
Abstract
Aims: Conflicting data exist on the impact on outcome of the use of different stent types during carotid artery stenting (CAS). The aim of this study was to evaluate clinical outcomes according to different carotid stent design among the population of the European Registry of Carotid Artery Stenting (ERCAS).
Methods and results: The present study was conducted in 1,604 patients who underwent neuroprotected CAS in ERCAS. All types of commercially available carotid stent were used. Open-cell design stents were classified according to free cell area into <7.5 mm2 or >7.5 mm2. A total of 713 closed-cell, 456 hybrid-cell, 238 <7.5 mm2 open-cell, and 197 >7.5 mm2 open-cell stents were implanted. Overall, the 30-day stroke and death rate was 1.37%. At 30 days, 19 strokes occurred (1.18%): eight in the group of patients treated with a closed-cell (1.12%), two in those with a hybrid-cell (0.44%), three in those with a <7.5 mm2 open-cell (1.26%), and six in those treated with a >7.5 mm2 open-cell stent (3.05%) (p=0.045).
Conclusions: Data of the present study suggest that, in the setting of neuroprotected CAS performed in high-volume centres by properly trained operators, the use of an open-cell design stent with a free cell area >7.5 mm2 may be associated with an increased 30-day stroke risk.
Introduction
Carotid artery stenting (CAS) is considered a less invasive treatment alternative to carotid endarterectomy (CEA) to reduce the risk of stroke in patients with significant carotid artery stenosis1. Although some predictors of adverse outcomes after CAS have been identified, the effects of device characteristics, including stent design, on neurologic adverse events have not been established. Carotid stents of different structural designs are available. “Open cell” and closed cell” stent designs differ not only in how the stent struts are connected but also in terms of the range of free cell area between the metal scaffolding2. Recent observational retrospective studies suggest that the use of closed-cell stents may be associated with lower stroke and death rates after CAS compared with open-cell stents3,4. In particular, a large retrospective, non-randomised study showed that, especially in symptomatic patients, who are known to have emboligenic plaque, the choice of a stent with a small free cell area resulted in a significant decrease in post-procedural events3. However, a randomised trial demonstrated no difference in the occurrence of cerebral microembolisations, as detected by diffusion-weighted magnetic resonance imaging (DW-MRI) and transcranial Doppler (TCD), when an open or a closed-cell design stent5 was used, but unfortunately the study was underpowered to detect difference in clinical outcomes. Therefore, at present, little and conflicting evidence exists on the impact on outcome of the use of different carotid stent types during neuroprotected CAS.
The aim of this study was to evaluate clinical outcomes according to different carotid stent design among the patient population of the European Registry of Carotid Artery Stenting (ERCAS)6.
Methods
STUDY POPULATION
From January to December 2007, 1,611 patients underwent CAS at eight different institutions6. The 1,611 patients represent all the CAS procedures performed at the selected centres during the trial period. The selection criterion was the stenosis grade determined based on echo duplex and pre-interventional angiography7,8 according to NASCET criteria:
1) Symptomatic stenosis of the internal carotid artery ≥50%, or
2) Asymptomatic stenosis ≥80% and estimated life expectancy of >5 years.
For the purposes of this study, “symptomatic” patients were defined as those with ipsilateral amaurosis fugax, ipsilateral hemispheric TIA(s), or ipsilateral ischaemic stroke without major disability (Barthel ≤60, National Institutes of Health Stroke Scale [NIHSS] <15, Rankin >3) within six months before intervention. Patients were considered at high surgical risk if presenting with at least one or more high-risk criteria in either medical comorbidities (age >80 years; CCS angina class III-IV or unstable angina; congestive heart failure NYHA Class III-IV; left ventricular ejection fraction <30%; presence of left main stem or ≥ two coronary artery severe stenoses; need for urgent [<30 days] heart surgery; recent myocardial infarction [<30 days]; severe chronic lung disease; severe renal disease) or anatomical criteria (high cervical lesion; lesion below clavicle, prior radical neck surgery, or radiation; CEA restenosis; contralateral carotid occlusion; tracheostomy; contralateral laryngeal nerve palsy)2.
CONCOMITANT THERAPY
All patients received aspirin (75-160 mg/day), and should have been on ticlopidine (250 mg twice daily) or clopidogrel (75 mg once daily) for at least seven days. Alternatively, patients received clopidogrel preload (300 mg) 24 hrs before the procedure. After the stenting procedure, thienopyridines were continued for three months, whereas aspirin was continued for life. For anticoagulation, 70-100 UI/kg of heparin was administered with the intention of achieving an ACT >250 s during the carotid intervention. Additional heparin was administered at the operator’s discretion.
CAS PROCEDURE
All procedures were performed percutaneously, with the patient under local anaesthesia. Each of the primary operators performing the procedures at each centre had to have fulfilled qualification for training and competence as described by the ICCS-SPREAD Joint Committee9, i.e., at least 150 procedures of supra-aortic vessel engagement (during diagnostic as well as interventional procedures) within two years, of which at least 100 were as the primary operator and at least 75 carotid stenting procedures, of which at least 50 were as the primary operator, within a two-year fellowship. The minimum requirement to maintain technical skill (competence) is 50 carotid stenting procedures performed and documented by each primary operator per year. CAS was performed percutaneously and according to each unit’s existing standards of care. All commercially available embolic protection systems (EPS) could be used: distal EPS (distal filters and distal occlusion devices) and proximal EPS (endovascular occlusion and flow reversal). Dedicated self-expanding carotid stents were used: nitinol stents (either closed, open or hybrid cell design) and stainless steel stents were used. Examples of strut design of different types of carotid stent are given in Figure 1. In each centre, an independent neurologist or NIHSS-certified operator evaluated all patients before the procedure and in case of any clinical event occurring during follow-up.
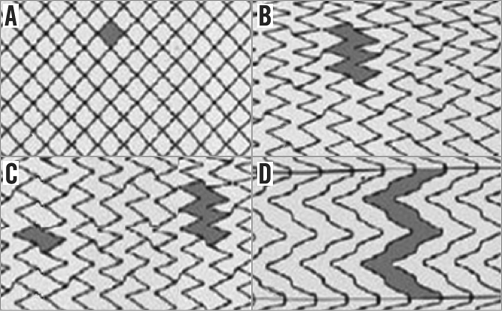
Figure 1. Examples of strut design of different types of carotid stent. A) Closed-cell stent. B) Open-cell stent with free cell area <7.5 mm2. C) Hybrid stent. D) Open-cell stent with free cell area >7.5 mm2.
DEFINITIONS
The primary endpoint of the present study was 30-day survival free of any stroke. The secondary endpoint was 30-day survival free of death and any stroke. Neurological events were classified as one of the following: 1) minor stroke defined as a new neurological deficit that either resolved completely within 30 days or increased the NIHSS by ≤3; 2) major stroke defined as a new neurological deficit that persisted for >30 days and increased the NIHSS by ≥48.
FOLLOW-UP
All patients were followed up at one month after CAS with a clinical examination and a structured questionnaire assessing overall general conditions, with specific emphasis on neurological symptoms, medication, hospitalisations, or any type of complication post procedure.
AUDITING PROCESS
A clinical committee, including three physicians (a neurologist, a cardiologist, and a vascular surgeon) not having any financial relationship with any of the institutions involved in the registry, reviewed all the charts in which any of the clinical events occurred. All of the centres adopted the same worksheet containing multiple entry fields for each treated patient. Ten patient charts were randomly selected in each centre and verified against the reported data. The level of discrepancy between reported data and charts was a measure of compliance. Typing errors were considered irrelevant. Lack of event reporting would have been considered a severe discrepancy leading to centre exclusion from the registry.
STATISTICAL ANALYSIS
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). Continuous variables were reported as mean±standard deviation. Other variables were expressed as absolute numbers and percentage. Comparisons were made by one-way ANOVA or χ2 test as appropriate. All statistical tests were two-sided. For all tests, a p-value <0.05 was considered statistically significant.
Results
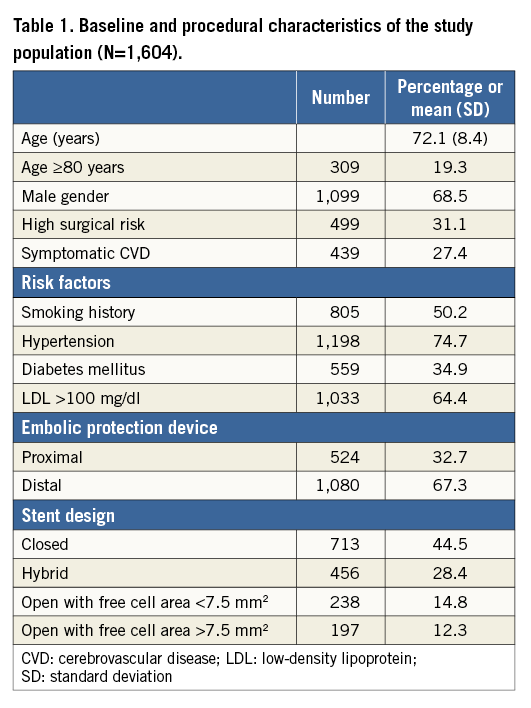
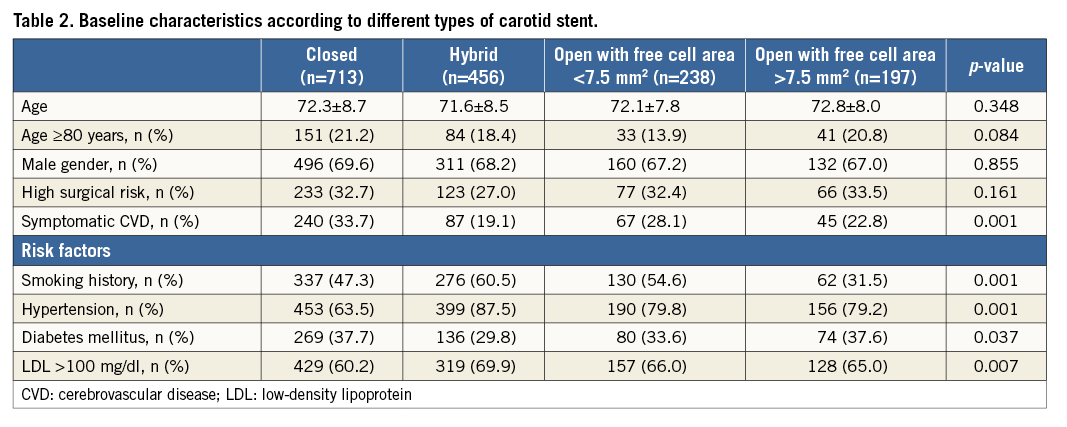
Demographic and procedure-related characteristics of the entire ERCAS registry study population (n=1,611) are reported elsewhere6. No clinically relevant discrepancies between reported data and charts were detected during the audit process. From the original registry population, seven patients were excluded (no events at follow-up), four because no embolic protection device was used, two because more than one stent was implanted, and one since no stent was implanted. Table 1 shows baseline clinical and procedure-related characteristics of the study population (n=1,604). The population enrolled exhibited a robust prevalence of cardiovascular risk factors. Octogenarians were quite numerous (19.3% of the total group) and almost one third of the patients were at high risk for CEA. Almost one third of the patients were symptomatic in the previous six months. Overall, 713 closed-cell, 456 hybrid-cell, 238 <7.5 mm2 open-cell, and 197 >7.5 mm2 open-cell stents were implanted. When comparing patient characteristics among the four cohorts receiving different carotid stents there were significant differences in cardiovascular risk factors (Table 2). Furthermore, symptomatic patients were more likely to receive a closed-cell stent (Table 2).
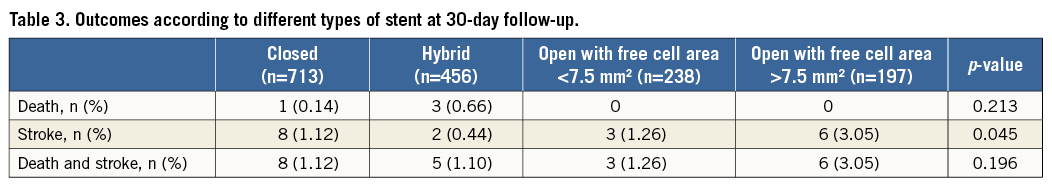
Overall in-hospital mortality was 0.06% (one patient), whereas the in-hospital stroke incidence was 0.50% (eight patients). Of these, six were major strokes and two were minor strokes. During the 30-day follow-up, three deaths (0.19%) and 11 strokes (0.69%) occurred (only one stroke was fatal). Among the strokes, three were major and eight were minor. All strokes were clinically considered ipsilateral to the treated artery and no haemorrhagic strokes occurred. Cumulative 30-day mortality was 0.25% (four patients) and stroke incidence was 1.12% (19 patients). Outcomes at 30-day follow-up according to stent type are reported in Table 3. No difference was observed among the groups for mortality and cumulative death and stroke incidence, whereas there was a significant difference in stroke incidence, with the lowest incidence in patients treated with a hybrid stent and the highest in those treated with an open stent with free cell area >7.5 mm2 (Table 3).
Discussion
The results of this registry suggest that the use of an open-cell design stent with a free cell area >7.5 mm2 may be associated with an increased 30-day stroke risk. The 30-day stroke/death rate in the present registry is comparable to other prospective, multicentre, controlled trials, or registries evaluating CAS in high-volume centres. Similar to other trials and registries, this registry6 confirmed a negative effect of age and of high surgical risk status on CAS outcomes10-12.
Conflicting evidence exists on the impact of the use of different carotid stent types on post-procedural neurologic adverse events. Observational retrospective studies suggest that the use of closed-cell stents was associated with reduced stroke and death rates after CAS compared with open-cell stents3,4. This effect was particularly evident in the treatment of symptomatic patients, who are known to have friable plaque, and the choice of a stent with a small free cell area has been shown to be associated with a significant decrease in post-procedural events3. On the other hand, another retrospective study conducted in 194 patients13 showed that an open-cell stent design was related to fewer cerebral ischaemic lesions following CAS without EPD. Moreover, another large retrospective study14, including a large consecutive number of patients (n=1,684) from 10 European centres, failed to identify any impact of stent design on patients’ clinical outcome, in either the early or the late post-procedure period. This was evident for both symptomatic (n=674) and asymptomatic (n=1,010) patients. Only one small randomised trial demonstrated no difference in the occurrence of cerebral microembolisation with closed-cell and open-cell stents in the setting of neuroprotected CAS, but unfortunately the study was underpowered to detect difference in clinical outcomes5. In line with these results, a large study evaluating the impact of open vs. closed-cell stent design (a registry involving 4,337 CAS patients treated with and without EPD) demonstrated that in-hospital and 30-day death/stroke/myocardial infarction rates were not significantly influenced by stent cell design15.
Operator experience is a well-recognised determinant of post-procedural outcomes12,16,17 and its variability may explain, at least in part, the conflicting findings observed in the above-mentioned studies3-5,13-15. In the ERCAS registry6, all of the operators fulfilled specific and complex criteria of training and clinical competence. This could have eliminated the confounding effect of operator experience on procedural outcomes and allowed us to investigate better the role of patient and procedural characteristics. Other issues may be involved in the conflicting results emerging from studies conducted up until now. First of all, the excellent results with both open and closed-cell stents may not have had enough power to detect a clinical difference between the groups studied. Also, the timing of adverse events could have influenced the different results observed in the literature. Indeed, from a technical point of view, the closed-cell stents may confer benefits in later follow-up while open stents may be more easily advanced through difficult anatomies, affecting in-hospital events.
The present study suggests that, in the setting of neuroprotected CAS performed in high-volume centres by properly trained operators, stent design may affect procedural outcomes. All commercially available carotid stents are able to contain the carotid plaque and avoid the possibility of post-procedural embolisation. However, in the present study, the use of stents with a large free cell area (>7.5 mm2) was associated with a higher risk of post-procedural strokes, probably because of a lower ability to contain the friable atherosclerotic plaque. This could protrude through the stent mesh and possibly embolise distally in the days following the endovascular procedure. In this regard, recent studies using high-resolution imaging modalities such as optical coherence tomography post CAS have shown that plaque prolapse was significantly more frequent in open-cell carotid stents compared to closed-cell design stents18,19.
Study limitations
Although this study involved a large cohort of CAS patients (>1,600), it is a retrospective non-randomised analysis, and the relatively small number of events makes it underpowered to come to definitive conclusions. However, it has to be acknowledged that the excellent results associated with neuroprotected CAS may not easily allow powerful differences to be found between subgroups in real-world settings.
Because patients were not randomised to receive different types of carotid stent and the physician decided at his/her discretion which stent should be used, a selection bias could have occurred. Indeed, in the present study, symptomatic patients were more likely to receive closed-cell design stents compared to asymptomatic ones. However, the fact that closed-cell stents were more likely to be used in patients at higher stroke risk (i.e., symptomatic patients) would, at least theoretically, have produced worse outcomes in the closed-cell stent group. Therefore, although affected by selection bias, the findings of the present study are strongly suggestive of a worse outcome with open-cell design stents with a free cell area >7.5 mm2. Furthermore, it is important to consider that in this registry a post-discharge neurologist’s evaluation was carried out only if a “clinical event occurred”. This is a potential weakness as it could have led to under-reporting of neurologic events. However, such a methodology is largely used in registries reporting CAS and CEA outcomes12. Moreover, it should be considered that these results are not representative of all-comer patients but, in most of the enrolling centres, operators selected upfront which patients would be suitable for CAS and which for CEA.
Conclusions
Data of the present study suggest that, in the setting of neuroprotected CAS performed in high-volume centres by properly trained operators, the use of an open-cell design stent with a free cell area >7.5 mm2 may be associated with an increased 30-day stroke risk. Future randomised and, given the low event rates, larger population trials would ideally be needed to confirm these findings.
| Impact on daily practice At present, little and conflicting evidence exists on the impact on outcome of the use of different carotid stent types during neuroprotected CAS. Data coming from the ERCAS registry suggest that, in the setting of neuroprotected CAS performed in high-volume centres by properly trained operators, the use of an open-cell design stent with a free cell area >7.5 mm2 may be associated with an increased 30-day stroke risk. Even if these findings need to be confirmed in larger, randomised trials, CAS operators should take into account that differences in stent design may affect outcome. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

