Abstract
Background: Renal denervation (RDN) is a guideline-recommended treatment to reduce blood pressure (BP) in patients with uncontrolled hypertension. However, it is unclear if there are patient characteristics that are predictive of greater BP reduction. Baseline systolic blood pressure (SBP) has consistently been identified as an indicator of BP reduction after RDN.
Aims: Our study aimed to quantify the expected SBP change after RDN based on baseline SBP.
Methods: Patients undergoing radiofrequency RDN were pooled from multiple clinical studies, including SPYRAL First-In-Human (n=50), SYMPLICITY HTN-3 (n=364), SYMPLICITY HTN-Japan (n=22), SPYRAL HTN ON-MED (n=206), and the Global SYMPLICITY Registry DEFINE (n=2,735). Office and 24-hour ambulatory BP were measured at baseline and 6 months. Linear regression modelled patient-level 6-month SBP changes against baseline SBP.
Results: The pooled cohort (N=3,377) had a mean age of 60±12 years, and 41% were female. Baseline office SBP (OSBP) and 24h ambulatory SBP (ASBP) were 171.8±20.5 mmHg and 155.9±17.3 mmHg, respectively. At 6 months, OSBP and 24h ASBP decreased by 16.3±24.0 and 7.5±16.7 mmHg, respectively. Patients were prescribed 4.4±1.5 antihypertensive drug classes at baseline and 4.3±1.5 at 6 months (p<0.0001). Higher baseline SBP correlated with greater SBP reductions (p<0.0001; r2=0.21 for OSBP; r2=0.13 for ASBP). Baseline OSBP of 150, 160, 170, and 180 mmHg were associated with 6-month reductions of 4.2, 9.8, 15.4, and 21.0 mmHg, respectively.
Conclusions: Baseline SBP was associated with 6-month SBP reductions after RDN in hypertensive patients. This relationship provides guidance for shared patient-clinician decision-making about what BP change to expect following radiofrequency RDN based on baseline SBP alone.
Several position papers and guidelines recommend renal denervation (RDN) as an adjunctive treatment approach in patients with uncontrolled hypertension12. In November 2023, the Symplicity Spyral radiofrequency (RF) RDN system (Medtronic) received approval from the U.S. Food and Drug Administration (FDA) as an adjunctive blood pressure (BP)-lowering treatment for patients with hypertension whose BP remains above treatment goals despite lifestyle modifications and antihypertensive pharmacotherapy. BP reduction after RDN may be associated with a reduction in cardiovascular events34, and model-based projections found significant reductions in major adverse cardiovascular events and low numbers needed to treat across 3 years of follow-up in a hypertensive population treated with RF RDN5. However, there is limited guidance on how clinicians can effectively assess individual response after RDN and in turn, inform shared decision-making with patients. Across multiple studies in different patient populations using different RDN techniques, the most consistent feature correlating with significant BP reductions was higher baseline BP6789. However, the observed relationship between baseline BP and subsequent BP reduction has also been observed in pharmacological studies and is not limited to “blood pressure per se”1011.This non-linear “law of initial value” was first described by Wilder10 and should be considered in addition to regression to the mean1011 and visit-to-visit BP variability12. Wilder’s law of initial value states that the response to a stimulus is related to the prestimulus level and is independent of both regression to the mean and BP variability11. Disentangling these potential effects remains a significant challenge to understanding the relationship between baseline BP and BP reduction after RF RDN, especially when pharmacotherapy alone has failed to adequately control hypertension. In this post hoc analysis of pooled clinical trials of RF RDN, we attempted to exploit this biological phenomenon of baseline BP being indicative of subsequent BP change after a therapeutic intervention and to delineate the probabilities of expected BP change after RDN based on baseline BP alone.
Methods
Patients with baseline systolic blood pressure (SBP) ≥140 mmHg who underwent RF RDN and were prescribed antihypertensive medications at baseline were pooled from the following SYMPLICITY and SPYRAL global clinical trials (N=3,377), either from multiple randomised, sham-controlled trials such as SYMPLICITY HTN-313 (n=364; ≥3 prescribed antihypertensive [AH] medications); SPYRAL HTN-ON MED14 (n=206; 1-3 AH medications); SYMPLICITY HTN-Japan15, a randomised (but not sham-controlled) study (n=22; ≥3 AH drugs); SPYRAL First-In-Human16 (FIH), a feasibility study (n=50; ≥3 prescribed AH drugs); and the Global SYMPLICITY Registry (GSR) DEFINE17, an all-comers study reflecting a real-world population (as of March 2023, n=2,735; including patients with baseline SBP ≥140 mmHg who were prescribed any number of AH drugs). Patients with baseline SBP <140 mmHg were not included in this analysis as patients in this category would have been limited to a select few from the GSR DEFINE study, and could have potentially biased results2. Details of the included study designs have been published previously1314151617. The SPYRAL HTN-OFF MED Pivotal Trial was not included because the study design required patients to discontinue AH medications before randomisation and permitted their reintroduction after 3 months, potentially confounding the 6-month results. At baseline, patient demographic and clinical characteristics were assessed, and office and 24-hour ambulatory SBP (ASBP) were measured according to guideline recommendations. Follow-up office and 24-hour ambulatory SBP were measured 6 months after RDN.
Procedures
Procedural details have been published previously14181920. Briefly, RF RDN was performed using the Symplicity G3 RDN RF generator with either the Symplicity Flex catheter or the Symplicity Spyral RDN multielectrode catheter (all Medtronic), the latter allowing circumferential ablation treatments of all renal arteries and branch vessels between 3 and 8 mm in diameter. Cases were performed by experienced proceduralists and, in the case of randomised controlled trials, were proctored according to predetermined treatment plans. Angiography was performed throughout the procedure to verify anatomy and catheter placement.
Statistical analysis
Statistical analyses were performed with SAS for Windows 9.4 (SAS Institute). Linear regression analyses were performed for office and 24-hour SBP to assess the correlation between baseline and 6-month change in SBP (SBP change=intercept+slope*baseline SBP). The resulting linear regression relationships were used to estimate (1) patient-level expected 6-month changes in SBP after RF RDN based on baseline SBP (increments of 10 mmHg) with 50%, 75%, and 90% confidence intervals and (2) the probabilities of BP change in 20 mmHg increments based on baseline SBP. The probabilities were determined using the model-predicted BP changes and standard errors for different baseline BP values to calculate the area under the regression curve (Central illustration). Categorical measures are expressed as percentages, and continuous measures are expressed as mean±standard deviation. 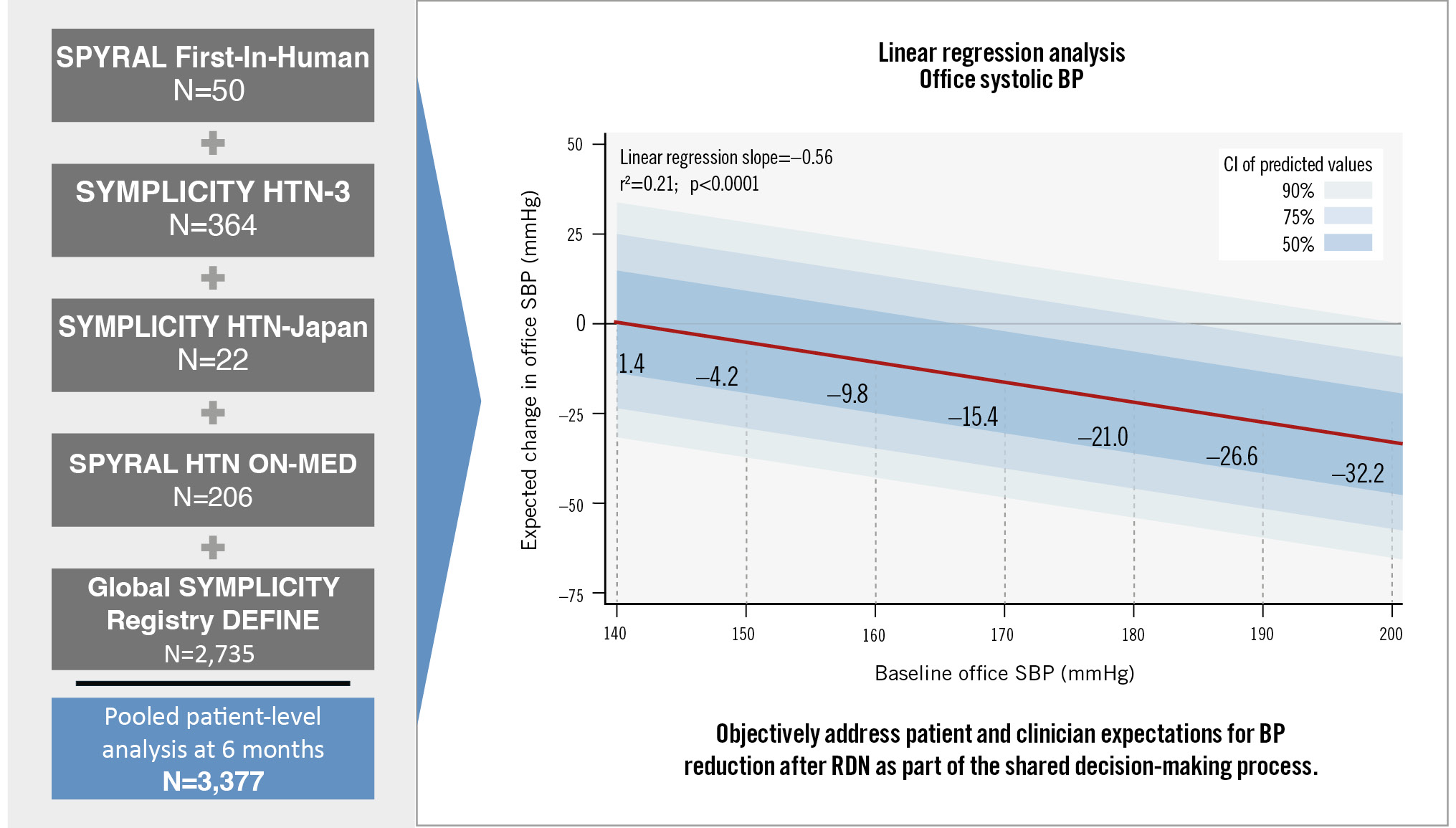
Central illustration. Blood pressure changes at 6 months following RF RDN based on baseline office SBP alone. BP: blood pressure; CI: confidence interval; RDN: renal denervation; RF: radiofrequency; SBP: systolic blood pressure
Results
The baseline demographic and clinical characteristics of the pooled cohort are summarised in Table 1. Patients were 60±12 years old, 41.0% female, with a body mass index of 31.3±5.9 kg/m2; 37.7% had type 2 diabetes mellitus, 8.4% had a history of myocardial infarction, and the estimated glomerular filtration rate was 76.5±24.1 mL/min/1.73 m². Baseline office SBP (OSBP) and 24-hour ASBP were 171.8±20.5 mmHg and 155.9±17.3 mmHg, respectively. Patients were prescribed 4.4±1.5 antihypertensive medications at baseline (Table 1). Six months after RF RDN, OSBP and 24-hour ASBP decreased by 16.3±24.0 and 7.5±16.7 mmHg, respectively. At 6-month follow-up, patients were prescribed 4.3±1.5 antihypertensive medications (p<0.0001 compared to baseline). Linear regression analysis of the relationship between baseline OSBP and 6-month change in OSBP showed that each 10 mmHg increase in baseline OSBP ≥140 mmHg was associated with a 5.6 mmHg (r²=0.21; p<0.0001) reduction in OSBP. Similarly, each 10 mmHg increase in baseline ASBP ≥140 mmHg was associated with a 4.3 mmHg (r²=0.13; p<0.0001) reduction in ASBP at 6 months. Baseline OSBP of 150, 160, 170, and 180 mmHg were associated with 6-month reductions of 4.2, 9.8, 15.4, and 21.0 mmHg, respectively. The expected 6-month reductions in both office and 24-hour ambulatory SBP varied substantially from the baseline values of 140 mmHg to 180 mmHg (Figure 1). The probabilities of expected SBP changes based on the separate linear regression models of baseline OSBP and ASBP were translated into a heat map, with higher probabilities in darker shades of green (Figure 2). For example, a patient with a baseline OSBP of 170 mmHg has a 35% probability of an OSBP change between 0 and –20 mmHg, a 29% probability of a change between –20 and –40 mmHg, etc. (Figure 2A). For baseline OSBP between 150 and 170 mmHg, the greatest probability of expected OSBP reduction is in the range of 0 to –20 mmHg (probabilities of 35% to 36%), and for baseline OSBP between 180 mmHg and 210 mmHg, the greatest probability of expected OSBP reduction is in the range of –20 mmHg to –40 mmHg (probabilities of 33% to 36%). A 12-month linear regression analysis revealed similar results to the 6-month analysis (Supplementary Figure 1), and is consistent with a recently published linear mixed model investigating long-term reductions after RDN21.
Table 1. Pooled cohort baseline characteristics.
| Baseline characteristics | Pooled cohort (N=3,377) |
|---|---|
| Age, yrs | 59.8±11.8 (3,377) |
| Male | 59.0 (1,994/3,377) |
| Type 2 diabetes mellitus | 37.7 (1,269/3,365) |
| Body mass index, kg/m2 | 31.3±5.9 (3,353) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 76.5±24.1 (3,220) |
| Prior myocardial infarction | 8.4 (255/3,036) |
| History of heart failure | 10.7 (326/3,036) |
| History of sleep apnoea | 21.3 (675/3,169) |
| History of atrial fibrillation | 11.0 (371/3,360) |
| History of smoking | 35.0 (1,180/3,374) |
| No. of antihypertensive drug classes prescribed | 4.4±1.5 (3,113) |
| Number of ablations | 20.8±17.7 (3,285) |
| Procedure duration, mins | 83.9±43.8 (3,199) |
| Office SBP, mmHg | 171.8±20.5 (3,377) |
| Office heart rate, bpm | 70.9±13.2 (3,224) |
| Office pulse pressure, mmHg | 78.4±19.4 (3,377) |
| 24-hour ambulatory SBP, mmHg | 155.9±17.3 (2,529) |
| 24-hour heart rate, bpm | 69.6±11.8 (2,194) |
| 24-hour pulse pressure, mmHg | 67.5±14.8 (2,529) |
| Data are mean±SD (N) or % (n/N). SBP: systolic blood pressure; SD: standard deviation | |
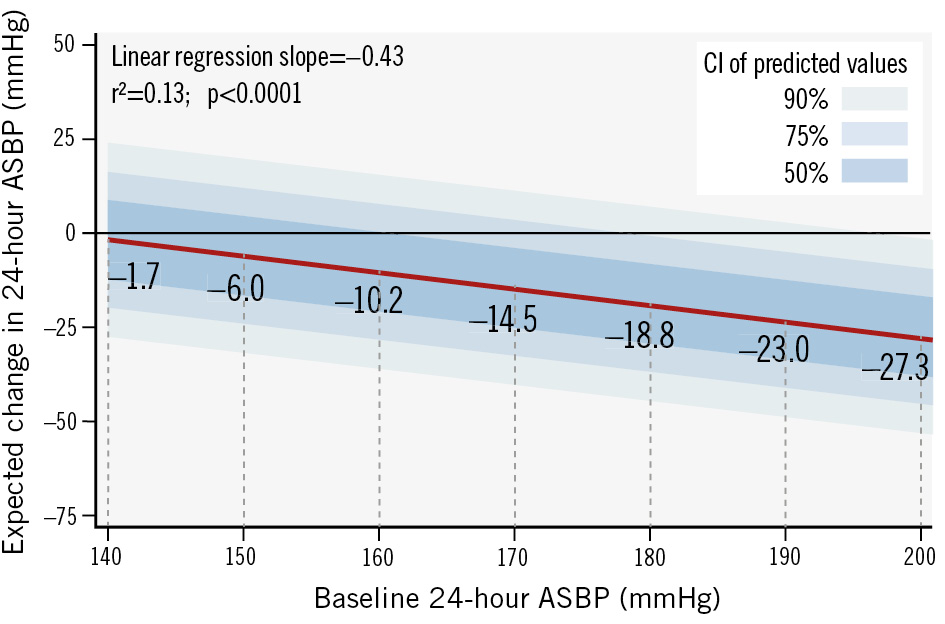
Figure 1. Expected change in 24-hour ambulatory systolic BP at 6 months after radiofrequency RDN. The red line is the linear regression line. Different shades of blue around the linear regression line represent different confidence intervals. ASBP: ambulatory systolic blood pressure; BP: blood pressure; CI: confidence interval; RDN: renal denervation
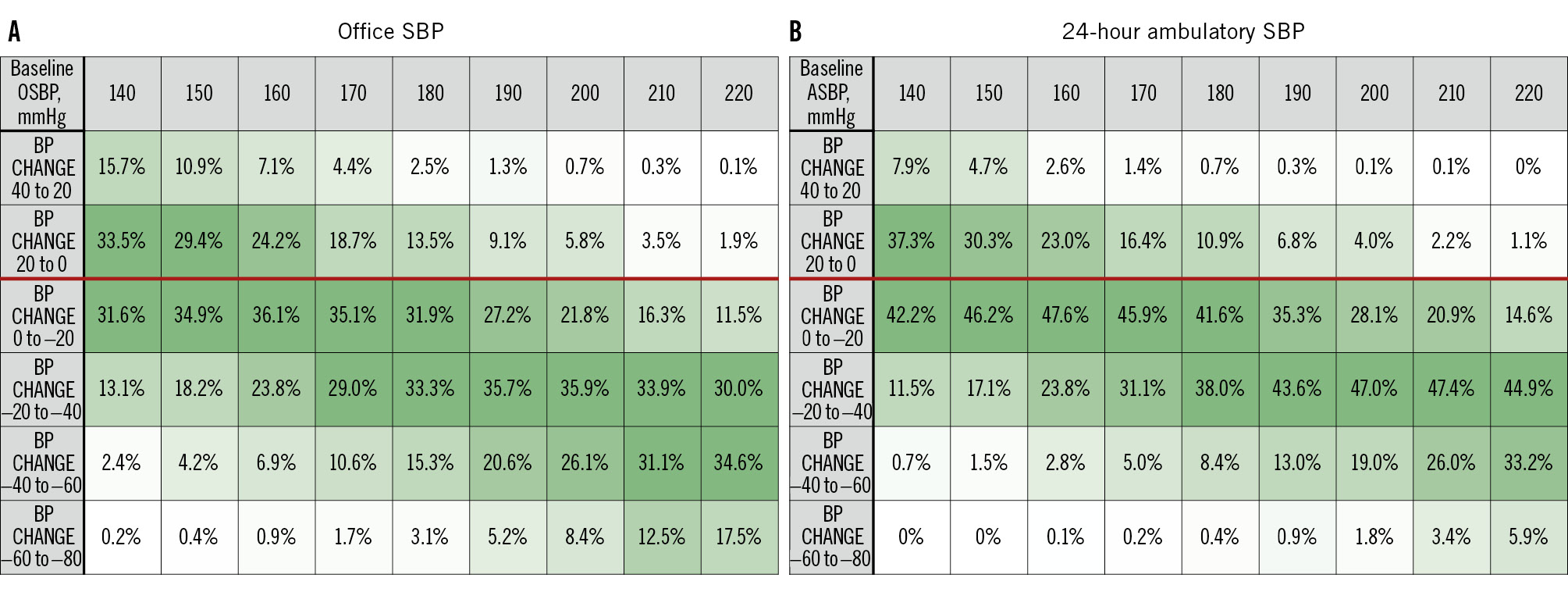
Figure 2. Probability heat map of the expected 6-month SBP changes by baseline SBP after RF RDN. Values within squares represent the probabilities of BP change (%). Darker green shades represent higher probabilities, and lighter green shades represent lower probabilities of the expected BP change range. The red line in each table separates increase and decrease in SBP change from baseline. A) Office SBP; (B) 24-hour ambulatory SBP. ASBP: ambulatory systolic blood pressure; BP: blood pressure; OSBP: office systolic blood pressure; RF RDN: radiofrequency renal denervation; SBP: systolic blood pressure
Discussion
The present analysis of a pooled database of 3,377 patients with uncontrolled office or 24-hour ambulatory SBP, despite treatment with antihypertensive medications, showed that higher baseline SBP was associated with greater SBP reduction at 6 months after RF RDN. However, it explained only 21% of the resulting BP reduction after RDN (r2=0.21 for OSBP). Despite strong statistical significance, linear correlations were modest (r² values<0.5), suggesting that additional factors also influence SBP change after RDN. Another factor influencing correlations is likely visit-to-visit BP variability22. Therefore, accurate individual “prediction” of BP response appears to be impractical. However, the present analysis does facilitate estimation of the relative likelihood of different BP reductions. For example, a patient with a baseline office SBP of 170 mmHg has a 76.5% probability of experiencing a BP reduction within 6 months after RDN. Similarly, a patient with a baseline SBP of 190 mmHg has an 88.7% chance of experiencing a BP reduction and a 62.2% chance of experiencing a BP reduction greater than 20 mmHg. This data-driven relationship between baseline BP and BP change after RF RDN could help both clinicians and patients better understand the potential benefits on an individual basis, and thus could provide better guidance in the shared decision-making process currently recommended by hypertension guidelines and RDN position statements1223. RDN in patients with uncontrolled hypertension is recommended by international guidelines for the treatment of arterial hypertension12. In clinical practice, there is a great need to better understand the expected BP change after an invasive therapy such as RDN, depending on the individual’s clinical characteristics. Currently, there is no single characteristic that accurately predicts the best BP response to RDN in all patients and that has been prospectively validated. This may reflect the lack of a single, uniform, non-arbitrary definition of “response” to RDN22. Several associations have been proposed, such as renal artery anatomy; BP variability; clinical and demographic conditions such as sex, presence of diabetes or obesity; and pathophysiological factors such as skin sodium levels, plasma renin activity, and vascular stiffness (invasive pulse wave velocity and aortic distensibility). However, each of these associations do not have a high enough accuracy to be used as a guide to predict BP reduction after RDN in clinical practice. Similarly, the genetic profile of patients with resistant hypertension was not associated with 24-hour BP reduction, which does not support the use of a genetic score to identify potential responders to RDN24. However, several studies have consistently shown that pretreatment or baseline BP has the greatest impact on the magnitude of BP reduction after RDN6789. Baseline BP is one of the easiest patient data points to collect in a real-world clinical setting. Our large, pooled database of RF RDN trials allowed us to show an expected range and mean BP change after RDN based on this single characteristic. A simple metric such as this may be useful as a guideline for primary care physicians and referring physicians, as it does not require complex and expensive investigations before referring patients to a specialist hypertension clinic for consideration of RDN. For patients at or near the threshold for guideline-recommended BP values for RDN treatment, a case-by-case approach is recommended, including incorporating patient preference as part of the shared decision-making process. Ultimately, RDN should be considered for patients at higher cardiovascular risk, including those with higher BP in whom target BP values at or below 130/80 mmHg are recommended. Indeed, further investigation is needed to identify other specific patient characteristics, in addition to baseline BP, that contribute to better BP predictability after RDN.
Limitations
BP changes at 6 and 12 months were evaluated and any correlation beyond 12 months was not evaluated. Analysis of BP reduction up to 36 months showed sustained and even amplified BP reductions after RF RDN, suggesting that the estimated expected BP reductions underestimate the long-term BP reductions after RF RDN5. The SPYRAL HTN-OFF MED study was not pooled in this study because the antihypertensive drug protocol differed significantly from the other pooled studies, due to a permitted uptitration of medications between 3 and 6 months. Not all patients were on the same antihypertensive drug regimen, and medication adherence was not tested for a large proportion of patients included in this study. In addition, where medication testing was available, many patients did not adhere to their prescribed medications throughout the follow-up period20, which may have influenced BP changes. This analysis pooled studies using different radiofrequency devices (first-generation Symplicity Flex and next-generation Symplicity Spyral catheters). Other variables not tested in this analysis may play an important role in SBP reduction after RF RDN.
Conclusions
In patients with uncontrolled hypertension, those with higher baseline office and 24-hour ambulatory SBP can expect greater SBP reductions after RF RDN at 6 months. Using baseline SBP as a guide for expected BP reduction after RDN may be useful in clinical practice and may serve as an aid in shared decision-making for RDN. Further research is needed to identify the best candidates for RDN.
Impact on daily practice
The findings from this pooled analysis of 3,377 patients indicate that higher baseline systolic blood pressure (SBP) is associated with greater SBP reduction at 6 months following radiofrequency renal denervation (RDN). This insight is important for interventionalists, as it provides a straightforward metric – baseline SBP – that can help estimate the potential benefits of RDN for patients. While the exact blood pressure (BP) response cannot be predicted, understanding the likelihood of significant BP reductions can enhance shared decision-making processes between clinicians and patients. This data-driven approach further supports the use of RDN for patients with uncontrolled hypertension.
Acknowledgements
Nicole Peterson, MBA, Maria Min-young Kim, MD, MRCP, PhD, and Benjamin Woods, PhD, all of Medtronic, provided editorial support under the direction of the first and last authors including the creation of tables, figures, and editing of text.
Conflict of interest statement
R.E. Schmieder reports grants and personal fees from Medtronic, Recor Medical, and Ablative Solutions. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung; Saarland University has received scientific support from Ablative Solutions, Medtronic, and Recor Medical; and until May 2024, he received speaker honoraria/consulting fees from Ablative Solutions, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, Recor Medical, Servier, and Terumo. G. Mancia has received speaker honoraria from Berlin-Chemie, Exicon Consulting, Menarini International, Merck Healthcare KGaA, Medtronic, Recordati, Sanofi, Servier, and Sun Laboratories. R.R. Townsend is a consultant for Medtronic, Axio, Regeneron, Bard, OBIO, and AstraZeneca; and has received royalties from UpToDate. D.E. Kandzari receives institutional research/grant support from Biotronik, Boston Scientific, OrbusNeich, Teleflex, Medtronic, and Ablative Solutions; he also receives personal consulting honoraria from Medtronic, Ablative Solutions, and DeepQure. K. Kario receives personal fees from Medtronic; receives grants from A&D Company, JIMRO, OMRON Healthcare, CureApp, Terumo, and Fukuda Denshi; receives honoraria from Otsuka Pharmaceutical and OMRON Healthcare; and participates on the advisory board of Fukuda Denshi. D.L. Bhatt reports the following relationships - advisory board: Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey & Company, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, and Stasys; board of directors: American Heart Association New York City, Angiowave (stock options), Bristol-Myers Squibb (stock), DRS.LINQ (stock options), and High Enroll (stock); consultant: Broadview Ventures and Hims; data monitoring committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute, and Rutgers University (for the NIH-funded MINT Trial); honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), and Wiley (steering committee); other: Clinical Cardiology (Deputy Editor); patent: sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither he nor Brigham and Women’s Hospital receive any income from this patent); research funding: Abbott, Acesion Pharma, Afimmune, Aker BioMarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; royalties: Elsevier (Editor, Braunwald’s Heart Disease); site co-investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; trustee: American College of Cardiology; unfunded research: FlowCo. M. Liu is a full-time employee of Medtronic. M. Böhm reports support from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, GlaxoSmithKline, Medtronic, Novartis, Recor Medical, Servier, and Vifor; and is funded by the German Research Foundation (DFG, TTR 219, S-01, M-03, project number 322900939). R. Whitbourn has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

