Abstract
Background: Reducing rates of permanent pacemaker implantation (PPI) after transcatheter aortic valve implantation (TAVI) is important for achieving the best procedural outcomes. The cusp overlap technique (COT) implements procedural steps including an overlap angulation of the right and left coronary cusp to mitigate this complication.
Aims: We investigated the incidence of PPI and complication rates following the COT compared to the standard three-cusp implantation technique (3CT) in an all-comers cohort.
Methods: A total of 2,209 patients underwent TAVI with the self-expanding Evolut platform from January 2016 to April 2022 at five sites. Baseline, procedural and in-hospital outcome characteristics were compared for both techniques before and after one-to-one propensity score matching.
Results: A total of 1,151 patients were implanted using the 3CT and 1,058 using the COT. At discharge, the rates of PPI (17.0 vs 12.3%; p=0.002) and moderate/severe paravalvular regurgitation (4.6% vs 2.4%; p=0.006) were significantly reduced with the COT compared with 3CT within the unmatched cohort. Overall procedural success and complication rates were similar; major bleeding was less common in the COT group (7.0% vs 4.6%; p=0.020). These results remained consistent after propensity score matching. In multivariable logistic regression analysis, right bundle branch block (odds ratio [OR] 7.19, 95% confidence interval [CI]: 5.18-10.0; p<0.001) and diabetes mellitus (OR 1.38, 95% CI: 1.05-1.80; p=0.021) emerged as predictors of PPI, whereas the COT (OR 0.63, 95% CI: 0.49-0.82; p<0.001) was protective.
Conclusions: The introduction of the COT was associated with a significant and relevant reduction of PPI and paravalvular regurgitation rates without an increase in complication rates.
Introduction
Transcatheter aortic valve implantation (TAVI) has become the standard treatment for the majority of patients with symptomatic severe aortic stenosis at intermediate or high surgical risk and is expanding towards younger and lower surgical risk patients12. Procedure-related complications have decreased over the past few years due to improved patient assessment, increased operator experience and successive technological developments of transcatheter heart valves (THVs).
However, the rate of permanent pacemaker implantation (PPI) remains a major concern. The rate ranges from 17.4-18.0% at 30 days when using self-expanding Evolut R/PRO devices (both Medtronic) in prospective, multicentre randomised studies13, and the occurrence of conduction disturbances might be associated with prolonged hospitalisation, higher costs, worse clinical outcomes and higher all-cause mortality4567. Conduction abnormalities at baseline, anatomical and clinical risk factors, the use of self-expanding Evolut THVs, and implantation depth (ID) have been linked to PPI after TAVI468.
In the standard three-cusp projection, the hinge point of the right coronary cusp (RCC) is aligned between the left coronary cusp (LCC) and non-coronary cusp (NCC) hinge points, which facilitates perpendicular positioning to the aortic annulus for valve deployment, defined as the standard three-cusp implantation technique (3CT). This approach is limited by device parallax, with difficulties in assessing the true device depth that have been related to the foreshortening of the left ventricular outflow tract (LVOT)9. The “cusp overlap” technique (COT) implemented a change in implant projection, applying an overlap projection of the LCC and RCC with isolation of the NCC. Furthermore, implantation procedures have been refined and minimised LVOT contact by starting the valve deployment from above the aortic annulus91011.
Smaller-scale studies have shown that the incidence of PPI ranged from 16.8% to 27.9% with the 3CT, and implementation of the COT dramatically reduced the incidence of PPI to 6.4% to 13.1% at discharge or 30 days1011121314. However, multicentre studies with larger longitudinal numbers of all-comer patients are lacking. They are needed to investigate real-world effects on PPI rates, rare but severe complications, and possible future improvements utilising this technique. Therefore, the aim of this study was to investigate the rates of PPI, occurrence of conduction abnormalities and complication rates using the COT.
Methods
Patient population
This study included patients who underwent TAVI with the Evolut R, PRO or PRO+ at five sites between February 2016 and April 2022. Supplementary Table 1 provides information on the total number of TAVI cases per centre and implementation time of the COT.
Indications for TAVI, approach and type of prosthesis were based on the decisions of an experienced interdisciplinary Heart Team. Exclusion criteria were age <18 years, prior surgical or transcatheter aortic valve replacement and prior PPI.
Baseline demographics, clinical and routine pre- and post-procedural echocardiographic characteristics, procedural information, and in-hospital outcome data were collected by the co-investigators at each institution using a dedicated electronic case report form.
Continuous telemetry monitoring was performed intraprocedurally and continued for at least 24 hours for all patients who had no conduction disturbances before and after TAVI. The presence of conduction disturbances, such as right bundle branch block (RBBB), new-onset left bundle branch block (LBBB) and high-degree atrioventricular block, resulted in continuous telemetry for at least 48 hrs at each centre following the current expert consensus4 and guideline recommendations15. The primary outcome for this study was the incidence of PPI at discharge. Bleeding, vascular complications, acute kidney injury, and stroke were defined according to Valve Academic Research Consortium-2 (VARC-2) criteria16. All patients gave their written informed consent for the procedure. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice and was approved by local ethics committees.
Preprocedural computed tomography imaging
Preprocedural computed tomography (CT) scans were performed according to standard of care based on recommendations17 and local practice to characterise aortic valve and aortic root anatomy. Modern single- or dual-source CT scanners with a minimum of 128 detector rows were used to conduct the scans. CT data acquisition was electrocardiogram-gated and typically reconstructed with a slice thickness and increments of 0.5 mm. Due to variations in the assessment methods in each centre and the complexity of the measurements, the patients were divided into high and low aortic valve calcification groups according to a median Agatston Score Unit of 2,948 from non-enhanced contrast scans (Syngo.via; Siemens Healthcare GmbH) and a median calcium volume of 490.8 mm³ derived from contrast-enhanced CT images (3mensio Structural Heart; Pie Medical Imaging). The percentage oversizing was calculated as (Ï×nominal THV size−annulus perimeter)/annulus perimeter×100.
Transcatheter heart valves
The self-expanding supra-annular Medtronic Evolut R, Medtronic Evolut PRO and Medtronic Evolut PRO+, covering the range for most annulus diameters, were included. These second-generation THVs have identical valve platforms, except for an exterior pericardial wrap around the valve inflow to improve annular sealing for the PRO family, but differ in delivery sheath size11819. Selection of prosthesis type and size was left to the discretion of the Heart Team of each participating centre and was based on a perimeter-derived diameter according to the manufacturer’s recommendations.
Implantation technique
Preoperatively, the three native cusps were annotated using CT scans. The standard three-cusp implantation technique is characterised by the alignment of the LCC, RCC and NCC in the same plane. For the cusp overlap projection, the hinge points of the LCC and RCC were overlapped, isolating the NCC (Central illustration). Both projections were calculated using the local software for pre-TAVI CT analysis and applied during the procedure. Implant procedures were performed according to the instructions for use and the centre’s standard procedures. Procedural steps like valve deployment starting from above the aortic annulus were implemented. Valve release was performed under fast or rapid pacing, with an optimal final ID of 3-5 mm. Since 2020, the manufacturer recommends a target ID of 3 mm, which was adopted in the participating centres. Pre- and/or postdilatation were performed at the discretion of the operating team.
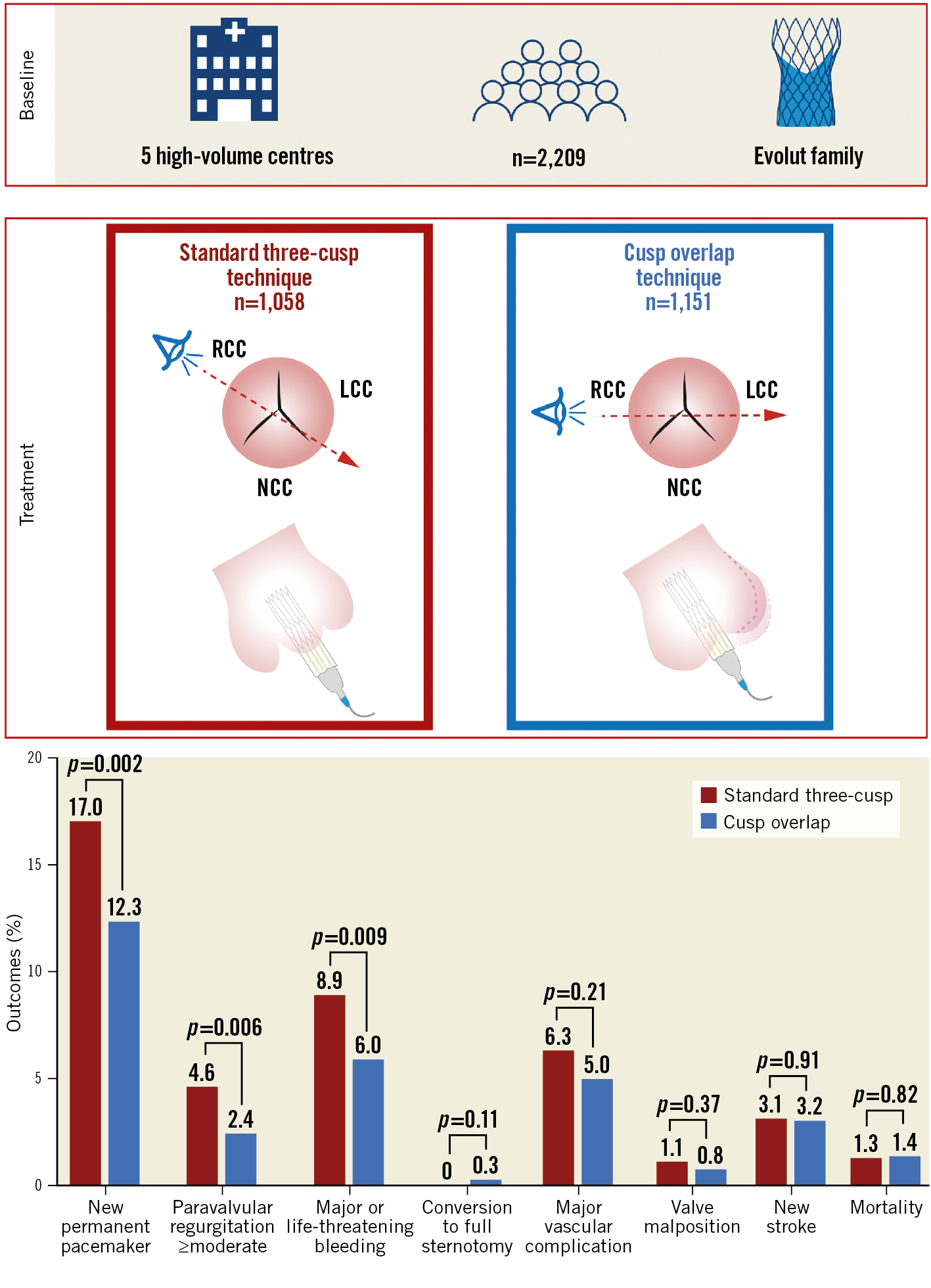
Central illustration. Clinical outcomes after TAVI according to implantation technique. LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp; TAVI: transcatheter aortic valve implantation
Statistical analysis
Continuous variables are expressed as median with interquartile range [IQR]. Categorical variables are reported as counts and percentages. Continuous variables were compared using unpaired and paired Wilcoxon rank-sum tests for the unmatched and matched samples. Categorical variables were compared using the chi-square statistic, Fisher’s exact test or McNemar’s test as appropriate. Additional details regarding the approach to imputation of missing values are provided in Supplementary Appendix 1. To account for the non-randomised design of the study, propensity score matching was used to adjust for baseline confounding variables between the COT and 3CT groups. The propensity score was calculated using a logistic regression model according to a non-parsimonious approach. Twenty-five explanatory variables were included in the logistic model to calculate the propensity score (Supplementary Table 2). The 1:1 nearest neighbour method with no replacement and a calibre width of 0.20 were used for propensity score matching with the R package MatchIt (R Foundation for Statistical Computing). Balance between the groups was estimated using standardised mean differences and variance ratios, and standardised mean differences <0.1 were considered as an acceptable balance between covariates. Baseline and procedural characteristics were used to perform a univariable and multivariable logistic regression model to evaluate the potential predictors of PPI. Predictors with a p-value <0.10 upon the univariable analysis were entered into the multivariable model. Odds ratios (OR) and their 95% confidence intervals (CI) were reported for the models. Two-sided p-values <0.05 were considered statistically significant. All statistical analyses were performed with R 4.2.1 (R Foundation for Statistical Computing).
Results
Study population
A total of 2,209 patients (54% female) with a median age of 82 years and median European System for Cardiac Operative Risk Evaluation (EuroSCORE) II of 3.6% [IQR 2.2-6.2%] were included in the study. Overall, 1,779 (80.5%) patients received an Evolut R prosthesis, and 1,058 (47.9%) patients underwent TAVI using the COT. Baseline characteristics for the unmatched and matched patients are shown in Table 1. Before propensity score matching, there were only minor differences between the groups, with lower surgical risk scores in the COT group and a higher prevalence of bicuspid valves in the COT group. In particular, no significant difference was found for age (82.7 years [IQR 79.0-86.0] vs 82.0 years [IQR 78.9-85.4]; p=0.080), preprocedural perimeter-derived aortic annulus diameter (24.1 mm [IQR 22.6-25.6 mm] vs 23.9 mm [IQR 22.3-25.4 mm]; p=0.077) or complete RBBB (8.2% vs 9.2%; p=0.40). Propensity score matching resulted in a well-matched and balanced cohort, with a standardised mean difference of <0.1 and 995 pairs (Supplementary Figure 1).
Table 1. Patient baseline characteristics.
| Overall,n=2,209 | Pre-matching | Post-matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Standard three-cusp, n=1,151 | Cusp overlap, n=1,058 | p-value | Standard three-cusp, n=995 | Cusp overlap, n=995 | p-value | |||
| Age (years) | 82.2 [79.0-85.9] | 82.7 [79.0-86.0] | 82.0 [78.9-85.4] | 0.080 | 82.0 [79.0-85.7] | 82.1 [79.0-85.5] | 0.68 | |
| Female sex | 1,193 (54.0%) | 634 (55.1%) | 559 (52.8%) | 0.29 | 531 (53.4%) | 529 (53.2%) | 0.96 | |
| Body mass index (kg/m2) | 26.1 [23.6-29.4] | 26.1 [23.5-29.2] | 26.1 [23.7-29.8] | 0.33 | 26.2 [23.8-29.4] | 26.1 [23.7-29.7] | >0.99 | |
| EuroSCORE II (%) | 3.6 [2.2-6.2] | 3.9 [2.4-6.5] | 3.3 [1.9-5.8] | <0.001 | 3.6 [2.3-5.8] | 3.3 [1.9-5.8] | 0.18 | |
| Society of Thoracic Surgeons Predicted Risk Of Mortality score (%) | 3.7 [2.5-5.6] | 3.8 [2.7-5.9] | 3.7 [2.4-5.4] | 0.023 | 3.6 [2.5-5.3] | 3.7 [2.4-5.5] | 0.39 | |
| New York Heart Association Functional Class | I | 81 (3.7%) | 41 (3.6%) | 40 (3.8%) | 0.44 | 40 (4.0%) | 38 (3.8%) | 0.61 |
| II | 527 (23.9%) | 264 (22.9%) | 263 (24.9%) | 241 (24.2%) | 244 (24.5%) | |||
| III | 1,430 (64.7%) | 763 (66.3%) | 667 (63.0%) | 650 (65.3%) | 629 (63.2%) | |||
| IV | 171 (7.7%) | 83 (7.2%) | 88 (8.3%) | 64 (6.4%) | 84 (8.4%) | |||
| Arterial hypertension | 1,964 (88.9%) | 1,029 (89.4%) | 935 (88.4%) | 0.44 | 887 (89.1%) | 883 (88.7%) | 0.83 | |
| Diabetes mellitus | 591 (26.8%) | 303 (26.3%) | 288 (27.2%) | 0.63 | 263 (26.4%) | 268 (26.9%) | 0.84 | |
| Extracardiac arteriopathy | 543 (24.6%) | 302 (26.2%) | 241 (22.8%) | 0.059 | 237 (23.8%) | 228 (22.9%) | 0.67 | |
| Chronic obstructive pulmonary disease | 419 (19.0%) | 246 (21.4%) | 173 (16.4%) | 0.003 | 168 (16.9%) | 167 (16.8%) | >0.99 | |
| Haemoglobin (g/dl) | 12.3 [11.0-13.5] | 12.2 [11.0-13.4] | 12.4 [11.0-13.5] | 0.25 | 12.3 [11.1-13.5] | 12.3 [11.0-13.5] | 0.95 | |
| Glomerular filtration rate (ml/min/1.73 m2) | 59.0 [43.0-74.3] | 58.0 [42.0-74.0] | 61.0 [44.0-76.0] | 0.030 | 59.6 [44.0-75.0] | 60.4 [44.0-75.0] | 0.82 | |
| Chronic renal replacement therapy | 66 (3.0%) | 35 (3.0%) | 31 (2.9%) | 0.88 | 29 (2.9%) | 30 (3.0%) | >0.99 | |
| Prior cardiac surgery | 193 (8.7%) | 84 (7.3%) | 109 (10.3%) | 0.012 | 78 (7.8%) | 93 (9.3%) | 0.25 | |
| Prior stroke | 283 (12.8%) | 150 (13.0%) | 133 (12.6%) | 0.75 | 129 (13.0%) | 124 (12.5%) | 0.79 | |
| Atrial fibrillation/flutter | 871 (39.4%) | 458 (39.8%) | 413 (39.0%) | 0.72 | 389 (39.1%) | 389 (39.1%) | >0.99 | |
| Prior left bundle branch block | 181 (8.2%) | 85 (7.4%) | 96 (9.1%) | 0.15 | 78 (7.8%) | 86 (8.6%) | 0.57 | |
| Prior right bundle branch block | 191 (8.6%) | 94 (8.2%) | 97 (9.2%) | 0.40 | 90 (9.0%) | 92 (9.2%) | 0.94 | |
| Left ventricular ejection fraction | ≥50% | 1,668 (75.5%) | 867 (75.3%) | 801 (75.7%) | 0.83 | 758 (76.2%) | 755 (75.9%) | 0.91 |
| <50% | 541 (24.5%) | 284 (24.7%) | 257 (24.3%) | 237 (23.8%) | 240 (24.1%) | |||
| Mean aortic valve gradient (mmHg) | 42.0 [32.0-51.0] | 41.0 [31.9-50.0] | 42.0 [33.0-51.9] | 0.058 | 41.8 [32.6-50.8] | 42.0 [33.0-51.0] | 0.58 | |
| Aortic valve area (cm2) | 0.7 [0.6-0.8] | 0.7 [0.6-0.9] | 0.7 [0.6-0.8] | 0.18 | 0.7 [0.6-0.9] | 0.7 [0.6-0.8] | 0.59 | |
| Bicuspid aortic valve | 48 (2.2%) | 18 (1.6%) | 30 (2.8%) | 0.041 | 18 (1.8%) | 21 (2.1%) | 0.75 | |
| Perimeter-derived annulus diameter (mm) | 24.0 [22.4-25.5] | 24.1 [22.6-25.6] | 23.9 [22.3-25.4] | 0.077 | 24.1 [22.5-25.5] | 23.9 [22.3-25.4] | 0.54 | |
| Annulus calcification (Agatston score) | 2,948.0 [1,855.0-4,041.0]/ (n=477) | 2,956.0 [1,695-4,049]/ (n=159) | 2,914 [1,913-4,027.2]/(n=318) | 0.74 | 3,028 [1,743-4,062]/(n=150) | 2,821 [1,898-3,868] (n=285) | 0.56 | |
| Annulus calcification (calcium volume, mm3) | 490.8 [268.8-833.8]/ (n=988) | 466.6 [248.4-820.8]/ (n=746) | 570.6 [329.0-923.7]/(n=242) | 0.005 | 488.8 [258.6-839.4]/(n=628) | 570.6 [326.3-924.0]/(n=236) | 0.060 | |
| Annulus calcification (pooled and imputed) | low | 1,113 (50.4%) | 599 (52.0%) | 514 (48.6%) | 0.10 | 499 (50.2%) | 489 (49.1%) | 0.68 |
| high | 1,096 (49.6%) | 552 (48.0%) | 544 (51.4%) | 496 (49.8%) | 506 (50.9%) | |||
| All data are median [interquartile range] or absolute number (percentage). Denominators vary due to some missing data. EuroSCORE II: European System for Cardiac Operative Risk Evaluation | ||||||||
Procedural characteristics
The procedural characteristics for the unmatched and matched patients are displayed in Table 2. Most cases were performed via a transfemoral approach (96%) under conscious sedation (91%). Utilisation of prosthesis type differed between groups due to the recent introduction of the Evolut PRO+ in the COT group. Radiation time and procedure time were both shorter in the COT group. Predilatation was more often performed in the COT group (57.4% vs 38.8%; p<0.001), whereas postdilatation was similar among both groups (29.1% vs 25.9%; p<0.001). The oversizing percentages were similar in both groups. However, the 34 mm valve size had a significantly larger oversizing percentage compared to the other valve sizes (all p<0.05).
In addition, a slightly higher volume of contrast medium was delivered in the COT group (100 mL [IQR 80-135 mL] vs 98 mL [IQR 75-123.5 mL]; p<0.001). Overall, the rates of valve migration/embolisation (0.8% vs 1.1%; p=0.37), conversion to surgery (0.3% vs 0%; p=0.11) and need for a second valve implantation (0.7% vs 1.3%; p=0.13) were similar in both groups.
Table 2. Procedural characteristics.
| Pre-matching | Post-matching | ||||||
|---|---|---|---|---|---|---|---|
| Standard three-cusp, n=1,151 | Cusp overlap, n=1,058 | p-value | Standard three-cusp, n=995 | Cusp overlap, n=995 | p-value | ||
| Anaesthesia | conscious sedation | 1,077 (93.6%) | 941 (88.9%) | <0.001 | 921 (92.6%) | 902 (90.7%) | 0.12 |
| general anaesthesia | 74 (6.4%) | 117 (11.1%) | 74 (7.4%) | 93 (9.3%) | |||
| Main access route | transfemoral | 1,112 (96.6%) | 1,010 (95.5%) | 0.17 | 959 (96.4%) | 953 (95.8%) | 0.56 |
| non-transfemoral | 39 (3.4%) | 48 (4.5%) | 36 (3.6%) | 42 (4.2%) | |||
| Valve type | Evolut R | 943 (81.9%) | 836 (79.0%) | 0.084 | 807 (81.1%) | 804 (80.8%) | 0.90 |
| Evolut PRO | 208 (18.1%) | 195 (18.4%) | 0.83 | 188 (18.9%) | 191 (19.2%) | 0.90 | |
| Evolut PRO+ | 0 (0.0%) | 27 (2.6%) | <0.001 | 0 (0.0%) | 0 (0.0%) | ||
| Valve size | 23 mm | 37 (3.2%) | 37 (3.5%) | 0.20 | 33 (3.3%) | 35 (3.5%) | 0.64 |
| 26 mm | 328 (28.5%) | 343 (32.4%) | 297 (29.8%) | 313 (31.5%) | |||
| 29 mm | 564 (49.0%) | 494 (46.7%) | 479 (48.1%) | 476 (47.8%) | |||
| 34 mm | 222 (19.3%) | 184 (17.4%) | 186 (18.7%) | 171 (17.2%) | |||
| Oversizing (%) | 19.7 [15.9-24.1] | 19.6 [16.0-23.8] | 0.47 | 19.6 [15.9-24.0] | 19. [16.0-23.8] | 0.84 | |
| 23 mm | 16.7 [13.8–21.4] | 17.9 [14.1-24.6] | 16.7 [13.8-21.4] | 17.9 [14.0-24.9] | |||
| 26 mm | 18.6 [15.0–23.2] | 18.2 [15.2-23.0] | 18.6 [15.0-23.0] | 18.4 [15.5-22.8] | |||
| 29 mm | 18.8 [15.5–22.3] | 19.1 [15.3-22.5] | 18.8 [15.6-22.1] | 19.1 [15.3-22.5] | |||
| 34 mm | 25.7 [21.7–28.2] | 24.2 [20.3-27.2] | 25.7 [21.7-28.3] | 24.5 [20.7-27.4] | |||
| Predilatation | 447 (38.8%) | 607 (57.4%) | <0.001 | 394 (39.6%) | 561 (56.4%) | <0.001 | |
| Postdilatation | 298 (25.9%) | 308 (29.1%) | 0.090 | 267 (26.8%) | 285 (28.6%) | 0.38 | |
| Procedure duration (min) | 61.0 [50.0-80.0]/ (n=1,139) | 57.0 [46.0-75.0]/ (n=1,039) | <0.001 | 60.0 [50.0-80.0]/(n=985) | 57.0 [45.0-75.0]/(n=977) | <0.001 | |
| Fluoroscopy time (min) | 14.5 [10.2-20.6]/ (n=1,139) | 13.0 [9.0-18.0]/(n=995) | <0.001 | 14.4 [10.0-20.7]/(n=983) | 13.0 [9.0-17.7]/(n=939) | <0.001 | |
| Contrast (ml) | 98.0 [75.0-123.5]/ (n=1,143) | 100.0 [80.0-135.0]/ (n=1,038) | <0.001 | 99.0 [75.0-125.0]/(n=987) | 100.0 [79.8-133.0]/(n=976) | 0.005 | |
| Total hospital stay (days) | 8.0 [7.0-12.0]/ (n=1,149) | 7.0 [5.0-10.0]/ (n=1,054) | <0.001 | 8.0 [7.0-11.0]/(n=993) | 7.0 [5.0-10.0]/(n=991) | <0.001 | |
| All data are median [interquartile range] or absolute number (percentage). Denominators vary due to some missing data. | |||||||
In-hospital outcomes
The outcomes for both implantation techniques before and after matching are listed in Table 3. In the unmatched cohort, fewer bleeding complications were observed in the COT group compared to 3CT. A similar trend was seen for vascular complications before and after matching. The haemodynamic performance either by the COT or 3CT did not differ significantly in the unmatched and matched samples, with a mean transvalvular gradient of 7 mmHg (IQR 5-10 mmHg; p=0.18). The rate of moderate/severe paravalvular regurgitation (PVR) was significantly lower in the COT group (2.4% vs 4.6%; p=0.006). The rate of moderate/severe PVR was slightly lower, without reaching statistical significance, between the Evolut R and Evolut PRO/PRO+ in the unmatched (3.2% vs 4.9%; p=0.085) and matched cohorts (3.2% vs 4.5%; p=0.20).
Table 3. Outcome characteristics.
| Pre-matching | Post-matching | ||||||
|---|---|---|---|---|---|---|---|
| Standard three-cusp, n=1,151 | Cusp overlap, n=1,058 | p-value | Standard three-cusp, n=995 | Cusp overlap, n=995 | p-value | ||
| New permanent pacemaker implantation | 196 (17.0%) | 130 (12.3%) | 0.002 | 169 (17.0%) | 118 (11.9%) | 0.001 | |
| tricuspid valve | 191/1,133 (16.9%) | 124/1,028 (12.1%) | 0.002 | 161/977 (16.5%) | 113/974 (11.6%) | 0.002 | |
| bicuspid valve | 5/18 (27.8%) | 6/30 (20.0%) | 0.72 | 5/18 (27.8%) | 5/21 (23.8%) | >0.99 | |
| New left bundle branch block* | 238/1,055 (22.6%) | 257/935 (27.5%) | 0.011 | 198/899 (22.0%) | 242/883 (27.4%) | 0.012 | |
| New right bundle branch block** | 18/1,047 (1.7%) | 27/936 (2.9%) | 0.082 | 16/894 (1.8%) | 26/879 (3.0%) | 0.074 | |
| Bleeding complication | none | 912 (79.2%) | 886 (83.7%) | 0.031 | 792 (79.6%) | 834 (83.8%) | 0.078 |
| minor bleeding | 137 (11.9%) | 109 (10.3%) | 115 (11.6%) | 105 (10.6%) | |||
| major bleeding | 80 (7.0%) | 49 (4.6%) | 0.020 | 68 (6.8%) | 44 (4.4%) | 0.025 | |
| life-threatening bleeding | 22 (1.9%) | 14 (1.3%) | 0.28 | 20 (2.0%) | 12 (1.2%) | 0.20 | |
| Vascular complication | none | 920 (79.9%) | 886 (83.7%) | 0.068 | 797 (80.1%) | 830 (83.4%) | 0.11 |
| minor vascular complication | 159 (13.8%) | 119 (11.2%) | 137 (13.8%) | 117 (11.8%) | |||
| major vascular complication | 72 (6.3%) | 53 (5.0%) | 0.21 | 61 (6.1%) | 48 (4.8%) | 0.23 | |
| Acute kidney injury | none | 1,002 (87.1%) | 927 (87.6%) | 0.97 | 873 (87.7%) | 868 (87.2%) | - |
| stage 1 | 109 (9.5%) | 97 (9.2%) | 92 (9.2%) | 95 (9.5%) | |||
| stage 2 | 19 (1.7%) | 17 (1.6%) | 15 (1.5%) | 16 (1.6%) | |||
| stage 3 | 21 (1.8%) | 17 (1.6%) | 15 (1.5%) | 16 (1.6%) | |||
| New stroke | 36 (3.1%) | 34 (3.2%) | 0.91 | 28 (2.8%) | 34 (3.4%) | 0.52 | |
| Conversion to open surgery | 0 (0.0%) | 3 (0.3%) | 0.11 | 0 (0.0%) | 2 (0.2%) | - | |
| Valve migration/embolisation | 13 (1.1%) | 8 (0.8%) | 0.37 | 9 (0.9%) | 8 (0.8%) | >0.99 | |
| Second valve implantation | 15 (1.3%) | 7 (0.7%) | 0.13 | 13 (1.3%) | 7 (0.7%) | 0.26 | |
| In-hospital mortality | 15 (1.3%) | 15 (1.4%) | 0.82 | 14 (1.4%) | 14 (1.4%) | >0.99 | |
| Mean aortic valve gradient (mmHg) | 7.0 [5.0-10.0]/(n=1,114) | 7.0 [5.0-10.0]/(n=1,000) | 0.18 | 7.0 [5.0-10.0]/(n=963) | 7.0 [5.0-10.0]/ (n=939) | 0.90 | |
| Mean aortic valve gradient (≥20 mmHg) | 11/1,114 (1.0%) | 19/1,000 (1.9%) | 0.077 | 9/963 (0.9%) | 19/939 (2.0%) | 0.11 | |
| Paravalvular regurgitation | none/trace | 584/1,140 (51.2%) | 501/1,042 (48.1%) | 0.003 | 511/984 (51.9%) | 478/980 (48.8%) | 0.022 |
| mild | 504/1,140 (44.2%) | 516/1,042 (49.5%) | 429/984 (43.6%) | 479/980 (48.9%) | |||
| moderate/severe | 52/1,140 (4.6%) | 25/1,042 (2.4%) | 0.006 | 44/984 (4.5%) | 23/980 (2.3%) | 0.018 | |
| All data are median [interquartile range] or absolute number (percentage). Denominators vary due to some missing data. *Excluding patients with left bundle branch block at baseline. **Excluding patients with right bundle branch block at baseline. | |||||||
Conduction disturbances and incidence of PPI
In the unmatched cohort, the frequency of new PPI was 12.3% in the COT group compared to 17.0% in the 3CT group (p=0.002) (Central illustration). Consistent results were observed in the matched cohort (COT: 11.9% vs 3CT: 17%; p=0.001). Excluding patients with prior LBBB, the occurrence of new LBBB was more frequent in the COT (27.5% vs 22.6%; p=0.011). A similar trend was observed with a higher incidence of new RBBB in the COT (2.9% vs 1.7%; p=0.082). Figure 1 displays the incidence of PPI with both techniques among the included centres.
Predilatation was used a lot more frequently in the COT group, which had lower PPI rates. Nevertheless, direct TAVI implantation yielded a favourable, but not significant, trend for decreased conduction system abnormalities in both groups (Supplementary Table 3). There was a high frequency of PPI among patients with bicuspid aortic valves; however, PPI rates could similarly be reduced with the COT, without reaching statistical significance due to the smaller sample size (Table 3).
Univariable and multivariable logistic regression analyses for the incidence of PPI according to the implantation technique are displayed in Table 4. Significant independent predictors of new PPI included pre-existing RBBB (p<0.001) and the presence of diabetes mellitus, whereas using the COT was protective in the unmatched cohort. Additionally, in the matched cohort, the presence of bicuspid valves and use of the 29 mm valve size compared to the 23 mm valve size reached statistical significance as predictors for PPI. Next, we examined the effect of the predictors for PPI according to the COT and 3CT within the univariable analysis (Table 5). Similarly, RBBB and a larger annulus perimeter showed a higher OR for PPI. In addition, patients treated in the last period across the tertiles of consecutive cases had a lower OR for PPI, but the trend was not significant.
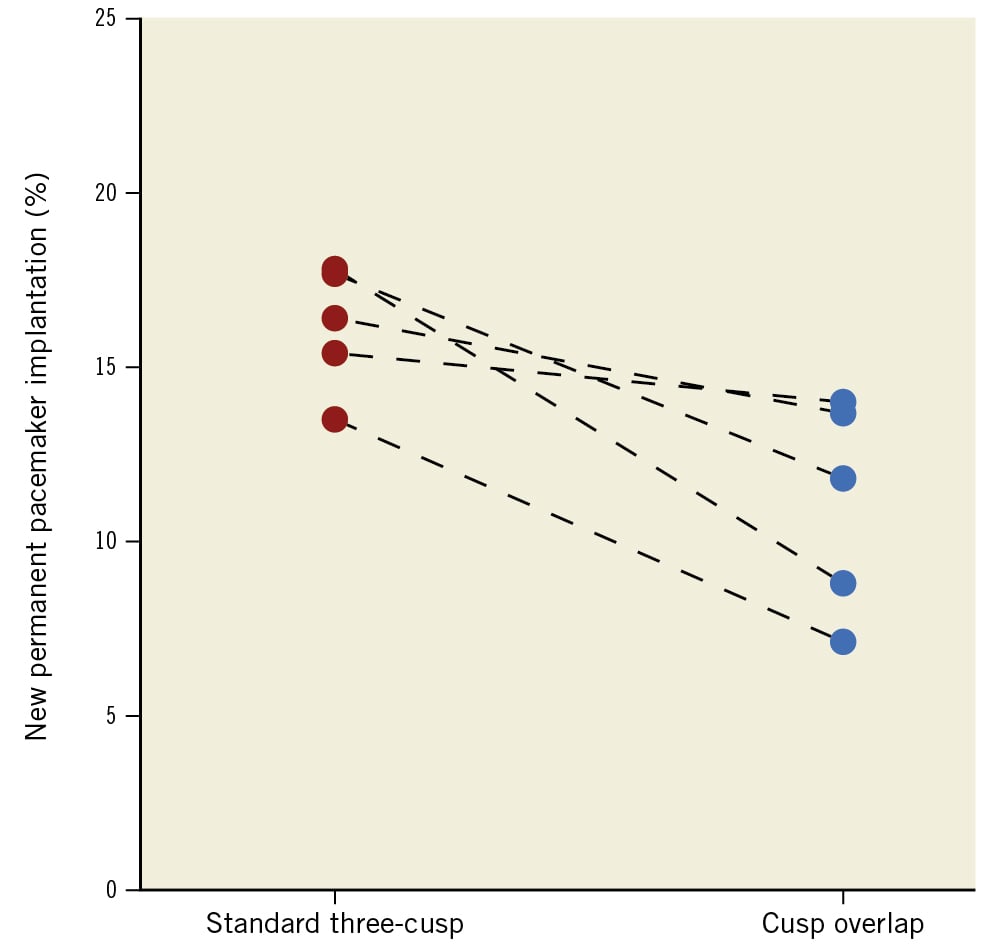
Figure 1. Change in new PPI incidence with the standard three-cusp and cusp overlap implantation techniques according to each centre. The PPI rate was significantly lower using cusp overlap than the three-cusp technique (17.0% vs 12.3%; p=0.002) in the entire cohort. The reduction in the incidence of PPI was different across centres, with an absolute difference of between 1.4 and 9.0 percentage points. PPI: permanent pacemaker implantation
Table 4. Univariable and multivariable logistic regression analysis for the prediction of permanent pacemaker implantation.
| Pre-matching | Post-matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable model | Multivariable model | Univariable model | Multivariable model | ||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Implantation technique | Cusp overlap vs standard three-cusp | 0.68 (0.54-0.87) | 0.002 | 0.63 (0.49-0.82) | <0.001 | 0.66 (0.51-0.85) | 0.001 | 0.65 (0.50-0.84) | <0.001 |
| Centre | 2 vs 1 | 0.76 (0.42-1.32) | 0.35 | – | – | 0.69 (0.36-1.22) | 0.22 | – | – |
| 3 vs 1 | 1.00 (0.73-1.38) | >0.99 | 0.91 (0.65-1.28) | 0.59 | |||||
| 4 vs 1 | 1.08 (0.79-1.49) | 0.62 | 1.03 (0.74-1.43) | 0.86 | |||||
| 5 vs 1 | 0.96 (0.61-1.48) | 0.87 | 0.88 (0.55-1.39) | 0.59 | |||||
| Age (≥75 years vs <75 years) | 1.60 (1.06-2.49) | 0.030 | 1.34 (0.88-2.14) | 0.19 | 1.62 (1.05-2.63) | 0.039 | 1.49 (0.97-2.38) | 0.082 | |
| Female sex | 0.69 (0.54-0.87) | 0.002 | 1.06 (0.78-1.45) | 0.70 | 0.67 (0.52-0.86) | 0.002 | 1.05 (0.77-1.42) | 0.78 | |
| Body mass index (kg/m2) | 1.01 (0.99-1.04) | 0.21 | – | – | 1.02 (0.99-1.04) | 0.14 | – | – | |
| EuroSCORE II (%) | 1.00 (0.97-1.02) | 0.89 | – | – | 1.00 (0.97-1.03) | 0.92 | – | – | |
| Diabetes mellitus | 1.32 (1.02-1.70) | 0.033 | 1.38 (1.05-1.80) | 0.021 | 1.36 (1.03-1.78) | 0.027 | 1.38 (1.05-1.81) | 0.020 | |
| Extracardiac arteriopathy | 1.04 (0.79-1.35) | 0.79 | – | – | 1.04 (0.77-1.39) | 0.77 | – | – | |
| Chronic renal replacement therapy | 1.43 (0.74-2.58) | 0.25 | – | – | 1.54 (0.77-2.84) | 0.19 | – | – | |
| Prior cardiac surgery | 0.72 (0.45-1.12) | 0.17 | – | – | 0.82 (0.50-1.29) | 0.41 | – | – | |
| Prior left bundle branch block | 0.83 (0.51-1.28) | 0.42 | – | – | 0.86 (0.52-1.36) | 0.54 | – | – | |
| Prior right bundle branch block | 7.08 (5.17-9.71) | <0.001 | 7.19 (5.18-10.0) | <0.001 | 7.16 (5.17-9.92) | <0.001 | 7.13 (5.14-9.90) | <0.001 | |
| Atrial fibrillation/flutter | 1.09 (0.85-1.38) | 0.50 | – | – | 1.07 (0.83-1.37) | 0.62 | – | – | |
| LVEF (<50% vs ≥50%) | 1.15 (0.87-1.49) | 0.32 | – | – | 1.17 (0.88-1.55) | 0.28 | – | – | |
| Bicuspid aortic valve | 1.74 (0.84-3.34) | 0.11 | – | – | 2.08 (0.96-4.18) | 0.049 | 2.15 (0.97-4.44) | 0.047 | |
| Annulus calcification (high vs low) | 1.18 (0.93-1.49) | 0.18 | – | – | 1.23 (0.95-1.58) | 0.11 | – | – | |
| Perimeter-derived annulus diameter (mm) | 1.12 (1.07-1.18) | <0.001 | 1.03 (0.92-1.15) | 0.64 | 1.13 (1.07-1.19) | <0.001 | 1.02 (0.91-1.14) | 0.77 | |
| Main access (non-TF vs TF) | 1.65 (0.95-2.73) | 0.060 | 1.45 (0.80-2.51) | 0.21 | 1.69 (0.95-2.88) | 0.061 | 1.49 (0.82-2.57) | 0.17 | |
| Valve size | 26 mm vs 23 mm | 2.04 (0.81-6.84) | 0.18 | 2.00 (0.74-7.03) | 0.21 | 2.54 (0.91-10.6) | 0.12 | 2.22 (0.83-7.79) | 0.15 |
| 29 mm vs 23 mm | 3.44 (1.40-11.4) | 0.018 | 2.80 (0.95-10.4) | 0.085 | 4.04 (1.47-16.7) | 0.019 | 3.29 (1.13-12.2) | 0.046 | |
| 34 mm vs 23 mm | 4.16 (1.66-14.0) | 0.007 | 3.39 (0.93-14.8) | 0.079 | 5.28 (1.89-22.0) | 0.006 | 3.73 (1.04-16.1) | 0.057 | |
| Valve type | Evolut R vs Evolut PRO | 0.76 (0.57-1.02) | 0.060 | 0.73 (0.53-1.02) | 0.058 | 0.81 (0.60-1.11) | 0.18 | – | – |
| Evolut PRO+ vs Evolut PRO | 1.64 (0.62-3.85) | 0.28 | 1.75 (0.61-4.52) | 0.27 | – | – | – | – | |
| Predilatation | 1.11 (0.88-1.41) | 0.37 | – | – | 1.13 (0.88-1.45) | 0.35 | – | – | |
| Postdilatation | 0.78 (0.59-1.02) | 0.071 | 0.75 (0.55-1.00) | 0.056 | 0.84 (0.62-1.11) | 0.22 | – | – | |
| CI: confidence interval; EuroSCORE II: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; OR: odds ratio; TF: transfemoral | |||||||||
Table 5. Univariable logistic regression analysis according to cusp overlap or standard three-cusp implantation technique for the prediction of permanent pacemaker implantation.
| Pre-matching | Post-matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cusp overlapOR (95% CI) | p-value | Standard three-cuspOR (95% CI) | p-value | Cusp overlapOR (95% CI) | p-value | Standard three-cuspOR (95% CI) | p-value | ||
| Centre | 2 vs 1 | 0.57 (0.13-1.70) | 0.38 | 0.73 (0.36-1.38) | 0.35 | 0.57 (0.13-1.70) | 0.38 | 0.63 (0.30-1.24) | 0.20 |
| 3 vs 1 | 1.19 (0.75-1.89) | 0.47 | 0.91 (0.57-1.45) | 0.70 | 1.06 (0.66-1.72) | 0.81 | 0.83 (0.51-1.35) | 0.46 | |
| 4 vs 1 | 0.72 (0.36-1.38) | 0.34 | 1.00 (0.69-1.48) | 0.98 | 0.70 (0.35-1.35) | 0.31 | 0.95 (0.63-1.44) | 0.81 | |
| 5 vs 1 | 1.21 (0.70-2.09) | 0.49 | 0.85 (0.31-2.00) | 0.72 | 1.11 (0.63-1.95) | 0.71 | 0.76 (0.25-1.92) | 0.59 | |
| Procedure timea | second vs first tertile | 0.70 (0.39-1.31) | 0.26 | 0.95 (0.69-1.31) | 0.76 | 0.69 (0.37-1.32) | 0.25 | 1.05 (0.74-1.49) | 0.77 |
| third vs first tertile | 0.73 (0.43-1.29) | 0.26 | 0.77 (0.39-1.42) | 0.43 | 0.72 (0.42-1.30) | 0.26 | 0.76 (0.36-1.47) | 0.44 | |
| Age (≥75 years vs <75 years) | 2.31 (1.21-4.99) | 0.019 | 1.14 (0.68-2.01) | 0.64 | 2.18 (1.10-4.96) | 0.040 | 1.22 (0.70-2.30) | – | |
| Female sex | 0.62 (0.42-0.89) | 0.011 | 0.73 (0.53-0.99) | 0.042 | 0.59 (0.40-0.86) | 0.007 | 0.73 (0.52-1.01) | 0.059 | |
| Body mass index (kg/m2) | 1.00 (0.96-1.03) | 0.90 | 1.03 (1.00-1.06) | 0.056 | 1.01 (0.97-1.04) | 0.79 | 1.03 (1.00-1.06) | 0.087 | |
| EuroSCORE II (%) | 0.99 (0.94-1.03) | 0.54 | 1.00 (0.97-1.03) | 0.97 | 0.99 (0.94-1.03) | 0.58 | 1.01 (0.97-1.05) | 0.71 | |
| Extracardiac arteriopathy | 0.83 (0.52-1.29) | 0.42 | 1.15 (0.81-1.62) | 0.42 | 0.79 (0.48-1.26) | 0.35 | 1.24 (0.85-1.80) | 0.26 | |
| Diabetes mellitus | 1.32 (0.88-1.95) | 0.17 | 1.33 (0.95-1.86) | 0.095 | 1.52 (1.01-2.28) | 0.043 | 1.25 (0.86-1.79) | 0.23 | |
| Chronic renal replacement therapy | 1.06 (0.31-2.76) | 0.92 | 1.72 (0.75-3.60) | 0.17 | 1.15 (0.33-3.01) | 0.80 | 1.90 (0.78-4.22) | 0.13 | |
| Prior cardiac surgery | 0.62 (0.28-1.19) | 0.18 | 0.88 (0.46-1.58) | 0.69 | 0.68 (0.30-1.36) | 0.31 | 0.98 (0.50-1.76) | 0.94 | |
| Prior left bundle branch block | 0.72 (0.33-1.39) | 0.36 | 0.96 (0.51-1.69) | 0.89 | 0.74 (0.32-1.50) | 0.44 | 0.98 (0.50-1.76) | 0.94 | |
| Prior right bundle branch block | 7.58 (4.78-12.0) | <0.001 | 7.09 (4.57-11.1) | <0.001 | 8.14 (5.06-13.1) | <0.001 | 6.65 (4.22-10.5) | <0.001 | |
| Atrial fibrillation/flutter | 1.30 (0.90-1.88) | 0.16 | 0.95 (0.69-1.30) | 0.75 | 1.17 (0.79-1.72) | 0.44 | 1.00 (0.71-1.40) | >0.99 | |
| LVEF (<50% vs ≥50%) | 1.17 (0.76-1.76) | 0.46 | 1.13 (0.79-1.59) | 0.51 | 1.32 (0.85-2.01) | 0.21 | 1.07 (0.72 -1.56) | – | |
| Bicuspid aortic valve | 1.82 (0.66-4.27) | 0.20 | 1.90 (0.60-5.09) | 0.23 | 2.38 (0.77-6.21) | 0.10 | 1.91 (0.60-5.13) | 0.23 | |
| Annulus calcification (high vs low) | 1.20 (0.83-1.74) | 0.33 | 1.19 (0.87-1.62) | 0.27 | 1.26 (0.86-1.86) | 0.24 | 1.21 (0.87-1.69) | 0.25 | |
| Perimeter-derived annulus diameter (mm) | 1.12 (1.04-1.21) | 0.004 | 1.12 (1.05-1.20) | <0.001 | 1.11 (1.02-1.20) | 0.016 | 1.14 (1.06-1.23) | <0.001 | |
| Main access (non-TF vs TF) | 1.70 (0.75-3.44) | 0.17 | 1.72 (0.78-3.47) | 0.15 | 1.52 (0.61-3.30) | 0.33 | 1.94 (0.87-3.97) | 0.084 | |
| Valve size | 26 mm vs 23 mm | 4.22 (0.87-76.1) | 0.16 | 1.31 (0.44-5.64) | 0.67 | 4.01 (0.82-72.4) | 0.18 | 1.81 (0.51-11.5) | 0.43 |
| 29 mm vs 23 mm | 4.79 (1.01-85.9) | 0.13 | 2.93 (1.03-12.3) | 0.078 | 4.26 (0.89-76.5) | 0.16 | 3.94 (1.16-24.6) | 0.064 | |
| 34 mm vs 23 mm | 8.46 (1.73-153) | 0.038 | 2.72 (0.92-11.7) | 0.11 | 7.53 (1.53-136) | 0.051 | 4.11 (1.17-26.1) | 0.060 | |
| Valve type | Evolut R vs Evolut PRO | 0.76 (0.49-1.22) | 0.25 | 0.74 (0.51-1.09) | 0.12 | 0.73 (0.47-1.18) | 0.18 | 0.87 (0.58-1.33) | 0.51 |
| Evolut PRO+ vs Evolut PRO | 2.09 (0.76-5.21) | 0.13 | – | – | – | – | – | – | |
| Predilatation | 1.41 (0.97-2.09) | 0.076 | 1.08 (0.79-1.47) | 0.64 | 1.40 (0.95-2.10) | 0.10 | 1.10 (0.78-1.53) | 0.60 | |
| Postdilatation | 0.67 (0.43-1.02) | 0.070 | 0.88 (0.61-1.26) | 0.50 | 0.75 (0.47-1.16) | 0.21 | 0.92 (0.62-1.33) | 0.65 | |
| afirst tertile (01/2016-04/2020), second tertile (04/2020-01/2021) and third tertile (01/2021-07/2022). CI: confidence interval; EuroSCORE II: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; OR: odds ratio; TF: transfemoral | |||||||||
Discussion
This study presents the largest patient series evaluating the use of the COT with the Evolut platform in patients with severe aortic stenosis so far. The main findings were that (1) the COT compared to the 3CT reduces the rate of PPI in a large, unselected, real-world cohort without increasing complication rates; (2) the reduction in PPI rate was consistent in both the overall and propensity score-matched analyses and consistent across all subgroups; (3) the COT was associated with a lower rate of moderate/severe PVR; and (4) the COT did not increase procedure and radiation time.
The incidence of PPI after TAVI ranged from 10.8-20.7% for the supra-annular self-expanding Evolut family across observational cohorts and randomised interventional studies1319202122 and (Harvey J. Decreasing Permanent Pacemaker Implantation Rates in the STS/ACC TVT Registry with a Supra-annular Self-expanding Transcatheter Heart Valve. TVT 2022. Chicago, IL, USA). In the Evolut Low Risk trial, PPI rates at 30 days were 3-fold higher in patients who underwent TAVI compared with those who had surgical aortic valve replacement (17.4% vs 6.1%)1. Among the Evolut device systems, the Evolut R and its successors, the Evolut PRO and PRO+, which have an additional outer pericardial skirt to improve valve-sealing performance and, therefore, reduce PVR, are in widespread utilisation.
It has been hypothesised that a reduced pressure per mm2 of tissue might be applied by the porcine pericardial wrap compared to the bare metallic frame of the Evolut R, leading to a lower incidence of PPI23. In contrast, rates of PPI were similar, with 15.3% for the Evolut R and 14.2% for the Evolut PRO in earlier reports of the Society of Thoracic Surgeons-American College of Cardiology Transcatheter Valve Therapy Registry22, at 30 days. Notably, in the same registry, the rate of PPI declined to 10.8% for all Evolut prostheses at 30 days in the second quarter of 2021 (idem Harvey J.). More recently, an interim analysis of the Optimize PRO study yielded a PPI rate of 9.2% for the Evolut PRO/PRO+ devices at 30 days (Grubb K, et al. Impact of standardised TAVI technique and care pathway in the Optimize PRO study. EuroPCR 2022. Paris, France).
However, the frequency of new PPI remained high for Evolut PRO/PRO+ devices with 17.9% and 15.6% compared to the SAPIEN 3 Ultra (Edwards Lifesciences; 10.1%) and the ACURATE neo2 (Boston Scientific; 7.7%) in contemporary studies2021 at 30 days.
PPI after TAVI might be associated with a long-term elevated risk of hospitalisation for heart failure and all-cause mortality67. Several non-modifiable risk factors elevate the risk for PPI, including conduction disturbances (especially RBBB), sex, age and anatomical factors24. However, potentially modifiable risk factors like ID might reduce the risk of PPI8. Hence, new implantation techniques may pose an important development to further improve TAVI outcomes.
Mechanistically, an implantation technique aiming at a higher implantation position may reduce PPI rates after TAVI. The COT was first described by Tang et al in 2018 with wide adoption in most centres since 20209. The key features of this approach are: 1) isolation of the NCC to visualise the most inferior point of the aortic annulus; 2) improved visualisation of the LVOT and aortic root; 3) the elimination of parallax; and 4) better anatomical alignment. Thereby, the technique enables a higher position for the implant. In addition, the valve deployment process is started above the aortic annulus, furthermore minimising LVOT and conduction system interactions.
Here, we analysed the largest real-world, multicentre study, so far, with regard to the COT in the Evolut platform. We observed an absolute reduction in the risk for PPI by 4.7% to a PPI rate of 12.3% with the COT at discharge (Central illustration).
Interestingly, the observed reduction in PPI rate when the COT was applied is lower compared to other recently published reports. Mendiz (COT group; n=156) et al showed a reduction from 17.8% to 6.4% (p=0.004), without an increase in adverse events13. Similarly, Pascual et al yielded a lower new PPI incidence (12.4% vs 23%; p=0.037)11 in a single-centre study and confirmed these findings (11.8% vs 21.7%; p=0.03) in a smaller propensity-matched cohort (COT group; n=161) from two centres at 30 days10. Doldi et al (COT group; n=61) yielded similar technical success between 3CT and COT patients with a reduction of the PPI rate from 27.9% to 13.1% (p=0.047)12. According to Maier et al (COT group; n=150), use of the COT significantly reduced the need for PPI (16.8 vs 8.0%; p=0.028) at discharge14.
To verify these partly striking findings, we designed this multicentre cohort to achieve larger sample sizes and analyse propensity score-derived matchings. The reduction of PPI incidence was observed in all subgroups. We observed a higher rate of predilatation in the COT group, which showed a trend towards higher rates of conduction disturbances and PPI. Nevertheless, studies reported conflicting results regarding predilatation and the frequency of PPI2526.
Petronio et al showed that a lower ID led to more conduction disturbances and higher rates of PPI8. The recommended ID was 3 to 5 mm of the prosthesis length below the annular plane, but since 2020, with the adoption of the COT, higher IDs with a target ID of 3 mm have been emphasised. We did not assess ID in this large-scale cohort, as there is no clear consensus for standard ID assessment. Furthermore, Vora et al have shown that angiography- and CT-based ID assessment following TAVI differ27. Of note, angiography of the ID records shallower assessments compared to CT. However, two studies have reported higher valve ID with the COT1014. By measuring from the NCC to the distal end of the THV, Pascual et al found a significant difference for the COT versus the 3CT with a mean ID of 4.2±2.1 mm vs 5.3±2.6 mm (p<0.001), whereas the deepest edge of the ID was not statistically different between implantation methods10. Of note, Attizzani et al found a shallower ID of 3.8±2.1 for the low-risk trial28. Nevertheless, in this randomised trial, the PPI rate was 17.4% (1). Barthélémy et al commented that differences in ID of <1 mm should be considered in view of the knowledge that angiographic spatial resolution is roughly 0.2 mm and parallax issues can occur29.
We found that predictors of PPI were baseline RBBB, diabetes and a larger aortic annulus diameter on CT, which is in line with previous studies2430. These strong predictors were not altered using the COT or 3CT in our cohort. Interestingly, a higher rate of LBBB was observed using the COT. This might have been caused by higher rates of interference with the conduction tissue without complete block of the atrioventricular conduction. However, multiple mechanisms are known to impact the appearance and duration of LBBB following TAVI31. The same trend regarding higher rates of LBBB without reaching statistical significance was observed in two studies by Pascual et al1011, with lower rates of LBBB (COT: 20.5% vs 3CT: 16.8% and COT: 20.6% vs 3CT: 14.7%, respectively). Other studies observed a lower incidence of new-onset LBBB using the COT1314.
Although a high implantation seems to lower the PPI rate, the risk of valve embolisation and aortic regurgitation might arise. As previously published, the COT did not increase the risk for valve embolisation and second valve implantations. Most interestingly though, we observed a decrease in moderate to severe PVR using the COT. It may be speculated that the COT leads to higher implantation, thereby applying more radial force on the annulus, as the Evolut platform has a conical valve design. This might result in a lower rate of significant PVR, which might translate into better long-term clinical outcomes32. Surprisingly, we observed a numerically higher rate of moderate or severe PVR with the Evolut PRO/PRO+ compared to recent publications2021 and (idem J. Harvey). One explanation could be the limited use of the more advanced PRO/PRO+ system in more complex anatomies and challenging cases. Furthermore, as only 20% of patients were treated with a PRO/PRO+ system, these findings should be interpreted with caution.
Additionally, there was no difference regarding transvalvular gradients among both groups. Whereas clinical event rates for life-threatening bleeding and vascular complications were slightly higher in all groups compared to previous TAVI studies31922, we did find a decrease in major bleeding rates with the COT. While registries and all-comer studies might be associated with increased complications due to less selected patient cohorts, the decrease in major bleeding and the trend of lower vascular complication rates might be associated with the refinement of vascular closure techniques over time. Also, ultrasound-guided puncture might be more common in recent COT cohorts with potential benefits33. We observed a non-significantly lower OR for PPI in patients treated at the end of the study period, therefore a learning curve might have played a role in the different outcomes.
Furthermore, additional intriguing variations in procedural and hospitalisation characteristics were found between the COT and 3CT. For example, procedural and fluoroscopy times were slightly lower for the COT compared to 3CT, respectively. Thereby, we can summarise that the COT should not be considered a more complex procedure. Most interestingly, patients treated with the COT had a shorter median length of stay, indicating optimised patient care. This could also potentially be due to the trend of earlier discharge in recent years.
Study limitations
First, our data were obtained during routine clinical care, representing a contemporary, real-world experience among multiple high-volume centres with highly experienced operators and were subject to the typical limitations inherent in this study design. Second, although we tried to reduce bias by reporting both propensity score-matched and -unmatched study results, we cannot exclude potential confounders deriving both from individual patient characteristics and operators’ decisions. Third, CT measurements can be prone to intersite and interobserver variability in assessment methods on valve calcification pattern/distribution, and the ID of the valve prosthesis was not measured. In addition, information regarding calcification was missing in 31.9% of cases and had to be randomly imputed. Fourth, the decision whether to implant a PPI was based on the decision of the treating physicians. Fifth, the assessment of novel conduction abnormalities was not prespecified. Rates are highly dynamic depending on the time of measurement, as numerous patients recover from conduction abnormalities. Sixth, our study was focused on the Evolut THV family, and the most often used device was the Evolut R prosthesis. Last, the study follow-up period was limited to in-hospital outcomes.
Conclusions
In this multicentre study, comprising the largest cohort of patients who underwent TAVI with self-expanding transcatheter aortic Evolut valves, the COT reduced PPI and significant PVR without increasing the rate of significant major adverse cardiac events compared with the 3CT.
Impact on daily practice
In this multicentre study with more than 2,000 patients undergoing TAVI for severe aortic stenosis with the Evolut self-expanding platform, the application of the COT lowered PPI rates from 17.0% to 12.3% compared to the standard 3CT. Both implantation techniques showed equally high performance regarding haemodynamic and clinical outcome parameters, while more than moderate PVR was less common in the COT group. Potential further advantages, such as commissural alignment, might also be more easily obtained with this technique but were not examined. As COT is applied by multiple centres in a systematic manner with reassuring results, an expertise-based randomised trial to compare the 3CT and COT might be difficult to conduct. Longer-term follow-up studies are required to confirm these beneficial outcomes.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) − Grant No. 397484323-TRR259.
Conflict of interest statement
M. Adam reports personal fees and speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic. S. Baldus reports lecture fees from JenaValve and lecture and speaker fees from Edwards Lifesciences. S. Bleiziffer reports speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. E. Kuhn reports consultancy and personal fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. O. Maier reports research funding from Medtronic. T. K. Rudolph reports speaker honoraria from Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic. A. Schaefer reports speaker honoraria from Abbott. V. Veulemans and T.Zeus report consulting fees, travel expenses, or study honoraria from Boston Scientific, Edwards Lifesciences, and Medtronic. S.Zimmer reports speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

