane
IVUS: intravascular ultrasound
LA: lumen area
LLL: late lumen loss
MLD: minimal lumen diameter
MSA: minimal scaffold area
OCT: optical coherence tomography
RVD: reference vessel diameter
SA: scaffold area
VA: vessel area
Introduction
Bioresorbable scaffolds (BRS) were developed to address the problems associated with the use of permanent drug-eluting metallic stents (DES), such as vascular inflammation, neoatherosclerosis, thrombosis, jailing of side branches and impairment of future surgical revascularisation options1,2. Up until now, multiple types of BRS have been studied for their in vivo performance with varying degrees of success2-6.
The Fantom BRS (REVA Medical, San Diego, CA, USA) is a desaminotyrosine (DAT)-derived polycarbonate sirolimus-eluting BRS with improved radiopacity and a strut thickness of around 125 microns. The device is made primarily from iodinated polycarbonate copolymer of tyrosine analogues (DAT) and biocompatible hydroxyl esters7-9. Due to the iodine atoms, the scaffold has a similar radiopacity to cobalt-chromium DES, precluding the need for additional tantalum or platinum radiopaque markers10. The polycarbonate is degraded by hydrolysis in I2DAT, CO2 and water. This initial degradation phase is followed by a resorption process that lasts four to five years.
The device was first assessed in the FANTOM I pilot study, followed by the FANTOM II study, in the which the use of the scaffold was associated with a major adverse cardiac event rate of 2.6% along with a late lumen loss of 0.25±0.40 mm in 117 patients included in cohort A with six-month follow-up10. The present report contains the intravascular ultrasound (IVUS) findings of patients enrolled in the FANTOM II study at baseline and either six or nine months.
Methods
FANTOM II is a non-randomised prospective multicentre trial, which enrolled patients at 35 sites in Australia, Belgium, Brazil, Denmark, France, Germany, the Netherlands and Poland, assessing the safety and efficacy of the Fantom BRS10. In brief, the study enrolled patients with stable or unstable angina and single de novo coronary artery lesions with an average reference vessel diameter of between 2.5 mm and 3.5 mm and an estimated lesion length of less than 20 mm. The use of intravascular imaging, including either IVUS and/or optical coherence tomography (OCT), was optional though encouraged at baseline, and mandatory at follow-up when it had been performed at baseline. A total of 240 patients were enrolled in two cohorts (cohort A with six- and 24-month angiographic follow-up, and cohort B with nine- and 48-month angiographic follow-up).
The present study reports the baseline and follow-up (six and nine months) IVUS findings of patients enrolled in cohort A and cohort B, respectively. Only paired analyses were assessed, resulting in 35 paired cases in cohort A and 26 cases in cohort B (Figure 1).
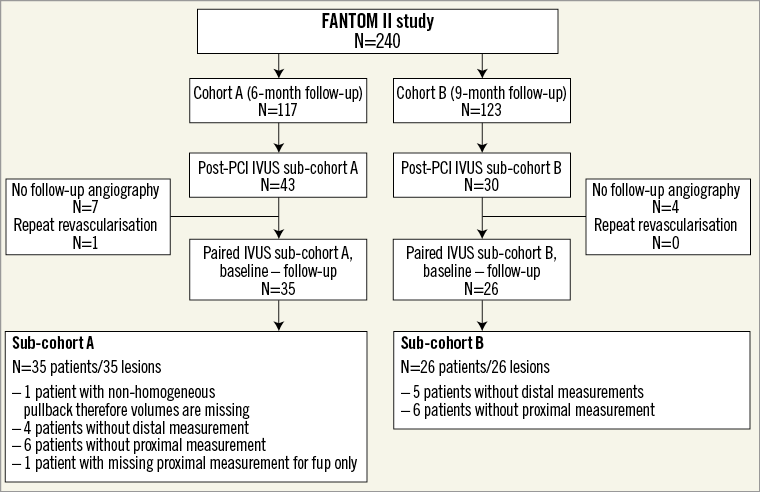
Figure 1. FANTOM II inclusion flow chart. fup: follow-up
QCA ANALYSES
Procedural and follow-up angiograms were assessed at an independent angiographic core laboratory (Yale Cardiovascular Research Group). In-scaffold late lumen loss (LLL) at six- and nine-month follow-up, as assessed by quantitative coronary angiography (QCA), was defined as the difference between the post-procedural minimal lumen diameter (MLD) and the MLD at follow-up. In-scaffold acute recoil was defined as (A-B)/A. A was the mean diameter of the stent delivery balloon at the highest pressure or, in case post-dilatation was used, the mean diameter of the post-dilatation balloon at the highest pressure. B was the mean post-procedural luminal diameter.
IVUS ANALYSES
In 10 of the 35 sites, post-procedural IVUS pullbacks were performed. Motorised IVUS pullbacks were performed after an intracoronary bolus of 200 µg nitroglycerine at 40 MHz (Boston Scientific, Marlborough, MA, USA, or Infraredx, Burlington, MA, USA) with a pullback speed of 0.5 mm/sec. The catheter was positioned distal to the stented segment, at least 10 mm from the distal stent edge. The automated pullback acquired footage from the distal reference segment to at least 10 mm proximal to the proximal scaffold edge. At follow-up, IVUS pullback was repeated in the same coronary segment, which was matched with post-procedural IVUS pullback using the fiducial anatomical landmarks. In case of a required target lesion revascularisation (TLR), preprocedural IVUS acquisitions were used for follow-up analyses.
All IVUS pullbacks were analysed by an independent core lab (Cardialysis BV, Rotterdam, the Netherlands). The region of interest beginning 5 mm distal to and extending 5 mm proximal to the treated segment was examined and analysed11. Three contours were delineated on IVUS: the endoluminal contour (lumen area [LA]), the leading edge of the struts (scaffold area [SA]) and the external elastic membrane (EEM) area (vessel area [VA]). Accordingly, four areas were quantified and assessed: the luminal area, the neointimal area between the lumen and the scaffold contours (= SA–LA), the plaque behind the struts area (= VA–SA) and the vessel area. The total plaque area was defined as: VA–LA 12. Incomplete apposition was defined as one or more scaffold struts separated from the vessel wall. An illustration of angiographic and IVUS footage at baseline, six- and nine-month follow-up is shown in Figure 2. Underexpansion or expansion rate was measured according to the MUSIC criteria and was defined as minimal scaffold area (MSA)/mean reference LA *100 13. The manufactured expected expansion rate was defined as MSA/(manufactured radius2π)*100 14. The acute recoil was defined as (maximal balloon diameter on angiography–MSA)/maximal balloon diameter on angiography *100 15.
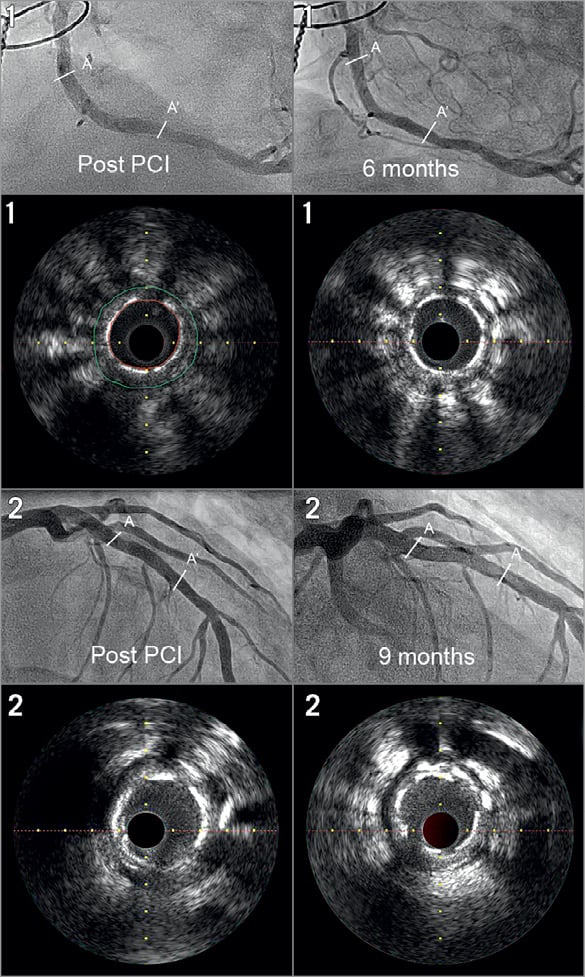
Figure 2. Angiographic and IVUS footage at baseline, six and nine months in two patients. The struts of the Fantom BRS are still clearly visible at six-month (patient 1) and nine-month follow-up (patient 2). A refers to the proximal stent edge while A’ refers to the distal stent edge. The yellow green line in the upper left IVUS still frame indicates the external elastic membrane area. The red line indicates the lumen area.
STATISTICAL ANALYSIS
Statistical analyses were performed using SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA). Categorical variables are expressed as counts and percentages. Differences in categorical variables between allocated cohorts were evaluated by applying chi-square tests, or Fisher’s exact tests. Continuous variables are described as mean±one standard deviation. Differences in continuous variables between allocated treatment groups were evaluated by applying the Student’s t-test. For the main analysis a paired sample t-test was used.
Results
Mean age was 59.7 years, and 75.4% were male. Diabetes was present in 19.7%. Baseline characteristics did not differ between the cohorts (Table 1).
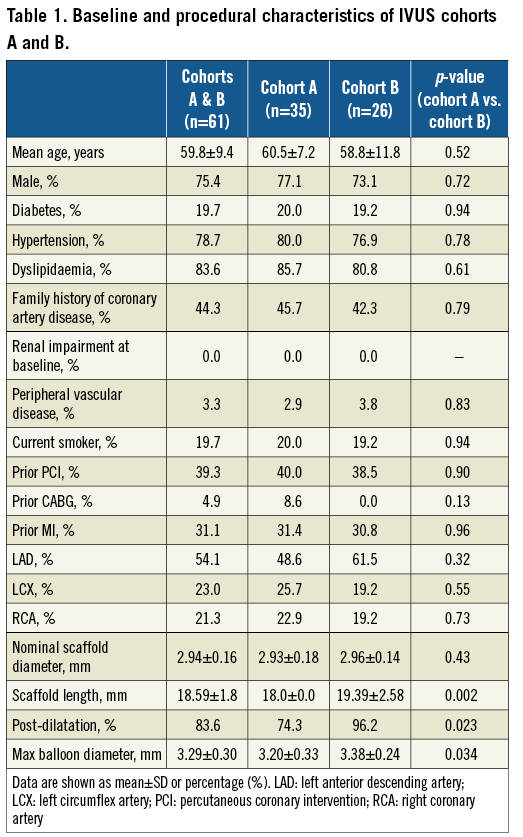
As compared to cohort A, patients included in cohort B had a slightly longer scaffold length (19.4 mm vs. 18.0 mm, p=0.002), more frequently underwent post-dilatation (96.2% vs. 74.3%, p=0.023), and the average maximum balloon diameter was larger (3.38 mm vs. 3.20 mm, p=0.034) (Table 1).
Preprocedural QCA analyses of the entire cohort were available for 238 patients, while angiographic follow-up was available in 100 and 105 patients in cohorts A and B, respectively. Preprocedural reference vessel diameter (RVD) was 2.71±0.37 mm, MLD 0.82±0.31 mm, percentage diameter stenosis 69.5±11.0% and acute recoil 4.0±8.3%. In-scaffold mean LLL was 0.25±0.40 mm at six months in cohort A and 0.33±0.36 mm at nine months in cohort B.
IVUS COHORT A (SIX-MONTH FOLLOW-UP)
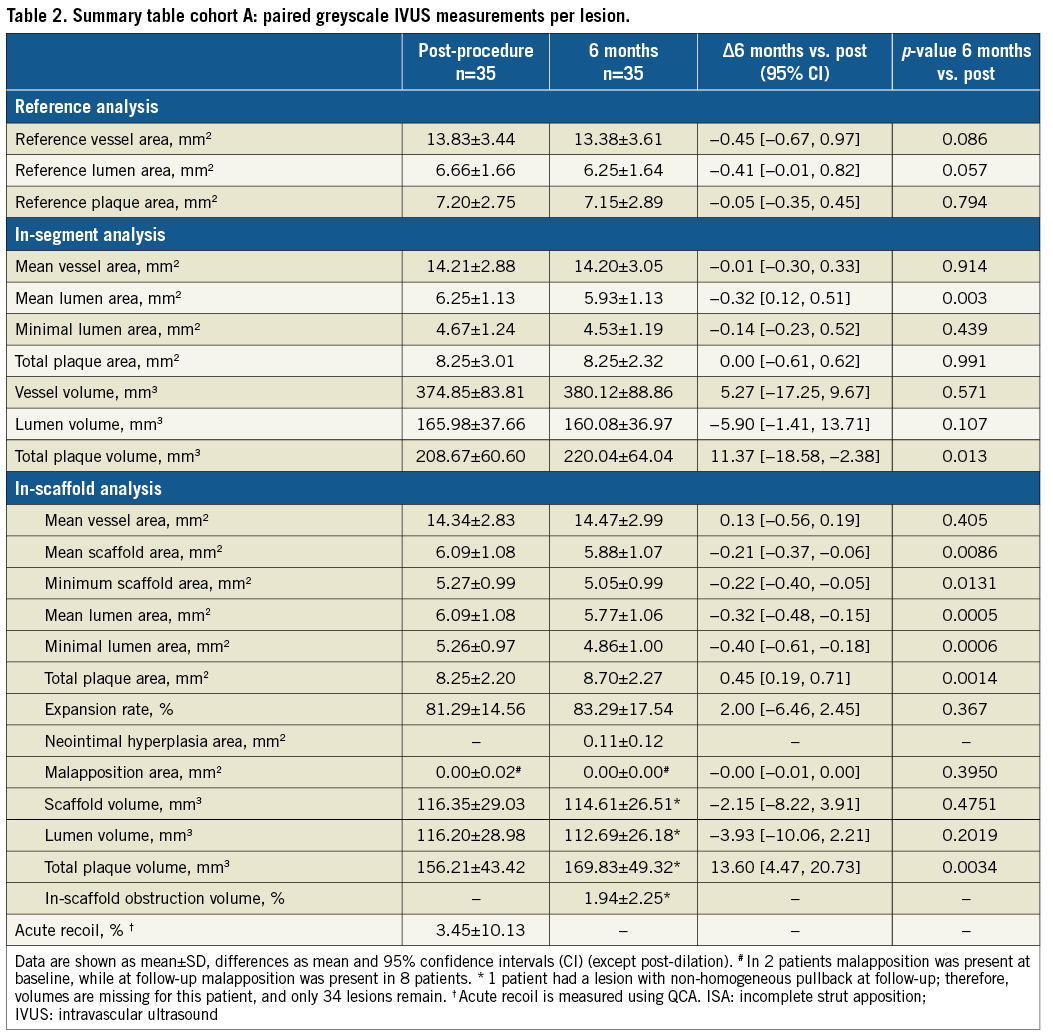
As compared to baseline, vessel area remained unchanged. At six months, mean scaffold area (SA) and MSA slightly decreased as compared to baseline (mean SA baseline: 6.09±1.08 mm2 vs. 5.88±1.07 mm2, p=0.009; baseline MSA: 5.27±0.99 mm2 vs. 5.05±0.99 mm2, p=0.01). Neointimal hyperplasia area was 0.11±0.12 mm2, resulting in an in-scaffold obstruction volume of 1.94±2.25%. Mean and minimum lumen area decreased from baseline to six months by 0.32 mm2 (p=0.005) and 0.40 mm2 (p=0.006), respectively (Table 2).
IVUS COHORT B (NINE-MONTH FOLLOW-UP)
At nine-month follow-up, struts were still visually recognisable on IVUS as highly echogenic material (Figure 2). Mean SA and MSA remained unchanged as compared to baseline (mean SA baseline: 6.46±1.11 mm2 vs. 6.38±0.96 mm2, p=0.35; MSA baseline: 5.45±1.00 mm2 vs. 5.36±0.86 mm2, p=0.32). Neointimal hyperplasia area was 0.20±0.21 mm2, resulting in an in-scaffold obstruction volume of 3.40±4.11%. Mean and minimum lumen area decreased from baseline to nine months by 0.27 mm2 and 0.49 mm2 (p<0.01), respectively (Table 3).
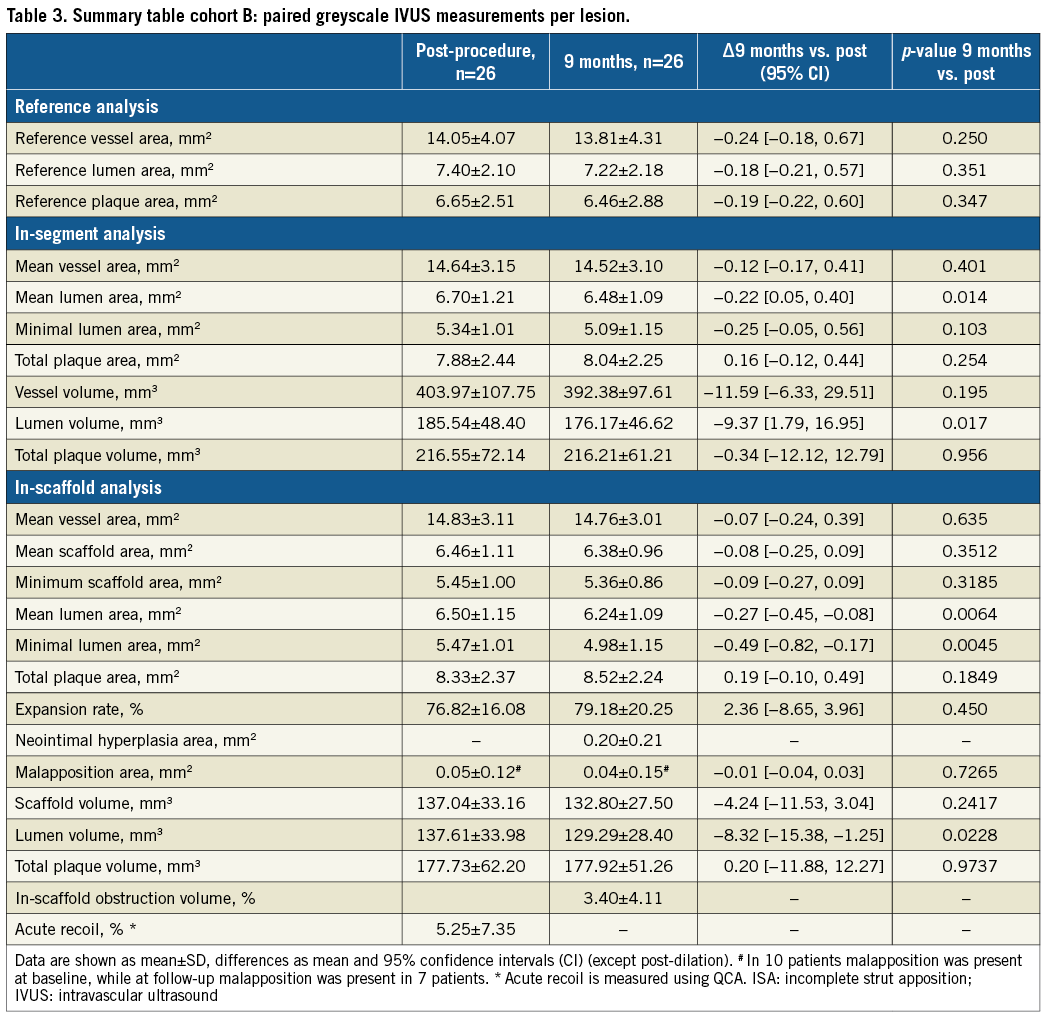
COMPARATIVE IVUS BASELINE FINDINGS OF COHORT A AND COHORT B
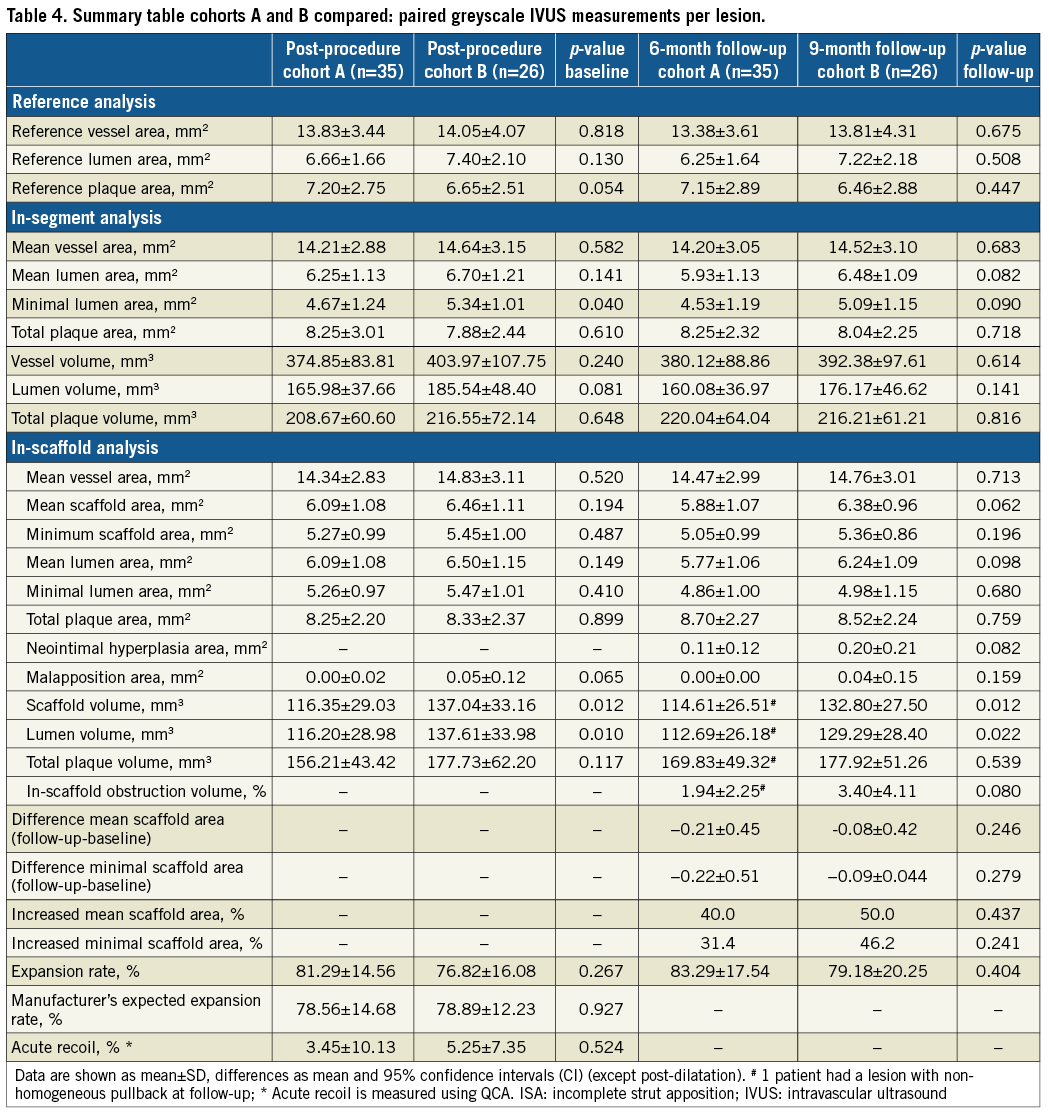
Expansion rates, manufacturer-expected expansion rates and acute recoil did not differ significantly between the cohorts, and expansion rates did not change between baseline and follow-up in either cohort. However, as compared to cohort B, a lower in-segment MLA at baseline was found in cohort A, a discrepancy which was no longer visible at follow-up (six and nine months compared) (Table 4).
Discussion
The present IVUS substudy of the FANTOM II study confirms the efficacy of the Fantom BRS in patients with stable coronary artery disease by successfully inhibiting neointimal hyperplasia at six and nine months as assessed by IVUS. With a backbone that is designed to be absorbed within four to five years, our results show obstruction volumes of 1.9% and 3.4% at six and nine months, respectively, strengthening the recently published clinical and angiographic findings with late loss of 0.25 mm10. Although the FANTOM II study only represents the performance of the scaffold in highly selected cases, the obstruction volumes found are comparable to contemporary DES such as the Resolute Onyx™ (Medtronic, Minneapolis, MN, USA) with reported obstruction volumes of 6.9% at eight-month follow-up16.
A slight decrease in mean SA and MSA was observed in cohort A (0.21 mm2 [3.45%] and 0.22 mm2 [4.17%], respectively) at six months, which was not seen in cohort B at nine months where mean SA and MSA remained unchanged. Although the apparent decrease in mean SA on IVUS in cohort A was merely 3.45% and expansion rates did not differ significantly between cohorts, post-dilatation was more often performed in cohort B (p=0.023) and larger balloon diameters were used for post-dilatation (p=0.034), resulting in a 0.18 mm2 larger MSA and 0.37 mm2 mean SA at baseline in cohort B as compared to cohort A (despite identical mean labelled nominal scaffold diameters). The latter supports the use of aggressive post-dilatation with high-pressure balloons17.
Irrespective of the minor differences between the cohorts in the present study, the performance of the Fantom BRS appeared comparable to earlier published findings on the Absorb™ BVS (Abbott Vascular, Santa Clara, CA, USA) and superior to the data on the Dreams 2G (Biotronik, Bülach, Switzerland)4,6. Following BVS implantation, both mean SA and minimal SA decreased over a period of six months after implantation (MSA: −0.27 mm2 [4.9%] and mean SA −0.14 mm2 [2.1%]), while, following implantation of the Dreams 2G, MSA decreased by 0.79 mm2 (14.6%) (mean SA remained unchanged at 0.03 mm2 [0.05%])4. Interestingly, in the 12-month results of the Absorb BVS, both MSA and minimum SA were back at baseline levels (−0.04 mm2 [0.08%] and +0.04 mm2 [0.06%], respectively) and, at three years, mean SA even increased further by 0.65 mm2 (10.1%) as compared to baseline, whereas the MSA remained unchanged12. A similar late increase in mean SA was seen 12 months after implantation of the DESolve® BRS (Elixir Medical Corporation, Sunnyvale, CA, USA) in which mean SA increased by 0.93 mm2 (15.7%)18. Conversely, this late restoration of scaffold dimensions was not seen 12 months after implantation of the magnesium Dreams 2G BRS in which a persistent decrease in minimal SA (−0.93 mm2 [16.1%]) and mean SA (−0.34 mm2 [5.2%] [p=ns]) as assessed by IVUS was found5,19. Unfortunately, in the latter studies, no information on the aggressiveness of post-dilatation and/or post-dilatation balloon diameters was provided. Prior in vitro research demonstrated 60-70% molecular weight loss after six to nine months post implantation. In the present study, on IVUS, scaffold struts were still well visible at both six- and nine-month follow-up without any apparent change in strut echogenicity (Figure 2). Although the latter might be due to the typical character of the materials, longer-term follow-up is needed to confirm the integrity of the Fantom scaffold at two and four years.
Limitations
Several limitations need to be mentioned. First, the results of the present study should be considered to be applicable to a highly selected patient population with stable or unstable coronary artery disease and non-complex coronary artery lesions. The external validity might not be as strong due to the strict inclusion and exclusion criteria of the study as well as the fact that not all participating sites included patients for the IVUS analysis. Second, although the use of intravascular imaging was encouraged, there was no predefined number of IVUS cases, resulting in a relatively small number of IVUS cases with matched baseline and follow-up imaging. Third, the matching of post-PCI and follow-up IVUS frames is prone to error, and we cannot ascertain whether discrepancies might have arisen. Finally, we hypothesised that the non-significant difference in baseline mean scaffold area and MSA in the subgroup of patients with baseline and follow-up IVUS was driven by more aggressive post-dilatation in cohort B. However, in the total Fantom population, no difference in post-dilatation strategy was found between the cohorts. The latter might suggest a play of chance. In addition, the comparison between follow-up dimensions (Table 4) between cohorts should be interpreted with caution, given the differences in follow-up duration.
Conclusions
The use of the Fantom BRS in stable coronary artery disease was effective with low rates of neointimal hyperplasia volume and in-scaffold volume obstruction at both six and nine months, as assessed by IVUS.
| Impact on daily practice The present findings strengthen and extend the recently published main clinical safety and efficacy data on the use of the Fantom sirolimus-eluting bioresorbable coronary scaffold in patients with stable coronary artery disease at six- and nine-month follow-up. The use of the Fantom BRS was associated with in-scaffold obstruction volumes of 3.40% at nine months, comparable to contemporary DES. These results support the safe use of the Fantom BRS with aggressive post-dilatation in daily practice in appropriate patients. Longer-term clinical and invasive imaging data are needed to confirm the current findings. |
Conflict of interest statement
L. van Zandvoort and J. Daemen have received an institutional grant from ACIST Medical. Ł. Kołtowski has received research grants and speaker fees from REVA Medical and speaker fees from Abbott. J. Kochman has received research grants and speaker fees from REVA Medical and speaker fees from Abbott. The other authors have no conflicts of interest to declare.

