Abstract
Pressure-derived fractional flow reserve (FFR) is an index of the haemodynamic significance of a coronary lesion. Numerous studies have provided robust evidence that FFR-guided percutaneous coronary intervention is associated with better clinical outcomes and reduces the need for repeat revascularisation. Although FFR is regarded as the gold standard for assessing lesion severity, it has limited clinical applications, mainly because it is a relatively expensive and time-consuming procedure. To overcome these limitations, several computational-based methodologies have been developed which enable estimation of the FFR in three-dimensional models derived from anatomic imaging data. Multislice computed tomographic coronary angiography and quantitative coronary angiography have been proposed for coronary reconstruction and computational evaluation of the FFR. In this review article, we describe the currently available methodologies for the computational estimation of FFR, present evidence derived from their clinical evaluations, stress their limitations, and discuss their potential value in clinical practice.
Introduction
Quantitative coronary angiography (QCA) has been the traditional method for estimating the extent and severity of coronary artery disease. However, cumulative evidence has shown that it has a modest accuracy in assessing the haemodynamic significance of intermediate lesions1-4. This limitation has been addressed by the introduction of fractional flow reserve (FFR). FFR provides a physiologic assessment of lesion severity and is regarded today as the gold standard for detecting stenoses that cause myocardial ischaemia. Numerous studies have demonstrated that FFR-guided percutaneous coronary intervention (PCI) is associated with better clinical outcomes than medical treatment, or angiographically guided PCI5-7. Hence, FFR has a IA indication in the currently published ESC guidelines for the assessment of the haemodynamic significance of a lesion in stable patients and for guiding PCI in patients with multivessel disease8.
Although there are robust data to support its use, FFR has limited applications in everyday clinical practice9. This discrepancy may be attributed to the fact that FFR measurement is a costly and time-consuming procedure, requires intravenous or intracoronary injection of adenosine which cannot be administered to all patients (e.g., in patients with asthma or severe hypotension, or in patients with second-degree atrioventricular block), and involves advancement of the FFR guidewire to the distal vessel, which may be a challenging procedure especially in side branches and in tortuous coronaries with complex anatomy10.
To overcome these limitations, several methodologies have been proposed that allow computational assessment of FFR in coronary models obtained by different imaging techniques (Online Table 1). The first approaches rely on the use of computational fluid dynamic (CFD) techniques in three-dimensional (3D) models obtained from coronary computed tomographic angiography (CTA)11-13 or 3D QCA3,14,15. The aim of this review article is to describe the currently available methodologies for the computational assessment of FFR, present the evidence from studies that evaluated their efficacy in a clinical setting, discuss their methodological limitations, and highlight their potential applications in clinical practice.
CTA- and CFD-based FFR
Fractional flow reserve can be derived from coronary CTA image data (FFRCT) acquired using standard acquisition protocols without the need for additional imaging, medication, or radiation. Computation of FFRCT requires the generation of a physiologic model of coronary blood flow. The physiologic model is based on three underlying principles which have been described in detail by Taylor et al16: 1) the total resting coronary blood flow can be quantified relative to the myocardial mass as assessed by CTA; 2) the microcirculatory vascular resistance at rest is inversely proportional to the size of the coronary arteries supplying the myocardium; and 3) the vasodilatory response of the coronary microcirculation to adenosine infusion is predictable, allowing computational modelling of the maximal hyperaemic state. Integration of these patient-specific mathematical models of coronary physiology to 3D CFD models enables the computation of coronary flow and pressure at each point in the coronary tree under hyperaemic conditions. Hence, FFRCT is calculated in a similar manner to when it is directly measured during invasive coronary angiography (ICA).
In three prospective multicentre trials including a total of 609 patients and 1,050 vessels with blinded comparison to FFR, computation of FFRCT has shown promising results in identifying lesion-specific ischaemia in patients with, or with suspected, stable coronary artery disease (CAD)11-13. The most recent and largest study, the “Analysis of Coronary Blood Flow Using CT Angiography: NeXt sTeps” (NXT) trial incorporated learning from the previous two trials, including use of the latest generation of FFRCT analysis software13. In this study an effort was made to optimise image acquisition by controlling patients’ heart rate and using pre-scan nitroglycerine. These modifications increased the per-vessel diagnostic accuracy of FFRCT (≤0.80) in predicting lesion-specific ischaemia to 86% (sensitivity 84%, specificity 86%) which was superior to anatomical assessment (stenosis severity >50%) by either coronary CTA or ICA (diagnostic accuracy 65% and 82%, respectively). In the NXT trial, there was a good direct correlation of FFRCT to invasively measured FFR (r=0.82), with a slight underestimation of FFRCT (0.03) when compared with FFR. Thus, FFRCT may encourage the use of coronary CTA with FFRCT as a “one-stop shop” providing high diagnostic sensitivity for anatomic evaluation of CAD and high specificity for ischaemia. Figure 1 displays a case with coronary obstructions and ischaemia.
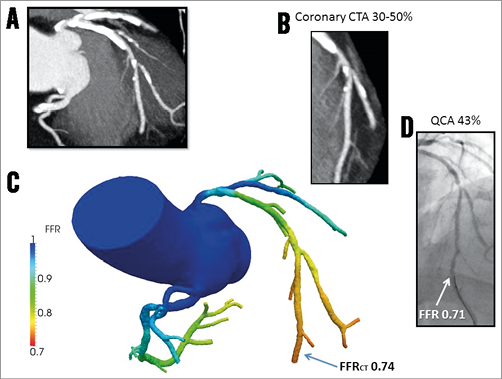
Figure 1. Computation of FFRCT by coronary computed tomographic angiography. A) Coronary CTA with severe calcification (Agatston score=2,314) of the LAD and LCx. B) Coronary CTA with magnified view of LAD showing a 30-50% stenosis. C) Computational FFRCT analysis revealed that the LAD lesion was haemodynamically significant with FFRCT=0.74, and with similar ischaemia in the diagonal branch. D) Quantitative coronary angiography demonstrated a 43% LAD stenosis with a corresponding FFR value of 0.71 indicating that the lesion was ischaemia-producing. FFR: fractional flow reserve; FFRCT: FFR calculated from coronary computed tomographic angiography; CTA: computed tomographic angiography; LAD: left anterior descending artery; LCx: left circumflex artery
Simulation analyses based on historical data indicate that the use of FFRCT to guide selection of ICA and coronary revascularisation may reduce costs and improve outcomes in stable CAD17,18. As for other non-invasive testing modalities, prospective data assessing the cost-effectiveness of FFRCT in clinical practice are lacking. The ongoing multicentre, prospective “Prospective LongitudinAl Trial of FFRCT: Outcome and Resource IMpacts” (PLATFORM) trial (ClinicalTrials.gov Identifier: NCT01943903) compares the effect of FFRCT-guided versus standard functional diagnostic evaluation on clinical outcomes, resource utilisation, and costs in patients suspected of CAD. One shortcoming of the FFRCT technique is that CTA data need to be sent off-site to a processing centre for subsequent analysis, and then the resulting FFRCT is returned to the hospital.
As the concept of non-invasive FFR derived from CTA may change the practice pattern for patients with suspected CAD, there have been many attempts to develop novel approaches19-21. Recently, the Siemens cFFR system (Siemens Healthcare, Forchheim, Germany) was developed, and its early clinical study results were released19. This system employs a hybrid methodology which couples reduced-order and full-order modelling to reduce the computational time. To simulate hyperaemia, the total coronary resistance was decreased, maintaining the boundary condition of the physiologic model. Baumann et al19 tested the clinical performance of this software in 36 coronary vessels. The mean total time for deriving cFFR including data-set processing and flow computation was 51.9±9.0 min per study and the final computational time was 3.9±0.8 min. The correlation between cFFR and invasive FFR was good (r=0.74), and 95% limits of agreement were between –0.11 and 0.15. In a larger study by Coenen et al21 including 189 vessels, the diagnostic accuracy of cFFR was 74.6% (sensitivity 87.5%, specificity 65.1%) and was better than that of CTA (accuracy 56.1%, sensitivity 81.3%, specificity 37.6%). The correlation between cFFR and invasive FFR in this study was moderate to good (r=0.59). This system may provide on-site, point-of-care CTA-based physiologic assessment. However, further prospective multicentre studies are needed to validate its applicability to real-world clinical practice. Furthermore, considering the nature of this technology, processing time may be a critical issue in its clinical application.
Estimation of patient-specific flow is critical in the calculation of CT-based FFR. Huo and Kassab22-24 developed and validated scaling laws that relate morphometric parameters (e.g., lumen diameter and area, vessel length and volume, etc.) to coronary blood flow and myocardial mass25. These structure-function relations can be used to determine the flow on a patient-specific basis given the specific anatomy and myocardial mass as obtained from CTA (coronary flow cannot be directly measured from CTA).
QCA- and CFD-based FFR
Morris et al15 reported a new model to compute FFR from two angiographic projections that were selected from a series of rotational angiographic images. Generic boundary conditions were applied to the CFD simulation in the inlet and outlet of the 3D model, and the downstream microvascular resistance was estimated using a Windkessel model. It took approximately 24 hours per case to compute FFR. The method was validated in 19 patients with relatively simple lesions, and the results obtained demonstrated the high accuracy, sensitivity and specificity of the proposed approach for detecting flow-limiting lesions (97%, 86%, and 100%, respectively). The authors concluded that FFR was reliably predicted without the need for invasive measurements or for inducing hyperaemia. It was admitted, however, that simplified assumptions on the downstream resistance were made. As a result, the computed FFR is more likely to deviate from the true FFR, when abnormal microcirculatory resistance or downstream collateral circulation is present.
Tu et al3 presented a new method for fast computation of FFR from coronary angiography. The anatomical model was reconstructed in 3D from two angiographic projections with angles ≥25º apart that were acquired by monoplane or biplane systems. Patient-specific volumetric flow rate at hyperaemia was calculated using the combination of TIMI frame count and 3D QCA, and subsequently applied to the inlet boundary in the CFD simulation. Reference diameter function and bifurcation angles by 3D QCA were used to determine the flow distribution at coronary bifurcations. Instead of using pulsatile flow and generic boundary conditions as applied by FFRCT and by Morris et al15, the mean flow and “fixed” boundary conditions were used in the CFD simulation. As a result, the computational time was significantly shortened. The entire analysis time including 3D QCA, TIMI frame count, and CFD simulation typically took less than 10 minutes per case. Figure 2 shows an example of FFR computation from X-ray angiography. The method was validated in 77 vessels with homogeneous intermediate lesions (mean diameter stenosis: 46.6±7.3%) from 68 patients. The computed FFR, or the so-called FFRQCA, correlated well with the pressure wire-based FFR (r=0.81, p<0.001). The overall accuracy of FFRQCA for determining FFR ≤0.80 was 88%, with sensitivity and specificity of 78% and 93%, respectively. Due to minimal requirement in data acquisition as compared to invasive FFR, FFRQCA can be straightforwardly incorporated into routine clinical practice, with the potential to reduce the barrier of a costly and time-consuming physiological assessment in the catheterisation laboratory26. The authors also observed wide scatter on the distribution of volumetric coronary flow reserve, especially in vessels with low baseline volumetric flow3, suggesting that baseline flow and coronary flow reserve vary widely between patients. These observations enforce the need for using patient-specific hyperaemic flow, rather than fixed hyperaemic flow rate or fixed coronary flow reserve, in computational FFR; nevertheless, the need for adenosine infusion constitutes a limitation of this approach and may affect the clinical applicability of the proposed methodology.
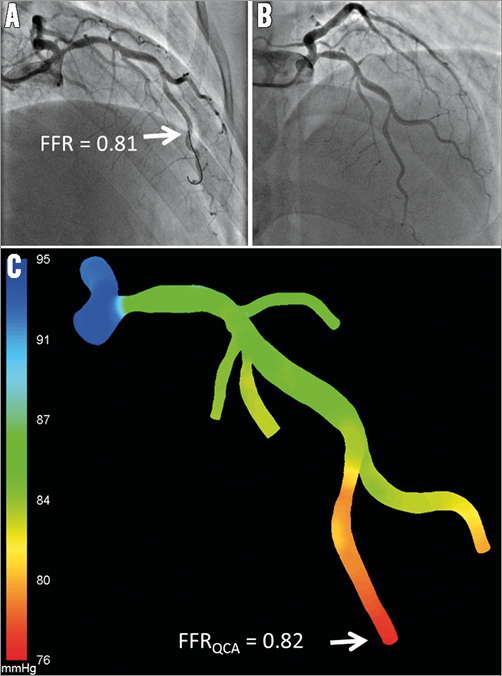
Figure 2. Computation of FFRQCA from coronary X-ray angiography. A) & B) X-ray angiography shows a series of lesions at the LAD. FFR measured by pressure wire at the distal LAD location (white arrow) was 0.81. C) Computational analysis shows that FFRQCA was 0.82, suggesting that the lesions were haemodynamically insignificant. FFR: fractional flow reserve; LAD: left anterior descending artery; QCA: quantitative coronary angiography
Papafaklis et al14 recently presented a simplified method of virtual functional assessment of coronary stenosis. Single-vessel 3D QCA was performed, and the reconstructed lumen geometry was processed by CFD. The arterial wall was considered to be rigid, while in the inlet of the model a pressure of 100 mmHg was imposed. Two separate simulations with a fixed steady flow of 1 ml/s and 3 ml/s were applied. The computed virtual functional index was validated on 139 vessels with mild and intermediate lesions (diameter stenosis: 38.8±10.9% by 3D QCA) from 120 patients. High accuracy, sensitivity, and specificity (88%, 90%, and 86%, respectively) in predicting FFR ≤0.80 was reported and the authors concluded that this “less invasive” approach could have important implications for patient management and cost.
Model-based FFR
To reduce the computational complexity, a new analytical model (AM) which does not rely on the use of CFD to quantify pressure drop, but on the dimensions of a lesion (i.e., the cross-sectional area along the lesion and the length of lesion) and on coronary flow measured by a flow probe with no empirical parameters –unlike previous models27-29– was developed and validated using in vitro and in vivo experiments and CFD23. Agreement between the analytical (FFRAM) model (real time) and CFD (FFRCFD) for known dimensions of a lesion was excellent (a near perfect identity line with r2=1), albeit the latter was much more costly to compute. In vitro (constrictions were created in isolated arteries using symmetric and asymmetric tubes as well as an inflatable occluder cuff) and in vivo swine experiments (constrictions were induced in coronary arteries of swine by an occluder cuff) were used to validate the proposed analytical model. The proposed model agreed very well with the experimental measurements. Flow pulsatility and stenosis shape (e.g., eccentricity, exit angle divergence, etc.) had a negligible effect on myocardial FFR because it is based on a mean pressure (pulsatility is averaged out) and the Reynolds number is small (and hence the detailed shape of the lesion is unimportant), while the entrance effect in a coronary stenosis was found to contribute significantly to the pressure drop which is accounted for in the analytical model23.
Recently, Schrauwen et al30 estimated pressure drop on geometrical features derived from computed tomography and intravascular ultrasound. Tapering and curvature added significantly to the total pressure drop. Using tapering angle, maximum area stenosis, and angularity of the centreline, the authors were able to estimate accurately the pressure drop in mildly diseased coronary arteries (mean difference between the computed and the CFD-derived pressure drop: 41±288 Pa). The authors advocated that tapering and 3D curvature should be included when estimating the pressure drop in human coronary arteries.
Tar et al31 investigated the distal laminar resistance in the calculation of FFR by fluid dynamic equations using contrast material velocities and the morphological data derived from 3D QCA. When the distal laminar resistances were incorporated in the computed model, the accuracy in the computed FFR was improved compared to the model without incorporating the resistances (mean error: –0.05 vs. –0.09, limits: –0.11 to 0.02 vs. –0.16 to 0.01).
Discussion
It is very apparent that there is an increased interest in the development of computerised methodologies that would allow a non-invasive or invasive (based only on 3D QCA) manner of evaluation of the haemodynamic significance of intermediate lesions. Non-invasive CTA-derived FFR can be used to identify, more accurately than CTA, patients with flow-limiting CAD who would benefit from further invasive investigations32, whereas QCA-derived FFR appears able to assess the haemodynamic severity of stenosis in high-risk patients undergoing coronary angiography. Thus, it is likely that these two computational-based approaches will have a complementary rather than a “competitive” role in real-world practice. Preliminary validation of CFD-derived FFR methodologies not only showed a good correlation between pressure wire-derived FFR and CFD-derived FFR, providing promise for the future, but also revealed the intrinsic limitations of these approaches.
The first CFD-based FFR methods require increased time to perform coronary reconstruction, blood flow simulation, and estimation of the haemodynamic significance of the lesions, limiting the applications of these approaches in a clinical setting. Of note, more recent approaches have significantly reduced the computational time, and model-based FFR methodologies have been proposed which appear fast and computationally inexpensive23,30,31. An extensive, prospective validation of these techniques in an all-comers population that will include patients with complex coronary artery disease (e.g., ostial lesions, chronic total occlusions, patients with a previous myocardial infarction or documented microvascular disease, etc.) is in progress and is anticipated to allow us to evaluate more accurately the error introduced by the theoretical assumptions made during the estimation of the FFR. These large-scale studies will provide a better understanding of the efficacy of a computationally estimated FFR, and critical assessment as to whether these techniques can replace the traditional pressure wire-based FFR or whether they should be used as an adjunctive first-line tool for the evaluation of lesion severity.
CTA-derived FFR has introduced a unique diagnostic potential as it appears able to assess the haemodynamic significance of flow-limiting lesions. Future advances in CTA imaging are likely to overcome the present limitations of this technology (e.g., the relatively low spatial resolution and the blooming artefacts introduced by calcium) in assessing luminal pathology and dimensions and improve the accuracy of CTA-derived FFR. Another limitation of CTA-based FFR is the assumptions that are made during the estimation of hyperaemic flow which can affect results, especially in patients with microcirculatory disease and collateral flow3,33,34.
Invasive coronary angiography offers a lumenogram with higher spatial resolution. In addition, patient-specific hyperaemic flow can be directly quantified using 3D QCA and TIMI frame count3. This can potentially improve the accuracy of estimating hyperaemic flow in the presence of abnormal microcirculatory resistance or downstream collateral circulation. However, this direct quantification requires administration of vasodilators, i.e., adenosine. Patients can experience some unpleasant angina during adenosine infusion, though these symptoms are generally tolerated35. In addition, the accuracy of QCA is inherently limited by vessel overlap when acquired at suboptimal projections36. Standardisation and optimisation of image acquisition protocols for 3D QCA need to be pursued for future investigations. The fusion with intracoronary imaging such as intravascular ultrasound37 and optical coherence tomography38 can further increase the accuracy of the lumen geometry. Studies involving both non-invasive and invasive imaging modalities are warranted to provide further insight into the incremental gain in accuracy by including invasive imaging in computational FFR.
Future perspectives
Several studies have provided evidence for the value of FFR in assessing the haemodynamic significance of bifurcation lesions39,40. It is often difficult, however, for the pressure wire to cross these complex stenoses10. It should be noted that assessment of the severity of bifurcation lesions is a challenge, as CT may fail to detect very short ostial lesions, while quantitative coronary angiography has limited accuracy in estimating the severity of ostial stenosis, especially when overlap is present41. New sophisticated algorithms may provide more reliable quantification of bifurcation anatomy, but operator skills in angiographic image acquisitions are generally required42.
The first validation studies used the pressure wire-based FFR as the gold standard to assess the accuracy of CFD-derived FFR approaches rather than clinical endpoints. However, pressure wire-based FFR has significant limitations, including the drift noted during FFR examination, difficulty in the interpretation of the readings (e.g., minimum FFR vs. a stable minimum FFR value), and the inability in some patients to achieve hyperaemic response during adenosine infusion. In addition, although the FAME II study may have provided robust evidence about the cost-effectiveness of FFR-guided PCI, the price of the pressure wire is high and adds cost and time to the procedure43. Moreover, pressure wire examination requires adenosine infusion which causes patient discomfort and occasionally bradycardia, and can be associated with a risk of complications (i.e., coronary dissection, wire perforation, etc.) which is minimal in simple lesions but increases in lesions with complex anatomy. Finally, FFR may provide inaccurate estimations in tortuous arteries where the advanced wire can change vessel geometry and cause spasm44. Therefore, future large-scale randomised controlled trials that compare computational-derived FFR against pressure wire-based FFR using clinical endpoints are needed in order to assess the clinical efficacy of these approaches and examine whether computational-derived FFR-guided PCI is cost-effective, and superior, equally effective or inferior to the traditional FFR-guided treatment.
Conclusions
Cumulative evidence has demonstrated that computer-based methodologies are able to assess accurately the haemodynamic significance of intermediate lesions in selected stenoses and groups of patients, highlighting their potential in the clinical setting. Further research is needed in order to validate invasive and non-invasive methods of computational FFR with clinical outcome studies in broad populations of patients with simple and complex coronary artery disease, before advocating their use in everyday clinical practice.
Funding
Currently, Shanghai Jiao Tong University receives institutional grant support on behalf of S. Tu from Medis.
Conflict of interest statement
S. Tu had an employment contract with Medis medical imaging systems bv until June 2014. J. Reiber is the CEO of Medis, and has a part-time appointment at LUMC as Professor of Medical Imaging. B. Nørgaard has received unrestricted research grants from Edwards Lifesciences and Siemens. The other authors have no conflicts of interest to declare.