
Introduction
Transcatheter aortic valve implantation (TAVI) in patients with bicuspid aortic valves (BAV) may be associated with suboptimal procedural and clinical outcomes due to inappropriate prosthesis sizing, anatomical difficulties or technical factors1. The narrowest part of the aortic root in BAV anatomy is evident at the supra-annular (SA) level, across the aortic valve leaflets, instead of the annular virtual basal ring (VBR)2. The main objective of our study was to identify a supra-annular plane in the pre-TAVI cardiac computed tomography (CT) scan that might predict the level of valve prosthesis anchoring in raphe-type BAV patients.
Methods
All patients with available CT scans before and after TAVI performed in three high-volume centres between January 2007 and March 2018 were screened retrospectively. After exclusion of patients with trileaflet aortic valve anatomy and patients with bicuspid type 0, all patients with BAV type 1 and 2 were included in the final analysis. Post-procedural CT scans had been performed due to enrolment in in-hospital clinical studies. Data collection at each participating site was performed according to the local institutional review board/ethics committee policies.
On the pre-TAVI CT scans, we identified the aortic root level where prosthesis anchoring was expected to occur, defined as the Level of Implantation at the RAphe (LIRA) plane, that takes into account BAV type and the following anatomical-functional assumptions:
1. In case of BAV type 1, the prosthesis should anchor at the level of the calcific/fibrotic raphe along the aortic root. Therefore, the LIRA plane was identified as the plane that cuts the raphe at its maximum protrusion along the aortic root (Figure 1A-Figure 1C).
2. BAV type 2 anatomy presents two raphes; therefore, the prosthesis should anchor at the level of the major raphe that was defined as the larger, in terms of mm in width and length, and with the greater amount of calcium. Therefore, the LIRA plane was identified as the level that cuts the major raphe at its maximum protrusion along the aortic root (Figure 1D).
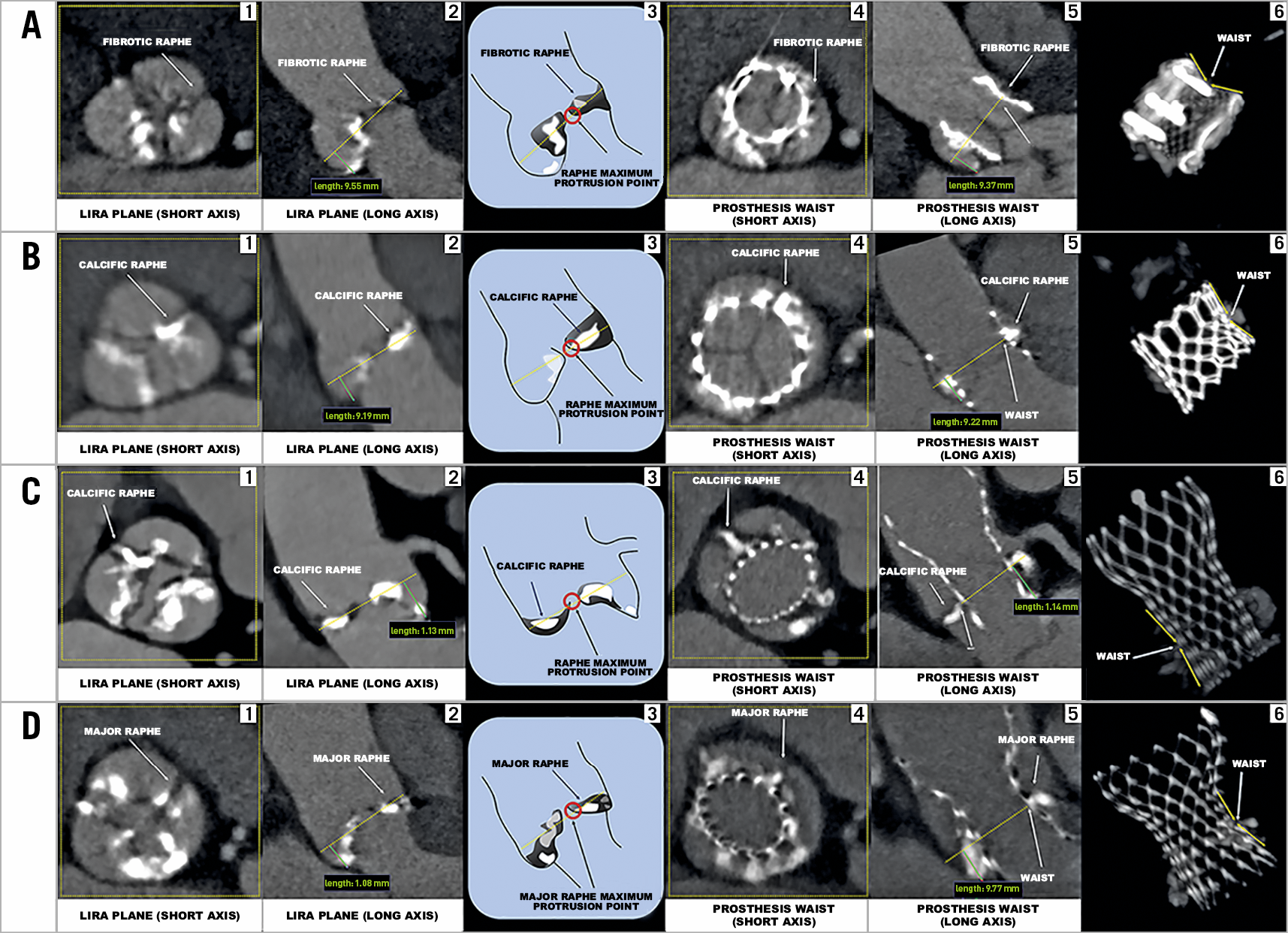
Figure 1. Determining the LIRA plane and the prosthesis waist in different BAV anatomies. A) (1-6): BAV type 1 with a fibrotic raphe where a Lotus™ valve (Boston Scientific, Marlborough, MA, USA) was implanted. B) (1-6): BAV type 1 with a calcific raphe where a SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) was implanted. C) (1-6): BAV type 1 with a calcific raphe where a CoreValve® (Medtronic, Minneapolis, MN, USA) was implanted. D) (1-6): BAV type 2 where a CoreValve was implanted. 1. LIRA plane with a fibrotic/calcific raphe on the short axis. 2. LIRA plane on the long axis corresponding to the cut at the raphe maximum protrusion level (dotted yellow line). 3. The raphe maximum protrusion point and the LIRA plane (dotted yellow line). 4. Prosthesis waist on the short axis. 5. Prosthesis waist on the long axis corresponding to the level where the valvular frame becomes less expanded in the transverse direction along the aortic root due to the compression by the surrounding anatomy (fibrotic/calcific raphe). 6. 3D image that indicates the prosthesis waist.
In post-TAVI CT scans, we identified the prosthesis anchoring level where the valvular frame becomes less expanded in the transverse direction along the aortic root (prosthesis waist) in relation to the segment of the prosthetic frame analysed and the degree of compression by the surrounding anatomy. Therefore, we evaluated whether the LIRA plane predicted the anchoring level of the prosthesis valvular frame by comparing the distance of the LIRA plane from the VBR (VBR-LIRA) in pre-TAVI CT scans with the distance of the prosthesis waist from the VBR (VBR-waist) in post-TAVI CT scans. To test for differences in means between groups with continuous variables, a Student’s t-test was used.
Results
A total of 65 patients with BAV were included. CT scans identified 59 patients (91%) with type 1 and six patients (9%) with type 2 BAV anatomies (Table 1). The VBR perimeter and area were significantly larger than the LIRA plane measurements (p<0.001), conferring the typical aortic root “volcano” shape of BAV disease (Table 1). Different types of TAVI valve were implanted (Supplementary Table 1), and the prostheses were sized according to the VBR. The VBR-LIRA distance in pre-TAVI CT scans was similar to the VBR-waist distance in post-TAVI CT scans (8.4±1.7 and 7.9±1.7 mm, respectively; p=0.10) (Figure 2).
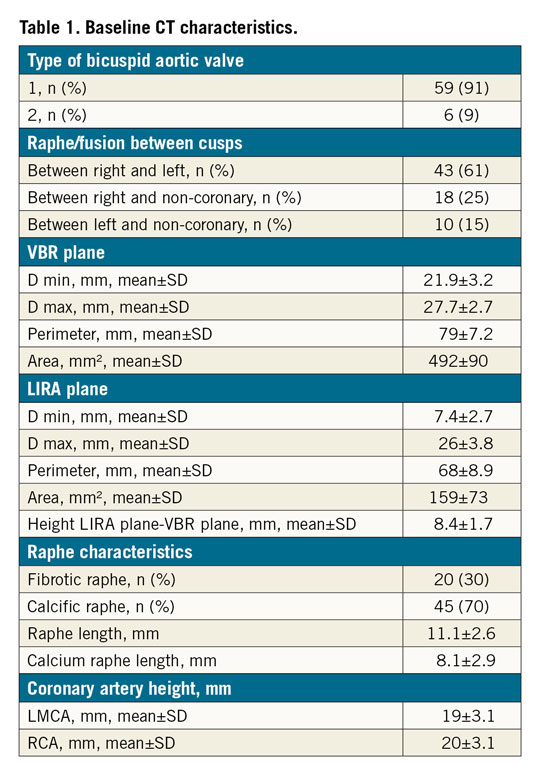
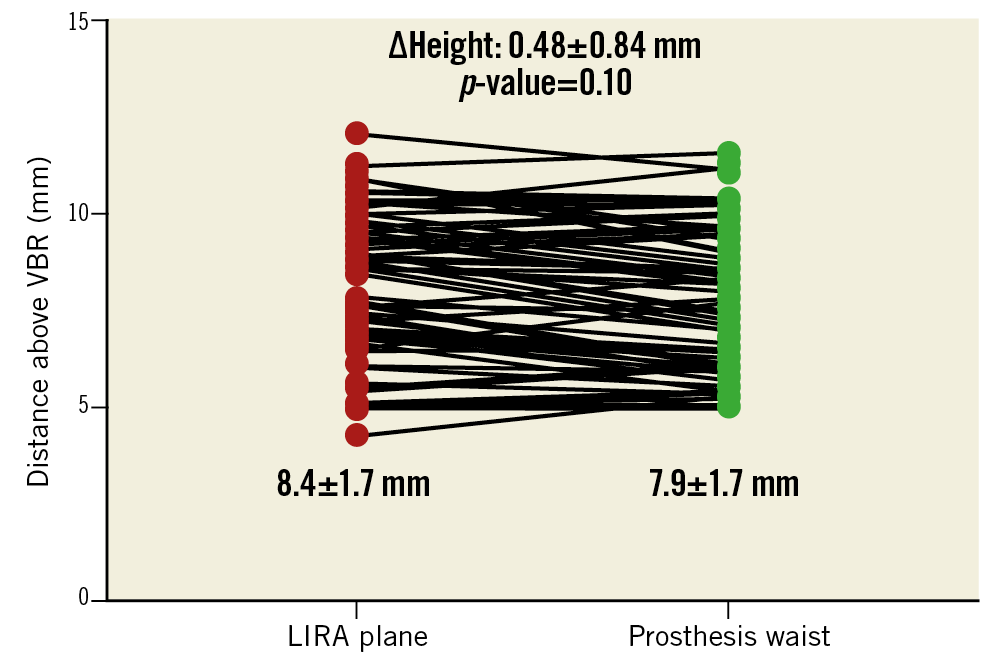
Figure 2. Distance of the LIRA plane from the VBR in pre-TAVI CT scan and distance between the prosthesis waist and the VBR in post-TAVI CT scan. Values are mean. Red dots represent the distance of the LIRA plane from the VBR in pre-TAVI CT scan. Green dots represent the distance between the prosthesis waist and the VBR in post-TAVI CT scan.
Discussion
The main finding of our study is the feasibility of a new method to localise the level of the aortic root at which prosthesis anchoring is expected in raphe-type BAV disease in pre-TAVI CT scans. The LIRA plane takes into account the most rigid anatomical structures in the aortic root (calcific/fibrotic raphes) that might facilitate valve anchoring during deployment. Pre- and post-TAVI CT scans show that the LIRA plane corresponds to the prosthesis waist. Sizing according to the LIRA plane would have suggested the selection of smaller valves in most of the cases considering the significantly smaller area and perimeter of the plane. Future studies will need to evaluate a prosthesis sizing method at the LIRA plane and its impact on procedural and clinical outcomes in raphe-type BAV patients.
Limitations
This was a selected population based on the availability of post-procedural CT scans. Secondly, we excluded type 0 BAV because of the absence in this type of anatomy of the raphe that might indicate the anchoring prosthetic level in pre-TAVI CT scans. Finally, a low number of BAV type 2 were included.
Conclusion
The LIRA plane is a new method to localise the level where TAVI prosthesis anchoring is expected to occur in raphe-type BAV disease. Future prospective studies evaluating a sizing method at the LIRA plane and its impact on procedural and clinical outcomes of patients undergoing TAVI in BAV anatomies are warranted.
|
Impact on daily practice Supra-annular assessment of BAV in TAVI is a concept not reproducibly demonstrated, and specific methods to localise a supra-annular plane where prosthesis sizing measurements can be adequately performed are not standardised among operators. We identified a novel supra-annular plane (LIRA plane) to localise the level where TAVI prosthesis anchoring is expected to occur in BAV disease. Future studies will need to evaluate a prosthesis sizing method at the LIRA plane and its impact on procedural and clinical outcomes in raphe-type BAV patients. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Appendix. Study collaborators
Liesbeth Rosseel, MD; Department of Interventional Cardiology, Rigshospitalet, University Hospital, Copenhagen, Denmark. Pal Maurovich-Horvat, MD, PhD; Department of Interventional Cardiology, Heart and Vascular Center, Semmelweis, Budapest, Hungary. Julia Karady, MD; Department of Interventional Cardiology, Heart and Vascular Center, Semmelweis, Budapest, Hungary.
Conflict of interest statement
O. De Backer has received consultancy fees from Abbott and Boston Scientific. L. Søndergaard has received consultancy fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic and Symetis. B. Prendergast has received institutional research grants from Edwards Lifesciences. A. Latib is a consultant for Medtronic and Abbott Vascular. M. Montorfano serves as a proctor for Edwards Lifesciences, Boston Scientific and Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

