Abstract
Aims: Non-compliant balloons provide uniform radial force along the vessel wall at any inflation pressure. As a result, the use of non-compliant balloons may reduce side branch complications and optimise stent deployment. We sought to investigate the impact of non-compliant balloons on the long-term clinical outcomes of patients undergoing a coronary bifurcation intervention.
Methods and results: A total of 2,897 patients treated with drug-eluting stents for bifurcation lesions were enrolled. Non-compliant balloons were used in 752 patients (26%). During a median three-year follow-up, major adverse cardiac events (MACE: cardiac death, myocardial infarction, or target lesion revascularisation) occurred less frequently in the non-compliant balloon group than in the compliant balloon group (8.2% versus 10.9%; p=0.03). After propensity score matching (710 pairs), the use of non-compliant balloons resulted in a lower rate of side branch dissection (0.1% versus 1.1%; p=0.046) and a higher rate of procedural success (79.0% versus 73.9%; p=0.01). The use of non-compliant balloons was associated with a lower risk of MACE (HR 0.64, 95% CI: 0.46-0.91; p=0.01) and cardiac death (HR 0.14, 95% CI: 0.03-0.64; p=0.01).
Conclusions: The use of non-compliant balloons was associated with favourable procedural and long-term clinical outcomes in patients receiving coronary bifurcation intervention. ClinicalTrials.gov number: NCT01642992
Abbreviations
COBIS II: COronary BIfurcation Stent II
DES: drug-eluting stent(s)
MACE: major adverse cardiac events
MI: myocardial infarction
MLD: minimum luminal diameter
MV: main vessel
PCI: percutaneous coronary intervention
RD: reference diameter
SB: side branch
TIMI: Thrombolysis In Myocardial Infarction
TLR: target lesion revascularisation
Introduction
A provisional side branch (SB) intervention after main vessel (MV) stenting is now regarded as the standard technique for the majority of coronary bifurcation lesions1,2. However, SB dissection is a serious procedural complication that can occur during ostial SB balloon dilatation or kissing balloon inflation following MV stenting. Theoretically, the use of a non-compliant balloon could assure uniform diameter expansion along the balloon length, prevent uncontrolled fast stretch of the vessel wall at any inflation pressure, and might avoid consequent SB injury3. In the case of double stenting of both the MV and SB, final kissing balloon inflation is a mandatory step4. The use of non-compliant balloons during final kissing balloon inflation might avoid underexpansion of the MV stent or overexpansion of the SB ostium (Figure 1), and it could facilitate optimal stent deployment and reduce the risk of SB injury1. Despite these theoretical advantages, there are limited data concerning the use of non-compliant balloons in patients with coronary bifurcation lesions5. Therefore, we sought to compare the long-term clinical outcomes of patients treated with non-compliant and those treated with compliant balloons on the basis of a large bifurcation registry.
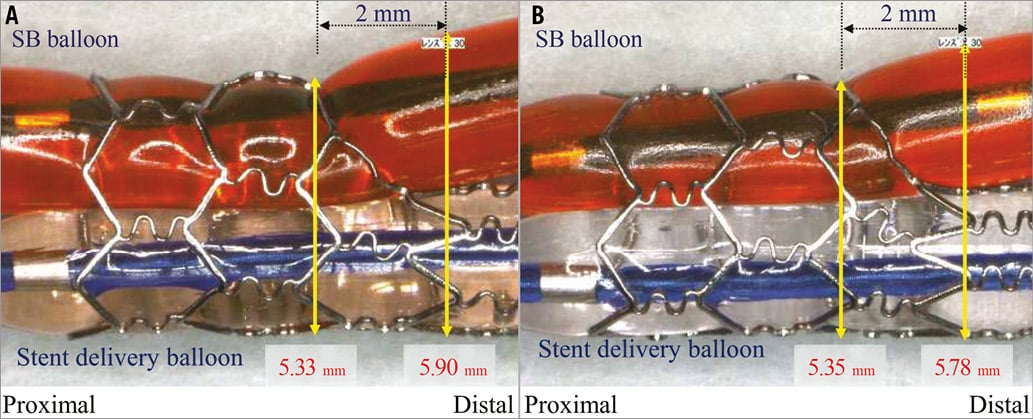
Figure 1. Bench tests of kissing balloon dilatation with compliant and non-compliant balloons. A) Semi-compliant balloon (Ryujin Plus; Terumo Corp. Tokyo, Japan), and (B) non-compliant balloon (Hiryu; Terumo Corp.) in a CYPHER stent (Cordis, Johnson & Johnson, Warren, NJ, USA). Non-compliant balloons avoid overexpansion of the side branch ostium. Image courtesy of Dr Yoshihisa Kinoshita, Division of Cardiology, Toyohashi Heart Center, Toyohashi, Japan.
Methods
STUDY POPULATION
The COronary BIfurcation Stent (COBIS) II registry is an observational, multicentre registry of patients treated with drug-eluting stents (DES) for coronary bifurcation lesions. We enrolled patients from 18 major coronary intervention centres in the Republic of Korea between January 2003 and December 2009. The inclusion criteria were: 1) coronary bifurcation lesions treated with DES alone, and 2) MV diameter ≥2.5 mm and SB diameter ≥2.3 mm by visual inspection. The exclusion criteria were: 1) the presence of cardiogenic shock or cardiopulmonary resuscitation, and 2) protected left main disease. Decisions regarding whether to use non-compliant balloons or not were made by the respective operators. The patients treated only with non-compliant balloons during the entire intervention except for stent deployment belonged to the non-compliant balloon group. The patients in whom compliant balloons were used in any steps were placed in the compliant balloon group. The protocol was approved by the local institutional review board at each hospital, and the need for informed consent for access to each institutional registry was waived. A detailed description of the study procedures is presented in the Online Appendix.
DATA COLLECTION AND ANGIOGRAPHIC ANALYSIS
Data were recorded using a web-based reporting system. Coronary angiograms were analysed at the angiographic core laboratory (Samsung Medical Center, Seoul, Republic of Korea) using an automated edge-detection system (Centricity CA1000; GE Healthcare, Waukesha, WI, USA). Bifurcation lesions were divided into three segments for quantitative coronary angiographic analysis: proximal MV, distal MV, and SB ostium6.
STUDY OUTCOMES AND DEFINITIONS
The primary outcome was major adverse cardiac events (MACE), which were defined as a composite of cardiac death, myocardial infarction (MI), or target lesion revascularisation (TLR) during follow-up. The secondary outcomes included individual components of the composite primary outcome and definite or probable stent thrombosis. A detailed description of study outcome definitions is presented in the Online Appendix.
STATISTICAL ANALYSIS
A detailed description of the statistical analysis is presented in the Online Appendix. Multivariable Cox regression analysis was performed to adjust for potential confounders. The results of the multivariable models were verified using propensity score matching methods. All p-values were two-tailed, and p<0.05 was considered to be statistically significant. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
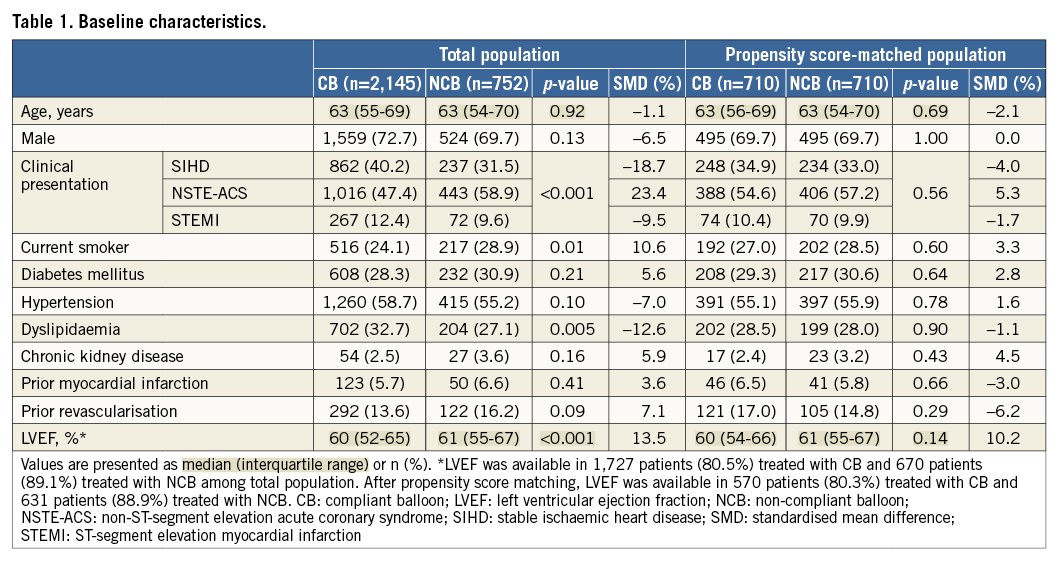
BASELINE CHARACTERISTICS
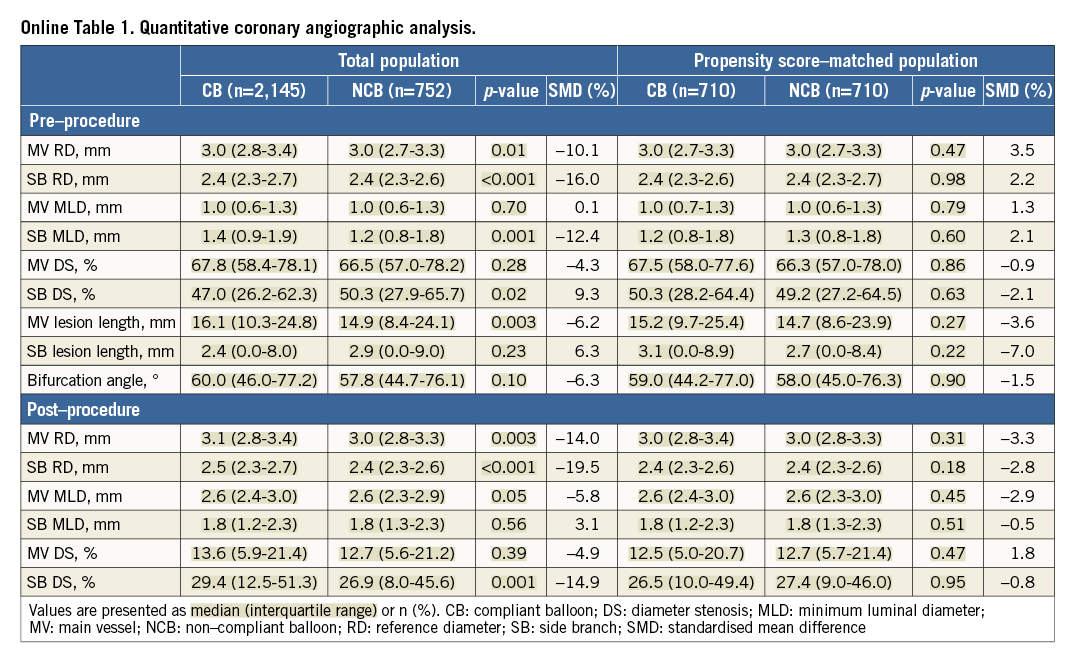
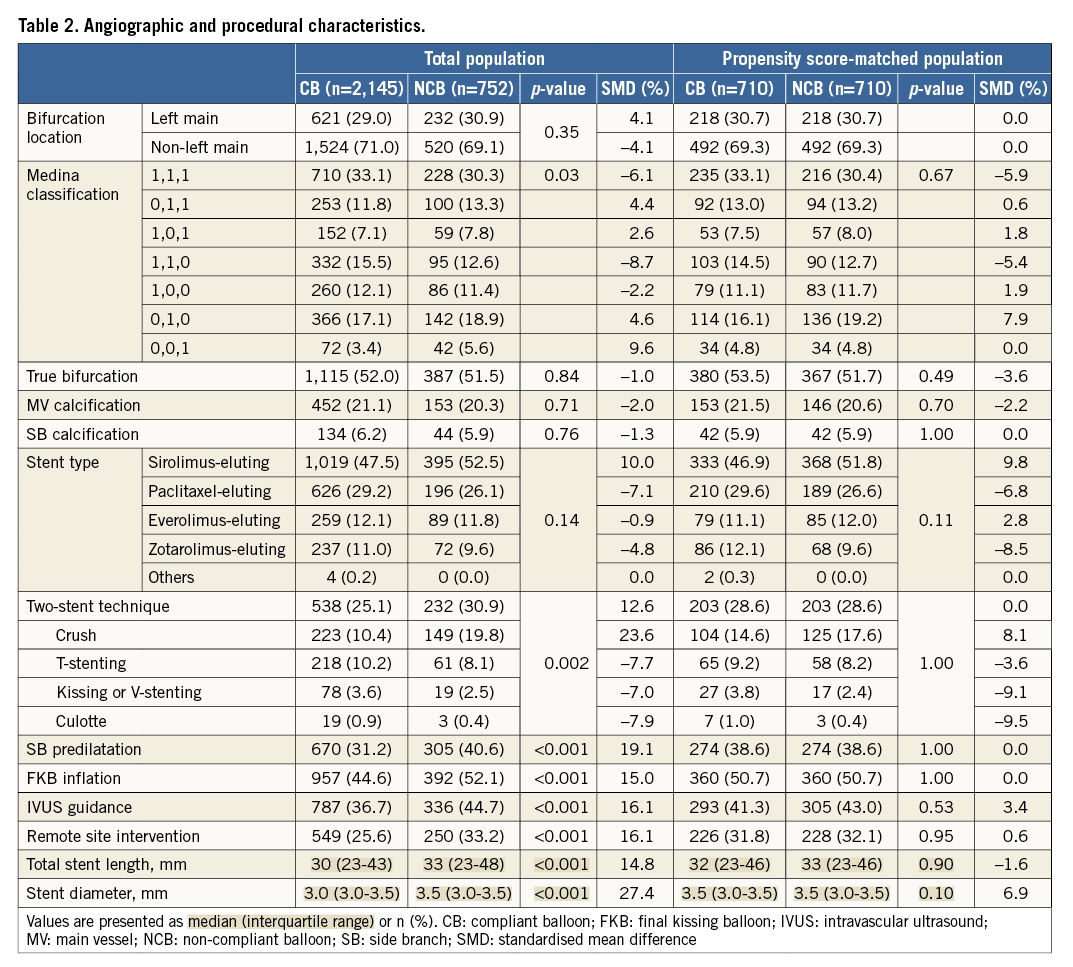
We enrolled 2,897 patients who visited 18 major coronary intervention centres in the Republic of Korea between January 2003 and December 2009: 752 patients (26%) were treated only with non-compliant balloons. The clinical, angiographic, and procedural characteristics of the two groups are shown in Table 1 and Table 2. The non-compliant balloon group had a higher prevalence of acute coronary syndrome at admission and of current smokers. Both balloons were similarly used for left main bifurcation lesions and true bifurcation lesions. A two-stent strategy, SB predilatation, final kissing balloon inflation, intravascular ultrasound, and remote site intervention were performed more frequently in patients treated with a non-compliant balloon. Quantitative coronary angiographic data are presented in Online Table 1. Patients with non-compliant balloons had a smaller pre-procedural reference diameter (RD) of the MV and SB, greater pre-procedural percent diameter stenosis of the SB, and a shorter lesion length of the MV compared to those with compliant balloons.
After propensity score matching, 710 matched pairs were created (Table 1, Table 2, Online Table 1). There were no significant imbalances in baseline variables of the matched population, except for left ventricular ejection fraction.
PROCEDURAL OUTCOMES
As shown in Table 3, SB dissection occurred less frequently in patients treated with non-compliant balloons. The non-compliant balloon group showed a significantly higher rate of angiographic and procedural success than the compliant balloon group. After propensity score matching, the use of non-compliant balloons was associated with a lower risk of SB dissection (non-compliant versus compliant, 0.1% versus 1.1%, p=0.046). The rate of SB angiographic success (79.7% versus 75.4%, p=0.03) and of procedural success (79.0% versus 73.9%, p=0.01) was higher in the non-compliant balloon group.
CLINICAL OUTCOMES
Complete clinical follow-up data were obtained in 96.3% of all patients. The median follow-up duration was 36 months (interquartile range, 27 to 39) in the non-compliant balloon group and 36 months (interquartile range, 24 to 38) in the compliant balloon group. Observed clinical outcomes according to the use of non-compliant balloons are shown in Table 4. The cumulative incidence of MACE was significantly lower in patients treated with non-compliant balloons (8.2%) than in those treated with compliant balloons (10.9%; p=0.03). The non-compliant balloon group also had a significantly lower incidence of cardiac death (Figure 2). Possible stent thrombosis occurred in 27 patients: this was the most common cause of cardiac death. Other causes of cardiac death were the following: definite stent thrombosis in three patients, probable stent thrombosis in six patients, recurrent myocardial infarction in one patient, cardiovascular procedure in two patients (one CABG, one PCI), lethal arrhythmia in two patients, and heart failure in one patient. In multivariable analysis, the adjusted risks for MACE were significantly lower in patients treated with non-compliant balloons than in those with compliant balloons. The use of non-compliant balloons was also associated with a lower risk of cardiac death. The adjusted risk of definite or probable stent thrombosis was similar in both groups, but the use of non-compliant balloons was associated with a lower risk of definite, probable, or possible stent thrombosis.
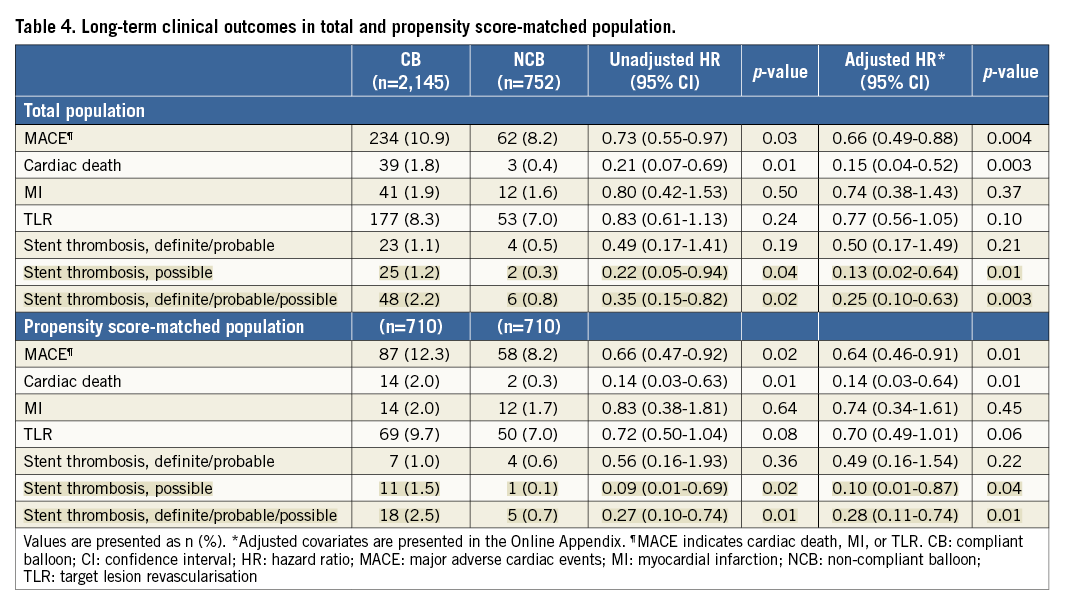
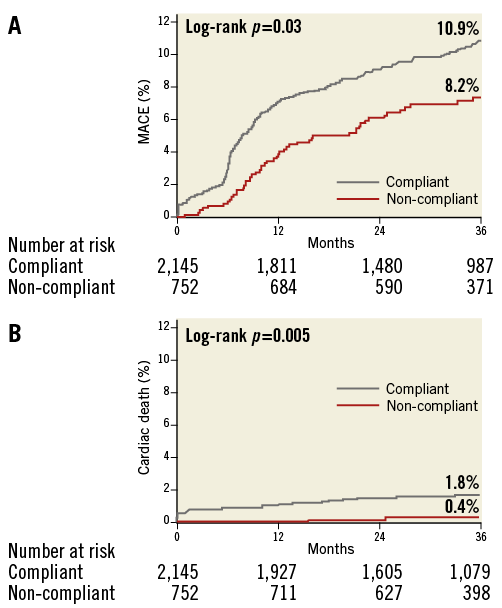
Figure 2. Clinical outcomes during a median three-year follow-up in the total population. Time-to-event curves of major adverse cardiac events (A) and cardiac death (B) in patients treated with non-compliant balloons or compliant balloons.
Figure 3 shows Kaplan-Meier curves for clinical outcomes according to the use of non-compliant balloons in the propensity score-matched population. After multivariable analysis, the risk of MACE was significantly lower in patients treated with non-compliant balloons than in those treated with compliant balloons. The use of non-compliant balloons was also associated with a lower risk of cardiac death and definite, probable, or possible stent thrombosis. The adjusted risk of TLR had a lower tendency in the non-compliant balloon group.
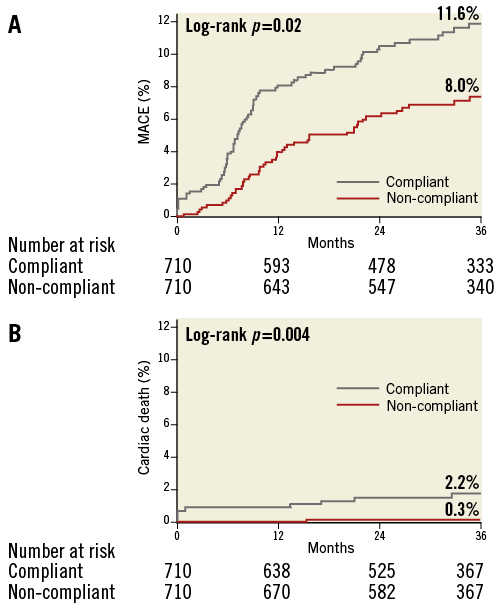
Figure 3. Clinical outcomes during a median three-year follow-up in a propensity score-matched population. Time-to-event curves of major adverse cardiac events (A) and cardiac death (B) in patients treated with non-compliant balloons or compliant balloons.
SUBGROUP ANALYSIS
The adjusted risk of MACE was consistently lower in patients treated with non-compliant balloons than in those treated with compliant balloons among subgroups (Figure 4). There were no significant interactions between the use of non-compliant balloons and MACE in terms of the seven subgroups.
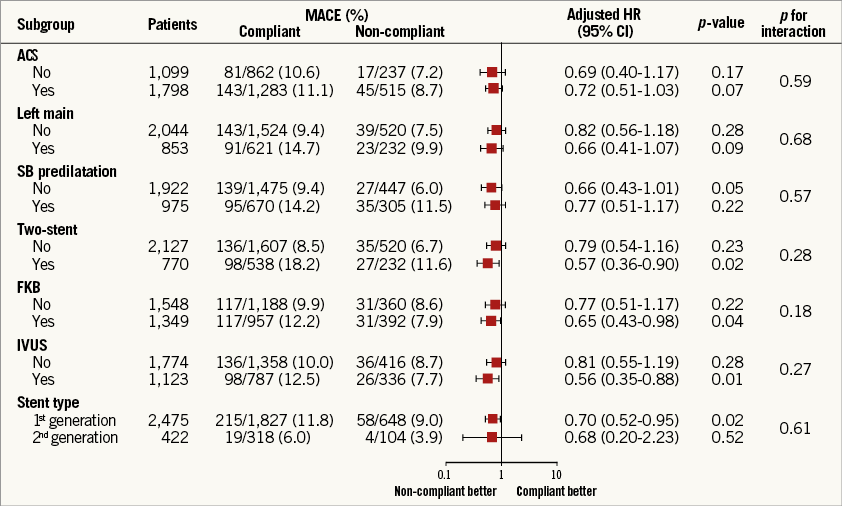
Figure 4. Adjusted hazard ratios of major adverse cardiac events for non-compliant balloon versus compliant balloon in the subgroups. CB: compliant balloon; CI: confidence interval; FKB: final kissing balloon; HR: hazard ratio; IVUS: intravascular ultrasound; MACE: major adverse cardiac events; NCB: non-compliant balloon; SB: side branch
Discussion
This is the first study to compare the long-term clinical outcomes of patients treated with non-compliant or compliant balloons during coronary bifurcation PCI. During a median follow-up duration of 36 months, the use of non-compliant balloons was associated with lower rates of MACE, mainly driven by the reduction of cardiac mortality. These results were validated in a propensity score-matched population. The impact of non-compliant balloon use on the risk of MACE was consistent across all subgroups.
Non-compliant balloons have been used as adjunctive balloons to optimise stent deployment7. In the Post-dilatation Clinical Comparative Study (POSTIT) using bare metal stents, adjunctive post-dilatation with non-compliant balloons increased the frequency of achieving optimal stent deployment from 21% to 42%8. However, in the DES era, the Post-stent Optimal Stent Expansion Trial (POET) failed to demonstrate the superiority of non-compliant balloons in obtaining optimal stent expansion compared with compliant balloons9. The major limitation of these studies was that coronary bifurcation lesions were not included8,9. It is possible that the technical benefit of non-compliant balloons might be more relevant with the treatment of coronary bifurcation lesions. Non-compliant balloons experience little change in volume and tolerate any inflation pressures, thus avoiding the risk of vessel overdilatation and subsequent injury when ostial SB dilatation is required during the one-stent technique3. In the case of final kissing ballooning during dual vessel stenting, non-compliant high-pressure ostial post-inflation was able to achieve full stent expansion and avoid overexpansion of the SB ostium1. Recently, one pilot study proposed the proof-of-concept regarding satisfactory procedural outcomes when using non-compliant balloons for final kissing inflation during treatment of bifurcation lesions5. This pilot study was intended only for specific situations, such as final kissing inflation during a provisional approach, and included a relatively small number of patients; the study also lacked a control arm. Therefore, we assessed the long-term comparative outcomes of treatment with non-compliant or compliant balloons during coronary bifurcation PCI using data from a large, multicentre, bifurcation-dedicated registry.
The use of non-compliant balloons resulted in lower rates of MACE and cardiac death at 36-month follow-up in the total population and in propensity score-matched populations. First of all, it is possible that these clinical differences in MACE and cardiac death might be derived from favourable procedural outcomes by the use of non-compliant balloons. In addition, theoretically, the use of non-compliant balloons during kissing balloon dilatation could facilitate optimal stent deployment, which partly explains the lower rate of MACE. Intravascular ultrasound studies have reported that stent underexpansion was prevalent in patients with stent thrombosis after DES implantation10. In coronary bifurcation lesions, intravascular ultrasound-guided stent optimisation was proposed as a major mechanism to reduce the risk of stent thrombosis11. A digital stent enhancement study showed that adjunctive non-compliant balloon post-dilatation optimised an additional 37% of stents12. Consequently, it is possible that the use of non-compliant balloons increases the achievement of optimal stent expansion, and might play a role in a lower rate of definite, probable, or possible stent thrombosis and cardiac mortality. However, our registry did not include the detailed data of intravascular ultrasound for evaluating stent expansion at the SB ostium before and after the use of non-compliant balloons. Further studies will be necessary to establish the exact mechanism of favourable outcomes of patients treated with non-compliant balloons.
The impact of non-compliant balloon use on the risk of MACE was consistent across all subgroups, including final kissing balloon inflation and stent techniques. It is well known that final kissing ballooning in two-stent techniques is associated with a lower risk of adverse clinical outcomes13. However, in patients treated with provisional SB stenting, final kissing balloon inflation failed to demonstrate clinical benefit14. A prospective pilot study showed that systematic kissing balloon inflation using non-compliant balloons after provisional SB stenting is associated with favourable procedural results and promising clinical outcomes5. In our study, long-term clinical benefits were associated with the use of non-compliant balloons in the overall population and did not have significant interactions in subgroup analysis according to final kissing balloon inflation or stent techniques. The role of non-compliant balloons for final kissing ballooning during provisional SB stenting will need to be re-evaluated by adequately powered studies.
Limitations
Our study could not avoid several of the limitations of a retrospective study. First, the use of non-compliant balloons was not randomised and might reflect individual operator preference. To reduce the selection bias for the use of non-compliant balloons and potential confounding effects, we used multivariable analyses; the results were also verified in a propensity score-matched population. Nevertheless, we were not able to correct for the unmeasured variables. It is difficult to predict how residual confounding can impact on clinical outcomes. Second, the patients in whom compliant balloons were used in any steps were placed in the compliant balloon group. Non-compliant balloons might have been used in the compliant balloon group and it is possible that the effect of compliant and non-compliant balloons might be mixed within this group. Third, information regarding balloon diameter, inflation pressure, and inflation duration at each step was not included in our registry. Therefore, we could not evaluate the procedural outcome of each step during PCI. Fourth, we analysed coronary angiograms using a single-vessel quantitative coronary angiography software, which might be inaccurate when used in bifurcation lesions due to specific anatomical characteristics of bifurcations. Bifurcation-dedicated software packages are superior to single-vessel software packages in terms of accuracy and reproducibility of quantitative assessment of bifurcations15-17. Fifth, the minimum luminal diameter (MLD) of the MV was defined as the minimum of the proximal and distal MV MLDs. This is not in line with the recommendations from the European Bifurcation Club and is conceptually incorrect18. Finally, large numbers of patients were treated with first-generation DES; the impact of non-compliant balloons on the outcomes of patients treated with newer-generation DES should be investigated.
Conclusions
In propensity score-matched analyses using a large, multicentre bifurcation registry, the use of non-compliant balloons was associated with favourable procedural and long-term clinical outcomes. The benefits of non-compliant balloons for coronary bifurcation intervention should be confirmed in future randomised controlled trials.
| Impact on daily practice The use of non-compliant balloons is associated with lower risks of cardiovascular events, mainly driven by the reduction of cardiac mortality. The benefits of non-compliant balloons on the risk of cardiovascular events are consistent across all subgroups, regardless of acute coronary syndrome, left main bifurcation, side branch predilatation, stent strategy, final kissing balloon inflation, use of intravascular ultrasound, or stent type. The use of non-compliant balloons might be considered in patients undergoing percutaneous coronary intervention for bifurcation lesions. |
Acknowledgements
We appreciate the excellent statistical support of Joonghyun Ahn, MS, and Keumhee Cho, PhD, at the Samsung Biomedical Research Institute.
Funding
This work was supported by the Korean Society of Interventional Cardiology, Seoul, Republic of Korea.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Appendix. Procedures, data collection and angiographic analysis, definition of study outcomes, statistical analysis
PROCEDURES
All patients received loading doses of aspirin (300 mg) and clopidogrel (300-600 mg) before percutaneous coronary intervention (PCI) unless antiplatelet medications had previously been prescribed. The treatment strategy, stenting techniques, type of drug-eluting stent (DES), use of intravascular ultrasound, and implementation of side branch (SB) predilatation or final kissing balloon inflation were determined at the operator’s discretion. Following PCI, the duration of dual antiplatelet therapy of aspirin and clopidogrel was at the attending physician’s discretion.
DATA COLLECTION AND ANGIOGRAPHIC ANALYSIS
Clinical, laboratory, angiographic, procedural, and outcome data were recorded using a web-based reporting system. Additional information was obtained by medical record review or telephone interview. Baseline and procedural coronary angiograms were analysed by the angiographic core laboratory at the Heart Vascular Stroke Institute, Samsung Medical Center, Seoul, Republic of Korea) using an automated edge-detection system (Centricity CA1000; GE Healthcare, Waukesha, WI, USA). Bifurcation lesions were divided into three segments for quantitative coronary angiographic analysis: proximal main vessel (MV), distal MV, and SB ostium6. We determined the minimum luminal diameter (MLD) and reference diameter (RD) for each segment. In the distal MV and SB ostium, MLDs were measured <5 mm distal to the SB take-off. The MV RD was the average of the proximal and distal MV RDs, and the SB RD was the distal reference lumen diameter. The MV MLD was the minimum of the proximal and distal MV MLDs. Percent diameter stenosis was calculated as 100×(RD–MLD)/RD. The bifurcation angle was defined as the angle between the distal MV and the SB at its origin using the angiographic projection with the widest separation of the two branches19.
DEFINITION OF STUDY OUTCOMES
The primary outcome was major adverse cardiac events (MACE), which were defined as a composite of cardiac death, myocardial infarction (MI), or target lesion revascularisation (TLR) during follow-up. The secondary outcomes included individual components of the composite primary outcome and definite or probable stent thrombosis. All deaths were considered cardiac unless a definite non-cardiac cause could be established. MI was defined as elevated cardiac enzymes (troponin or myocardial band fraction of creatine kinase) greater than the upper limit of the normal value that occurred alongside ischaemic symptoms or electrocardiography findings indicative of ischaemia unrelated to the index procedure. TLR was defined as repeat PCI of the lesion within 5 mm of stent deployment or bypass graft surgery of the target vessel. Definite, probable, or possible stent thrombosis was assessed according to the definitions of the Academic Research Consortium20. All bifurcation lesions were classified according to the Medina classification, in which the proximal MV, distal MV, and SB components of the bifurcation are respectively allocated a score of 1 or 0 depending on the presence or absence of >50% diameter stenosis by operator’s visual inspection21. Lesions with Medina classifications (1,1,1), (1,0,1) and (0,1,1) were included in the true bifurcation lesions. SB dissection was described according to the National Heart, Lung and Blood Institute classification system for coronary artery dissection types. SB occlusion was defined as development of a Thrombolysis In Myocardial Infarction (TIMI) flow grade <3 during the procedure. Angiographic success was defined as a TIMI flow grade 3, <30% residual stenosis in the MV, and <50% residual stenosis in the SB, by visual estimation. The occurrences of cardiac death, ST-elevation MI, and emergent bypass graft surgery during the initial hospital stay were defined as in-hospital MACE. Procedural success was the achievement of angiographic success in the absence of any in-hospital MACE. The information about vital status was validated from the National Population Registry of the Korea National Statistical Office using a unique personal identification number.
STATISTICAL ANALYSIS
Categorical variables were summarised as frequencies with percentages and were compared using the chi-square test or Fisher’s exact test. After performing the Shapiro-Wilk test for normality, continuous variables were presented as mean±SD for normally distributed variables or median (interquartile range) for non-normally distributed variables, and were compared using the t-test or Wilcoxon rank-sum test, respectively. Time-to-event hazard curves were presented with Kaplan-Meier estimates and were compared using a log-rank test. To minimise the impact of selection bias and any potential confounders, we adjusted for differences in baseline characteristics using multivariable Cox regression analysis. For multivariable models, covariates included those with p-values <0.2 on univariable analysis, and were the following: diabetes mellitus, chronic kidney disease, previous revascularisation, left ventricular dysfunction (defined as left ventricular ejection fraction of less than 50%), left main bifurcation, true bifurcation, type of stent used, two-stent technique, SB predilatation, use of intravascular ultrasound, lesion lengths of MV and SB, pre-procedural reference diameter of MV, and pre-procedural diameter stenosis of SB. Furthermore, the following variables were also added to the multivariable model since they were deemed clinically relevant: acute coronary syndrome, current smoking, dyslipidaemia, remote site intervention, final kissing ballooning, pre-procedural reference diameter of SB, pre-procedural diameter stenosis of MV, and the angle between MV and SB. The results of the multivariable models were verified using propensity score matching methods. The propensity score, which represents the probability of a non-compliant balloon, was estimated without regard to outcome using multiple logistic regression analysis. A fully non-parsimonious model was developed that included all variables shown in Table 1, Table 2, Online Table 1. The pairs were matched using the nearest neighbour method with a caliper width of 0.4 times the SD22. A covariate was considered balanced if the standardised mean difference of each variable shown in Table 1, Table 2, Online Table 1 was less than 10%.
Within the COBIS II registry, most covariates were complete except for left ventricular ejection fraction, which was 82.7% complete. A multiple imputation procedure, which takes the correlation between all potential predictors, was used to generate five multiply imputed data sets. We then used an average of these imputation values to fill in for missing data in all subsequent analyses. All analyses were performed for the imputed data sets, and compared with results based on complete subset data, which gave much the same results. To perform subgroup analyses for the overall population, a propensity score for non-compliant balloon versus compliant balloon was estimated in each subgroup and included in the multivariable Cox regression model as a covariate in order to estimate the adjusted hazard ratio. All p-values were two-tailed, and p<0.05 was considered to be statistically significant. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).