Despite two decades of progress in transcatheter aortic valve implantation (TAVI), postprocedural conduction abnormalities and the requirement for pacemaker implantation (PPI) remain irritatingly frequent. Compared to surgery or balloon-expandable TAVI, the rate of new PPI has historically been higher with the self-expanding Evolut (Medtronic) platform12.
The depth of transcatheter heart valve (THV) implantation is variable and is a modifiable risk factor for PPI, which can be adjusted according to operator and system preference. The need for standardisation of the implant procedure, to yield a safe and reproducible depth of implant is, therefore, axiomatic. Piazza developed the “double S-curve” (S-curves for annular plane and THV delivery catheter) which provides a single implant view (right anterior oblique/caudal in 90%), eliminating parallax between patient anatomy and the catheter and facilitating an accurate implant depth34. This cumbersome technique was subsequently simplified into the cusp overlap technique (COT), which overlaps the left and right coronary cusps, isolating the non-coronary cusp, which is located immediately above the membranous septum and the conduction tissue45. Among other advantages (Figure 1), the COT results in a higher implantation depth than a traditional three-cusp implantation technique (3CT) and reduces the incidence of PPI without increasing the risk for other adverse outcomes6.
In this issue of EuroIntervention, Wienemann et al investigate the effectiveness of the COT compared to 3CT in reducing both the incidence of PPI and complication rates with Evolut (R, Pro, PRO+)7. In this multicentre, observational, retrospective study, the authors used propensity score matching (PSM) to compare implant techniques. The study included 2,209 TAVI recipients at five German sites between 2016 and 2022 (1,151 with 3CT; 1,058 with COT). The PSM (995 pairs) resolved between-group differences in baseline characteristics and procedural technique, except for a higher rate of predilation in the COT group (57.4% vs 38.8%; p<0.001). The authors report a lower rate of PPI with the COT than the 3CT in both the unmatched (12.3% vs 17.0%; p=0.002) and matched analyses (11.9% vs 17.0%; p=0.001), as well as a lower rate of new left bundle branch block (22.6% vs 27.5%; p=0.011). The COT was protective of PPI (odds ratio 0.63, 95% confidence interval: 0.49-0.82; p<0.001) in the multivariable analysis. The COT cohort also had a lower rate of moderate/severe paravalvular regurgitation (PVR) compared to the 3CT cohort (2.4% vs 4.6%; p=0.006). The current study also reports a lower rate of major bleeding (4.6% vs 7.0%; p=0.020) with the COT, a result that suggests residual unmeasured confounding in the analysis.
The study by Wienemann et al is the largest available experience investigating the use of the COT with the Evolut platform (20% treated with PRO/PRO+) and is consistent with meta-analyses of observational studies6. It is also in line with the preliminary results of the multicentre, prospective Optimize PRO study, where an optimised care pathway and strict adherence to the COT with the Evolut system reduced the rate of PPI at 30 days to 9.8% and further, to 5.8%, when a 4-step COT implant was performed8. The consistency of these results across multiple experiences is particularly significant given that previous studies have found PPI rates with the Evolut PRO/PRO+, of 10-20%, much higher than those for the SAPIEN 3 Ultra (~10%; Edwards Lifesciences) and the ACURATE neo2 (~7%; Boston Scientific) platforms9. Using the COT aligns the PPI rate of the Evolut with other commercially available platforms.
Several limitations should be noted including the study design itself (retrospective, observational) and the lack of prespecified protocols for PPI. It is also likely that the 3CT patients represent an historic cohort, with the COT patients being more contemporary. Other factors influencing PPI rates were not assessed, such as valve and left ventricular outflow tract calcification.
The need for new PPI continues to be an “Achilles heel” of TAVI and has economic and healthcare-related implications. The longer-term prognostic implications of new PPI remain a matter of debate, with some studies suggesting a higher risk of heart failure hospitalisation and mortality, while others report no significant association1011. These discrepancies may stem from variability in pacing indications, pacing dependency, and patient characteristics across different studies, and in older and frailer historic TAVI populations, death is an important competing risk. The deleterious effects of right ventricular pacing are more likely to present in younger patients subjected to a high ventricular pacing percentage over a longer period of time. Reducing PPI remains an important evolution in the TAVI story and is timely given the extension to younger and lower-risk patients.
While valve design and implantation technique play a considerable role in the risk of PPI post-TAVI, there remain several non-modifiable risk factors: pre-existing conduction disturbances and anatomical variables (short membranous septum). While the COT does not imply a higher implantation of the THV per se, in reality it usually results in a higher implantation versus the 3CT. As shown in the current manuscript, the COT does not increase the risk of THV embolisation, but there may be important future implications from a higher deployment of the Evolut platform, or any TAVI system. Reaccess to the coronary arteries and safe TAV-in-TAV are likely to be negatively impacted by higher implants in a proportion of patients. The impact of a higher implantation may well play out in the coming decades as younger TAVI recipients present with acute or chronic coronary syndromes or THV failure. Notwithstanding this, the study by Wienemann et al represents another important step in the evolution of TAVI as it confirms the COT as the standard implantation technique for patients receiving Evolut TAVI.
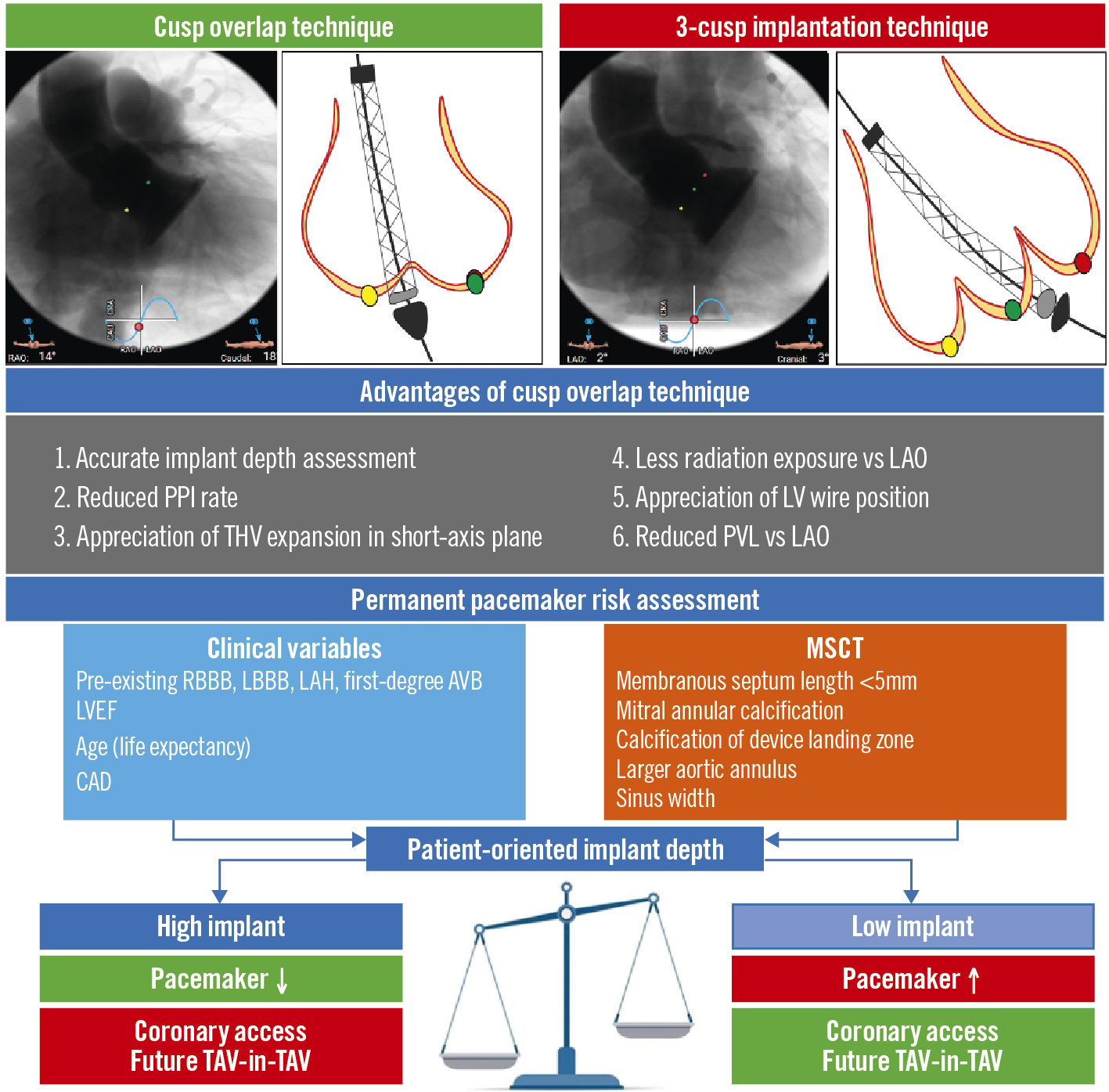
Figure 1. Cusp overlap technique versus 3-cusp coplanar view: advantages and implications of a precise, tailored valve implantation depth. AVB: atrioventricular block; CAD: coronary artery disease; LAH: left anterior hemiblock; LAO: left anterior oblique; LBBB: left bundle branch block; LV: left ventricle; LVEF: left ventricular ejection fraction; MSCT: multislice computed tomography; PPI: permanent pacemaker implantation; PVL: paravalvular leak; RBBB: right bundle branch block; TAV: transcatheter aortic valve; THV: transcatheter heart valve
Funding
This work was supported, in part, by grants from Science Foundation Ireland (15/RP/2765) to S. Fezzi.
Conflict of interest statement
D.Mylotte has served as aconsultant for Medtronic, Boston Scientific, and MicroPort. The other authors have no conflicts of interest to declare.

