
Abstract
A virtual aortic annular plane that is built using the three hinge points, known as the hinge point-based annular plane (HPAP), is routinely used during transcatheter aortic valve replacement (TAVR). Abnormal aortic cusps (AAC) with unequal length and size influence the relationship of the HPAP to the aortic root axis significantly and pose challenges to valve deployment, leading to paravalvular leak and valve embolisation. Obtaining a centreline-based aortic annular plane in addition may help to understand valve deployment behaviour in AAC better.
Introduction
Understanding the orientation of the aortic annular plane in relation to the body axis aids in optimal placement in transcatheter aortic valve replacement (TAVR) and helps to avoid complications such as paravalvular leak (PVL) and valve embolisation. The hinge point-based annular plane (HPAP), the transverse plane that connects the most basal attachments of the leaflets (Figure 1), forms the basis for optimal angiographic angulation during TAVR1. Abnormal aortic cusps (AAC), such as unequal cusps with varying cephalocaudal length and size, may have implications on valve deployment when using HPAP, as described below2.
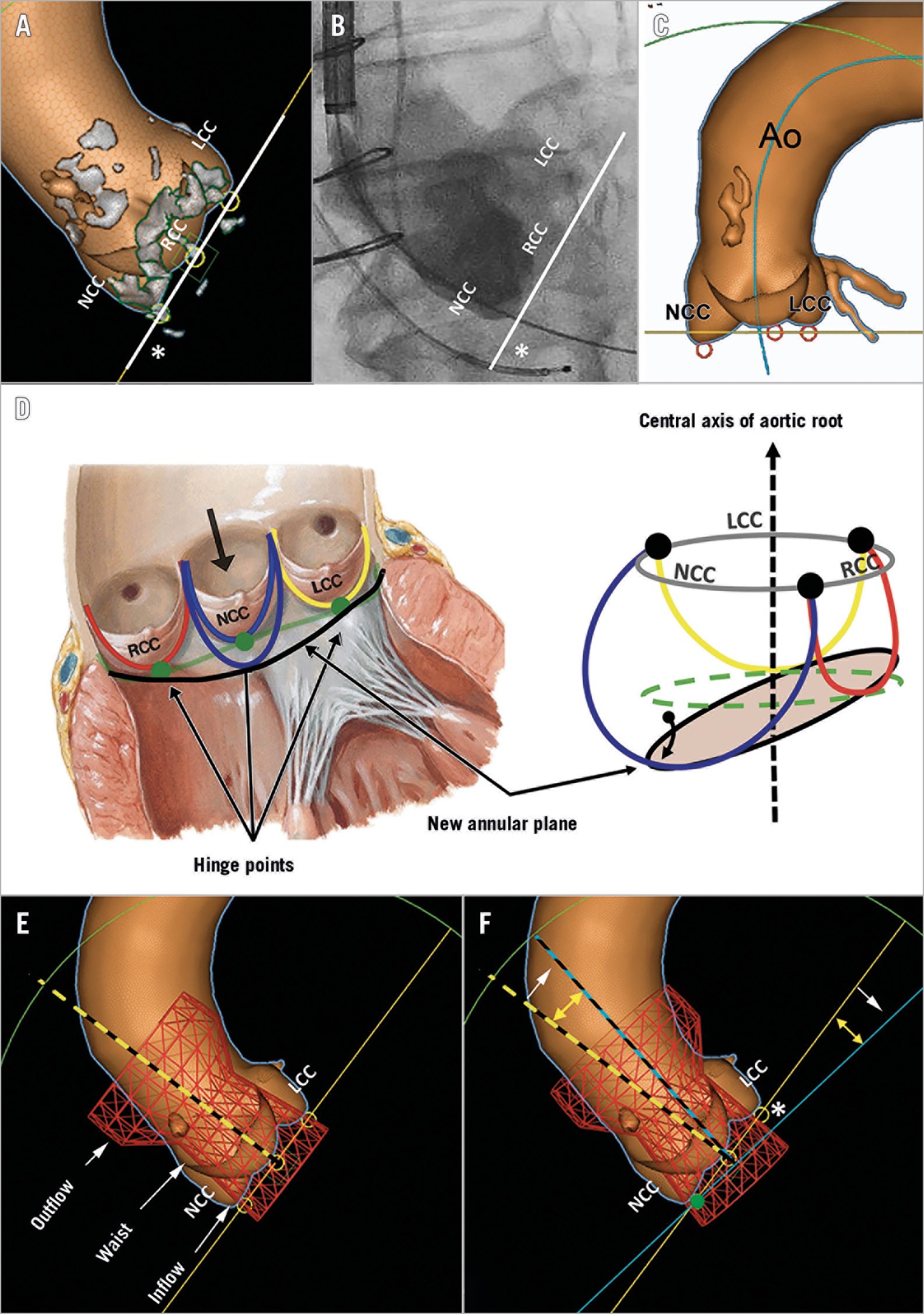
Figure 1. Hinge point-based aortic annular plane in abnormal aortic cusps. The hinge point-based annular plane (HPAP) connects all three hinge points (yellow circles) of the aortic cusps (A). The HPAP can be replicated in the cath lab by aortic root angiography (B). A 3D computed tomography reconstruction shows a large NCC that is more caudal than the other two cusps (C). The aortic valve leaflets are attached throughout the length of the aortic root forming semilunar aortic cusps (D). The basal hinge points (green dots) of the cusps form a virtual ring (green line) that separates the left ventricular outflow tract and the aortic root. Due to a large NCC, the new virtual annular ring (black line) is slanted and is not perpendicular to the central axis of the aortic root. For the deployment of the Evolut R valve, the HPAP (yellow line) is built based on the lowest nadir points of the aortic cusps (E). The long axis of the simulated valve (dashed yellow line) seen perpendicular to the HPAP does not fit in the ascending aorta. Hence, on release, the valve tends to “self-orient” towards the centreline of the ascending aorta (blue dashed line) (F). Due to this rotation, the contralateral side of the deformed aortic cusp has a relatively “lower” valve implantation depth. Ao: ascending aorta; LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp. Panel D is modified from Figure 1 in Kasel et al4. With permission.
Methods
HINGE POINT-BASED VIRTUAL AORTIC ANNULAR PLANE IN AAC
While the assumption that the HPAP is perpendicular to the central axis of the aortic root is true in most cases, large deformation of the cusps may alter this anatomical relationship. An isolated increase in the cephalocaudal length of the cusps would tilt the HPAP, affecting its perpendicularity to the central axis of the aortic root (Figure 1).
SELF-ORIENTATION OF SELF-EXPANDING TRANSCATHETER AORTIC VALVE
Most often, the long axis of the self-expanding valve (Evolut™ R; Medtronic, Minneapolis, MN, USA) does not fit into the ascending aorta, hence the valve tends to “self-orient” towards the centreline of the ascending aorta on release (Figure 1). In AAC, the contralateral side of the deformed aortic cusp may have a relatively “lower” valve implantation depth.
Results
IMPLICATION OF AAC ON PARAVALVULAR LEAK
HPAP was used in a patient (Supplementary Figure 1) with a large non-coronary cusp (NCC). The transcatheter heart valve (THV) was deployed, starting from the NCC to the left coronary cusp (LCC), that self-oriented to the central axis of the aortic root. However, a transthoracic echocardiogram (TTE) showed severe PVL at the junction of the right coronary cusp (RCC) and the LCC. The 3D computed tomography angiography (CTA) reconstruction with virtual THV simulation showed that the placement of the THV was at a relatively deeper position congruous to a low deployment, at the junction of the RCC and the LCC. The tapered waist section that has lesser radial force conformed to the annulus at this junction, resulting in a large PVL. The location of the predicted PVL coincided with that seen on the TTE, supporting this hypothesis. Hence, the presence of an AAC posed a challenge in identifying an ideal annular plane and increased the risk of PVL.
IMPLICATION OF AAC ON VALVE DEPLOYMENT
In a similar patient (Supplementary Figure 2) with a large NCC, THV deployment was attempted in the HPAP. The starting depth at the NCC was adjusted to aim for a high implant. As the THV was unsheathed and released, there was a tilt of the outflow portion of the valve towards the lesser curvature of the aorta, leading to embolisation of the valve from the abnormal NCC. A second THV was deployed using the centreline-based annular plane (CAP) that was overlaid on fluoroscopy. The CAP predicted the expected behaviour of the valve better. The final axis of the THV was perpendicular to the CAP. Hence, there is a possibility of underestimating the tilt of the THV, potentially leading to valve embolisation, with the use of the HPAP in AAC.
Discussion
The limitations of the HPAP in AAC can be mitigated with a CAP approach which can help to predict the final position of the THV better.
CENTRELINE-BASED ANNULAR PLANE APPROACH
A centreline-based approach in coronary CTA, referred to as a “multiplanar reformation (MPR)” display, is used to create a “curved” MPR in long and tortuous coronary arteries and depicts a single image3. A curved MPR can also be used to reconstruct an aortic root to a single two-dimensional image. By adjusting the position of “seeds” on two perpendicular planes, the centreline and CAP which is perpendicular to the centreline can be obtained (Figure 2).
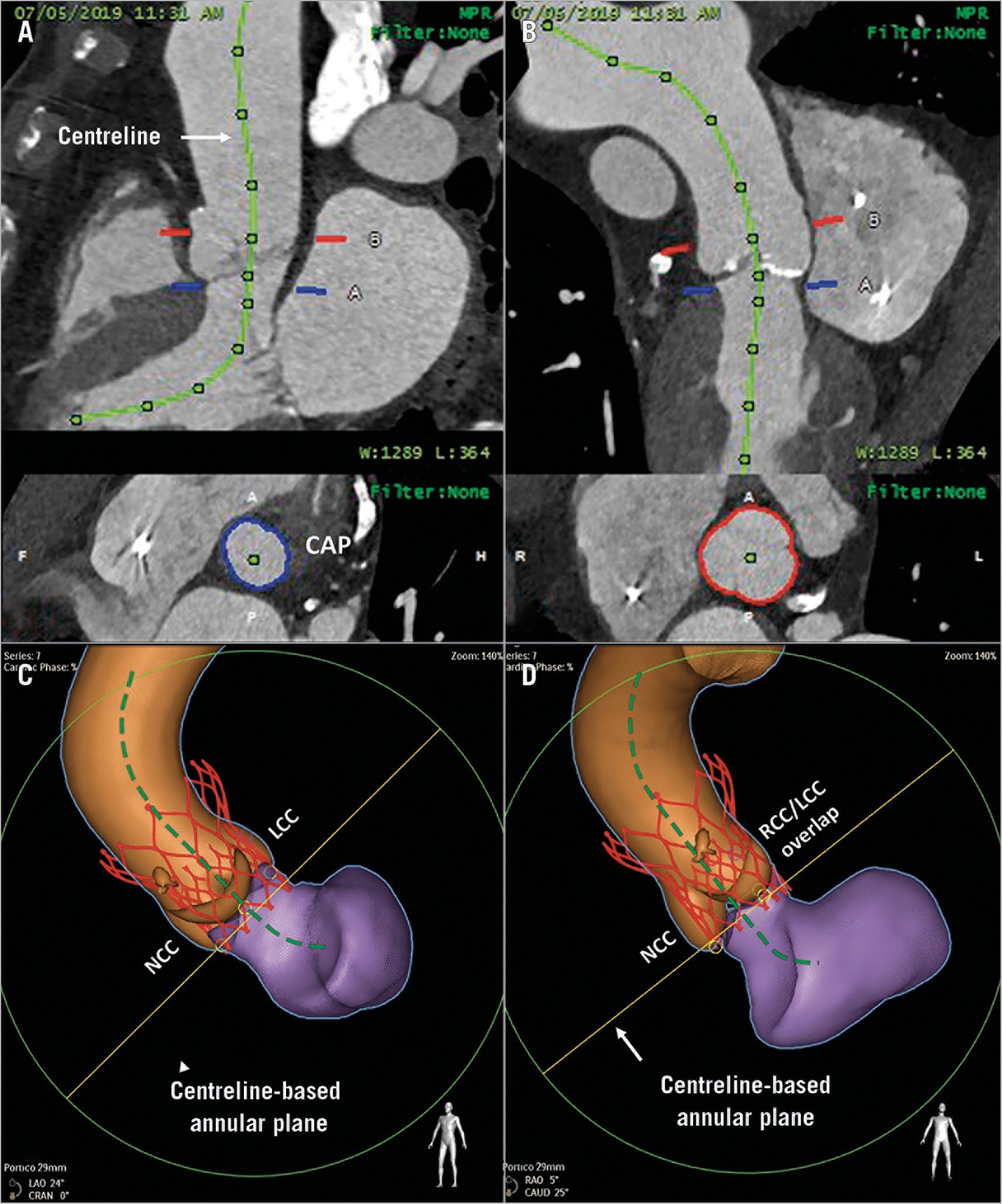
Figure 2. Centreline-based aortic annular plane approach. A centreline is drawn at the centre of the aortic root by adjusting the position of the seeds on two perpendicular planes (A & B). The centreline-based annular plane is represented perpendicular to the centreline at the level of the aortic annulus (blue plane). The co-planar angle for deployment is obtained by orienting this annular plane such that the non-coronary cusp is at the left of the screen as in the traditional three-bulbi view (C). A virtual simulation of the self-expanding valve is shown in its final position, oriented to the central axis of the aorta (C). An alternative co-planar angle with the non-coronary cusp on the left of the screen and an overlap of the right and left coronary cusps at the right is shown (D). The possible final position of the virtually simulated valve is shown in both views.
The co-planar angle of the CAP is adapted from the traditionally used three-bulbi view where the NCC is placed to the left. Operators should be mindful of the initial delivery catheter position. If it is at the greater curvature, a deeper position is preferred; conversely, if the delivery catheter is at the lesser curvature, unsheathing should be performed from a shallower depth. If a larger tilt is anticipated, higher deployment (shallow depth) is preferable to minimise the relative “low depth” on the contralateral side while being mindful of potential embolisation of the THV. An alternative co-planar angulation with the NCC to the left of the screen and the “overlap of the right and left coronary cusps” to the right can be used where the depth of implantation can be adjusted based on the lower abnormal NCC.
Limitations
Using the CAP approach requires expertise in post-processing software and an understanding of the THV behaviour in a non-traditional view.
Conclusion
In severely AAC, traditionally used HPAP is less reliable in the prediction of THV deployment behaviour and can lead to PVL or valve embolisation. The CAP approach is better at predicting the THV behaviour and should be routinely performed in AAC.
|
Impact on daily practice The limitations of the HPAP approach in AAC include a high risk of PVL and valve embolisation. These risks can be countered by using the CAP approach which helps to understand THV behaviour better. |
Conflict of interest statement
T.K.R. Pasala has received institutional grants from Medtronic and Philips Healthcare and speaker fees from Philips Healthcare. V. Jelnin has received an institutional grant from Philips. C. Sahyoun is an employee of Philips Healthcare. C.E. Ruiz has received institutional grants from Medtronic, Philips and Abbott/St. Jude Medical. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

