Abstract
Studies evaluating the diagnostic performance of coronary computed tomography angiography (CTA) are consistent in demonstrating a high negative predictive accuracy, but only a modest positive predictive accuracy for the detection of significant coronary artery disease. Consequentially, there has been a considerable effort made to enhance the diagnostic capability of coronary CTA by developing scanner technology and also post-processing algorithms for coronary stenosis evaluation. Of these new developments, the proposition of being able to measure non-invasive fractional flow reserve by coronary computed tomography angiography (FFRct) has generated much recent interest. Initial reports indicate that the application FFRct not only correlates well with invasive fractional flow reserve but also has the potential to enhance substantially the positive predictive accuracy and overall accuracy of coronary CTA. Although it is theoretically possible to measure FFRct using complex computational fluid dynamics adapted from the aeronautical industry, this approach is likely to face a number of challenges prior to it being accepted into the mainstream as an adjunct to coronary CTA. The aim of the current review is to provide an overview of: 1) the fundamental engineering principles behind computational fluid dynamic modelling of coronary arterial blood flow; 2) the difficulties faced from an engineering perspective in developing a truly representative model; and 3) the challenges this technology is likely to face as it attempts to enter the clinical domain.
Introduction
Since the introduction of 64-slice CT scanners in 2005 numerous studies have compared the ability of coronary CTA to detect coronary artery disease (CAD) with invasive coronary angiography (ICA). There is now a wealth of evidence from retrospective studies demonstrating that coronary CTA is associated with a high sensitivity (91%-99%) and specificity (74%-96%) for detecting significant stenosis1-3. Similarly, from three major prospective multicentre studies, these results have been replicated4-6. The Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) Trial4 evaluated the diagnostic performance of 64-slice CTA in detecting and excluding significant coronary artery stenosis (≥70%) in 229 patients from 16 different institutions presenting with chest pain. In this study, the reported sensitivity, specificity, positive predictive value and negative predictive value were 91%, 84%, 49% and 98% (AUC 0.95), respectively, against a gold standard of invasive coronary angiography. Importantly, this study did not exclude patients based on resting heart rate, BMI or coronary calcium score. In a multicentre multivendor study Meijboom et al5 addressed whether coronary CTA was able to detect or rule out significant coronary disease (luminal stenosis ≥50%) in 360 patients with symptomatic acute and stable anginal symptoms. The authors demonstrated results similar to the ACCURACY trial with coronary CTA having a sensitivity, specificity, positive predictive valve and negative predictive value of 99%, 64%, 86% and 97%, respectively, in predicting the presence of significant coronary stenosis (≥50%). The third prospective multicentre trial to evaluate the diagnostic accuracy of 64-slice coronary CTA to rule out significant CAD was the CORE-64 trial6. In this study of 291 patients with suspected coronary artery disease and coronary calcium score of 600 Agatston units or less, 64-slice coronary CTA had a sensitivity, specificity, positive predictive value and negative predictive value of 85%, 90%, 91% and 83%, respectively, for detecting or ruling out significant coronary stenoses (≥50%) in vessels >1.5 mm.
These studies indicate that, although coronary CTA has particular strengths in excluding the presence of significant coronary disease, it performs less well in terms of its positive predictive accuracy. This is recognised in major international guidelines that currently indicate coronary CTA as being primarily appropriate for patients at a low to intermediate risk of coronary disease7. If coronary CTA is to extend its role, improvements are required in its overall diagnostic accuracy. Currently, efforts to achieve this have been largely focused on developing scanner technology in an attempt to improve coronary visualisation and to eliminate the influence of artefacts upon coronary segmental interpretation. The other major area of interest has been the development of sophisticated post-processing algorithms to evaluate the functional significance of coronary stenoses. There are now emerging reports indicating that fractional flow reserve (FFR) may be evaluated by coronary CTA (FFRct) and that this new approach may reduce the false positive rate of coronary CTA by up to 70%. Although this is an exciting and welcome development in the field of coronary CTA, there remains a cloud of scepticism amongst many as to whether this technology will work reliably, and where it will fit into the current arsenal of cardiac imaging and functional tests available. This current report reviews the status of FFRct, the principles upon which it is based, and the difficulties such an approach may be faced with as it attempts to emerge into the mainstream.
Factors governing the coronary circulation
In its simplest form the coronary circulation can be considered to consist of two main components, the first being epicardial vessels, or conductance vessels, that provide no resistance to flow, and the second being vessels <400 um, that may be considered as being “resistive vessels”. In the presence of a coronary stenosis it is the resistive vessels that influence myocardial blood flow.
The main parameters that govern circulatory function are flow, pressure and resistance. Whereas flow and resistance are dependent on the myocardial mass being perfused, pressure generally remains constant over the entire course of the epicardial coronary artery, even during maximal hyperaemia. Thus, in contrast to the size and flow within the coronary arteries that may vary, the pressure remains constant down the length of a normal coronary artery. This constant pressure gradient is maintained even though the absolute pressure may change with a patient’s age, systemic haemodynamics and coronary microvasculature8,9.
Fractional flow reserve – invasive coronary angiography
Fractional flow reserve is defined as the ratio between maximal blood flows achievable in a stenotic artery compared to normal maximal blood flow to flow in the same vessel. Because flow is proportional to pressure, if resistance is kept minimal and constant (Ohm’s law), pressure may be used as a surrogate for flow during maximal hyperaemia, which minimises resistance. Since pressure in a normal coronary artery is equal to pressure within the ascending aorta, FFR is simply calculated as the ratio between the pressures distal to a coronary stenosis when compared to pressure within the ascending aorta, during maximal hyperaemia. One of the main advantages of FFR compared to other indices of coronary circulation such as coronary flow reserve and the index of microvascular resistance is that its derivation attempts to exclude the confounding influences of the effects of the microcirculation, changes in haemodynamics or contractility10. FFR values <0.75 are generally considered to be abnormal and are associated with ischaemia detected by functional testing11, whilst values >0.8 are generally associated with negative ischaemic results12.
Clinical validity
There are now a number of studies documenting the clinical validity of invasive FFR. The DEFER study13 evaluated 325 patients scheduled for percutaneous coronary intervention for intermediate coronary lesions, all of whom underwent FFR measurements. Patients with an FFR ≥0.75 were randomly assigned to percutaneous coronary intervention (90 patients) or deferred treatment (91 patients). For those patients with an FFR <0.75, PCI was performed as planned. At five-year follow-up those patients in the deferred treatment group had an excellent outcome with the risk of death or myocardial infarction being <1% per year and not being decreased by stenting. In the landmark Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) study14, 1,005 patients with multivessel coronary disease were randomly assigned to undergo drug-eluting stent implantation guided by invasive angiography or FFR measurements in addition to invasive angiography. The authors showed that the routine use of FFR in patients with multivessel coronary disease to guide coronary stent insertion reduced the total number of stents required and also the composite endpoint of death, myocardial infarction and repeat revascularisation at one year (angiography group 18.3% vs. 13.2% in the FFR group, p=0.02). A later economic evaluation of the FAME study15 also showed that the mean overall costs in the FFR-guided arm were less than in the angiography-guided arm, thus indicating that invasive FFR not only improves patient outcomes but is also a cost-effective strategy for evaluation of patients with multivessel coronary disease.
Fractional flow reserve by coronary computed tomography
Based on these prior studies of invasive FFR, it would be an attractive prospect if it were possible to couple coronary CTA with FFRct and thus enhance its diagnostic capability. HeartFlow Inc. (Redwood City, CA, USA) have pioneered developments in this field and indicate that this is now a reality by using technology based on the 3-D volumetric analysis of the coronary tree using supercomputers, and sophisticated computational flow dynamics (CFD) derived originally from the aeronautical industry. Although this technology is currently only available for investigational purposes, provisional data are emerging that demonstrate that this innovation has much promise.
The Diagnosis of Ischaemia-Causing Stenoses Obtained Via Non-Invasive Fractional Flow Reserve (DISCOVER-FLOW) trial16 prospectively enrolled 103 patients from five international centres and identified 159 lesions with a stenosis of at least 50%. All patients subsequently underwent invasive angiography with FFR measurements and CT angiographic mapping with FFRct. The authors reported a 70% reduction in false positives, a twofold increase in true negatives and a 25% increase in overall accuracy when using the FFRct measurements. Additionally, there was an excellent correlation with invasive FFR, indicating that FFRct presents an alternative and non-invasive method for assessing coronary lesions that currently can only be obtained by invasive coronary angiography. Recently, the results from the Diagnostic Accuracy of Fractional Flow Reserve from Anatomic CT Angiography (DeFACTO) study have also emerged17. In this international multicentre prospective study involving 252 patients from 17 different centres, the authors compared the diagnostic accuracy of FFRct and coronary CT against invasive FFR as the gold standard. On a per-patient basis the diagnostic accuracy for FFRct plus coronary CT against invasive FFR was 73%, the sensitivity 90%, specificity 54%, positive predictive value 67% and negative predictive value 84%. Although the area under the receiver operating characteristic curve was greater for FFRct (0.81) when compared to coronary CT alone (0.68) for the discrimination of significant coronary disease by invasive FFR, the study did not achieve its primary endpoint of per-patient diagnostic accuracy. The results of the DISCOVER-FLOW and DeFACTO trials indicate that there is promise that FFRct may emerge as a useful technique in the future but that further refinements in accuracy may be required prior to clinical use.
Can coronary blood flow be measured using computational fluid dynamics?
WHAT IS COMPUTATIONAL FLUID DYNAMICS?
Computational fluid dynamics (CFD) is a numerical modelling technique which predicts and analyses the mechanical properties of fluids18,19. It is commonly used in the study of flow dynamics in silico. In the current state of the art, it is based on the solution of the Navier-Stokes equations, which account for most of the mechanical properties of fluids. The Navier-Stokes equations are the most popular model to describe the motion of fluids. They are used as the fundamental basis for studying haemodynamic factors of blood flow, such as flow velocity and pressure gradient and wall shear stress distribution along vessel walls20,21. In the context of estimating coronary blood flow by means of coronary CTA, given a set of input physiological conditions (i.e., functional information available from clinical or statistical data, such as intravascular pressure/velocity of the blood flow at the root of the artery), the CFD technique can be employed to estimate the haemodynamic parameters of the blood fluid in a patient-specific geometric model of the coronary artery.
The main steps of performing a bioflow simulation are as follows: segmentation of 3-D vascular structures from medical images, formulation of initial conditions and definition of modelling equations describing the physical laws of bioflow, and further numerical solutions by means of computational fluid dynamics (CFD) techniques using dedicated computer software. In this context, the term solution implies computation of haemodynamic parameters, e.g., flow rate, pressure, and velocity inside the vascular structure. Finally, the obtained solution is analysed and visualised (Figure 1). Using this technique it is theoretically possible to measure FFRct at any point along the coronary tree and to simulate flow and pressure characteristics in vessels with and without haemodynamically significant coronary stenoses (Figure 2 and Figure 3).
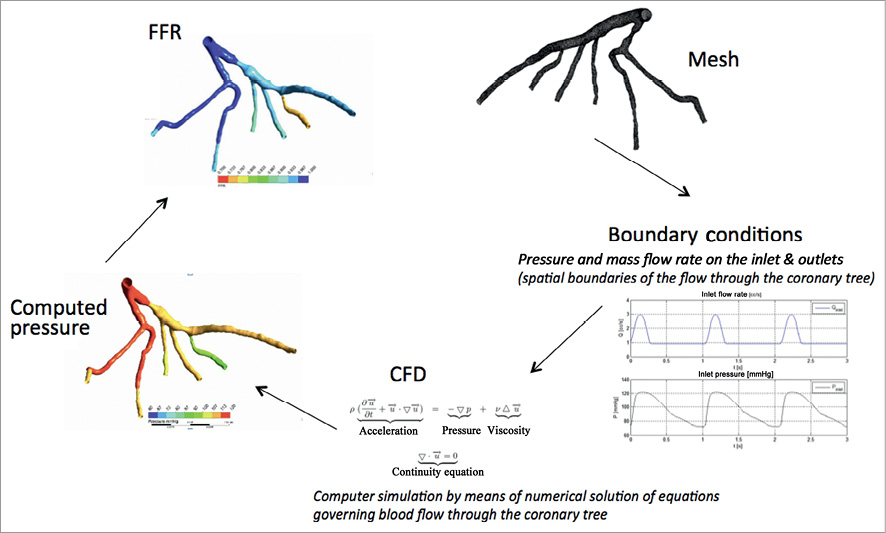
Figure 1. Example of FFR assessment flow chart.
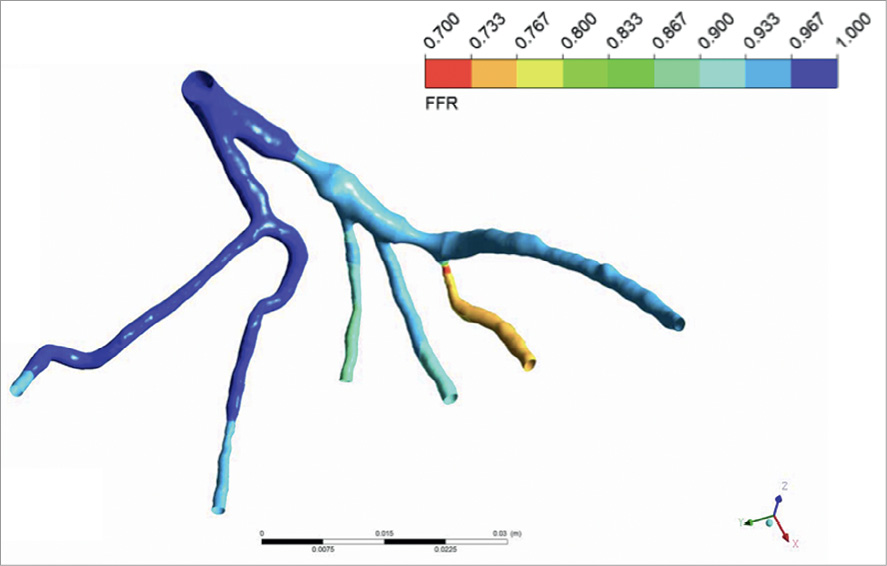
Figure 2. Example of FFR calculated for the vessel tree with artificially generated stenosis.
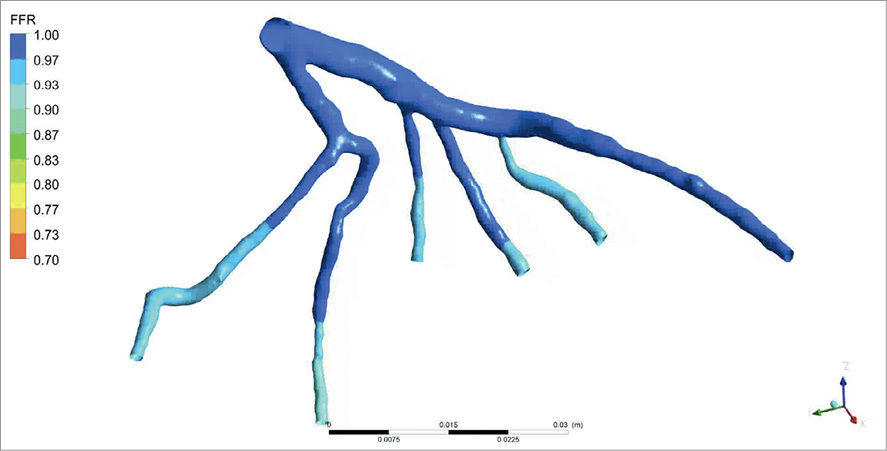
Figure 3. Example of FFR calculated for the vessel tree without stenosis.
Modelling of the geometry of the coronary arteries is a prerequisite in CFD calculations, as it defines the physical bounds of the problem. During the past decade, there have been major advances in the segmentation of vascular structures (i.e., identifying the voxels occupied by vasculatures) from static coronary CTA images. Numerous commercial and laboratory-based systems have been developed based on various vessel segmentation algorithms, which allow for the analysis of patient-specific geometric models of the arteries, subject to providing input from the user22, or even in a fully automated manner23,24. However, there are no accounts of systems that can produce an anatomically correct geometry in all patient cases. Erroneous segmentations are usually encountered in clinical practice, due to the presence of coronary pathologies (e.g., stents and calcification) and image artefacts25,26. In particular, the blooming effect, a partial volume artefact (perceived as blurring) affecting high radiation attenuation objects introduces an ambiguity with regard to the true size of such objects, thus posing additional difficulties in the construction of anatomically accurate coronary artery models. In fact, establishing the true boundaries of luminal regions in the vicinity of such objects is a challenging task even for an experienced clinician, and remains a topic for further research27.
Discretisation
Since there is generally no analytical solution to the Navier-Stokes equations, numerical techniques, primarily based on finite element (FEM)28 or finite volume methods (FVM)29, are commonly utilised in solving the blood flow equations. The entire volume occupied by the coronary artery is discretised into a large number of small subdivisions (known as elements or volumes), which are generally described by a collection of vertices, edges and faces that define the shape of each element. The discretisation process is known as meshing, which allows for the solution of the blood flow problem at the location of each element rather than the entire volume (Figure 4). However, this meshing process is not trivial, as it usually involves a series of geometrical operations, such as smoothing and re-meshing, to ensure numerical stability of the final biofluid simulation30. For instance, the level of smoothing is determined by the CFD specialist, thus posing additional difficulties to clinicians and potentially introducing further uncertainty in blood flow modelling.

Figure 4. Discretisation – 3-D mesh representation of the proximal ascending aorta and coronary tree.
Boundary conditions
It is impractical to take all of the vessels in the cardiovascular system into account in the haemodynamic simulations based on conventional coronary CTA. Firstly, small branches of the arteries (diameter <0.6 mm) cannot always be imaged appropriately with coronary CTA. Secondly, it is not possible to perform haemodynamic simulations in the entire coronary circulation system, as this would require immense computational resources (hardware) and computationally efficient algorithms (software)31. An interactively processed 3-D geometric model, containing only the vessel(s) of interest, is commonly used in haemodynamic analysis studies. Hence, the resulting geometric model of the arteries is restricted to the domain of interest and requires prior knowledge regarding the behaviour of the flow in the boundaries (known as boundary conditions, e.g., the central aortic pressure and the viscosity of the flow along the vessel boundary, etc.) (Figure 5). Although the central aortic pressure is commonly used as the inlet boundary condition in simulating blood flow in coronary arteries, unlike PC-MRI and ultrasound, blood pressure cannot be directly measured by coronary CTA. Brachial blood pressure is often used as a surrogate for pressure in large arteries, even though there is still controversy as to whether brachial pressure is a good estimate of blood pressure in large arteries. Contrary to inlet flow that can be assigned based on the functional information coming from clinical data, the outlet boundary condition, which models the effect of the distal vascular system, such as small arteries, microcirculatory vessels and veins, returning blood to the heart, is difficult to determine in practice. Commonly used outflow boundary conditions, using zero/constant pressure or prescribed velocity field, can lead to inaccurate estimation of blood pressure in the arterial model32,33. Recently, biofluid research has emerged into coupling the lumped parameters (determined by sophisticated methods), which approximate the haemodynamic conditions of the distal vascular system, thus improving the overall performance of the CFD simulation in predicting blood flow and pressure in vessels34. The estimated flow distribution of each of the major coronary arteries is a consequence of the relationship between vessel size and resistance, while cardiac output is based on the measurement of myocardial mass that is derived from the cardiac CT dataset. Furthermore, the coronary venous resistance is calculated based on the assumption of mean coronary blood flow. The introduction of these and other such assumptions needs to be made to facilitate a workable model of FFRct that can produce results within an acceptable timeframe. This approach to modelling, however, is at the expense of incorporating patient-specific factors, and their clinical application is otherwise still under investigation.
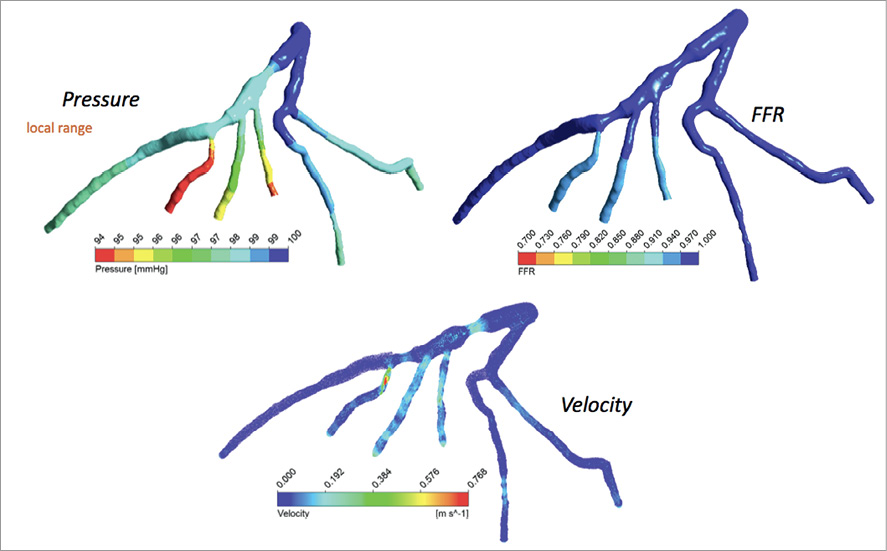
Figure 5. Computed velocity, pressure and FFR during diastole for ideal boundary conditions.
Recent advances in image-based blood flow simulation
Most studies in image-based blood flow assume that blood is a Newtonian fluid, and flow computations are carried out in static and rigid vessels. However, vessels are elastic bodies, which exhibit a certain degree of deformation, when blood passes through, which subsequently influences the mechanics of blood flow. Recent advances in fluid structure interaction (FSI) research have allowed for modelling the effect of such deformations in haemodynamic simulations35,36. However, the mechanical properties of the vessel wall are difficult to determine in practice (particularly on a patient-specific basis), due to the presence of coronary pathologies and the limited resolution of CTA. It should be noted that plaque deposits on the vessel wall have a fundamental effect on the mechanical properties of the vessel wall37-39.
Perspective
There is promise that FFRct will enhance the diagnostic capability of coronary CTA by improving its positive predictive ability and overall accuracy. If this is proven, this would represent a significant advancement in the field of coronary CTA. For the first time it would be possible to assess non-invasively the coronary anatomy and physiology simultaneously. This could potentially result in an extended role for coronary CTA in clinical practice. Whereas currently coronary CTA is usually indicated for patients at a low-intermediate likelihood of coronary disease, it may be possible to extend its role to patients within other risk categories. Coronary stent evaluation may become more robust, as may the evaluation of coronary artery bypass graft insertion points. Furthermore, and perhaps more importantly, it may reduce the number of patients being referred for invasive coronary angiography with non-obstructive plaque disease, following an indeterminate coronary CTA as a result of significant calcified plaque limiting accurate coronary stenosis evaluation.
Despite this ideology for FFRct, there are a number of issues that will require resolution before its true clinical applicability can be determined. Perhaps most important is that it is currently unclear where this new technology will integrate amongst the currently existing functional tests available. Dobutamine stress echocardiography, adenosine stress first-pass perfusion MRI and myocardial single photon emission tomography (SPECT) already have an extensive evidence base, provide an immediate result and have been proven to be cost-effective techniques for assessing myocardial ischaemia. In contrast, FFRct is at an early stage in its validation and involves a time delay (4-6 hours) prior to the results being available. In a climate of escalating healthcare costs, it will be increasingly important that comparative effectiveness research and cost-effectiveness studies are performed to help guide clinicians as to the optimal investigative strategies for their patient populations.
Conclusions
From a scientific perspective, it is clear that the measurement of FFRct is possible by CFD. Nevertheless, there are a number of challenges that this approach is likely to face as it attempts to enter the clinical domain. From an engineering perspective, issues are likely to arise as a result of the necessary introduction of a number of physiological assumptions, and the potential oversimplification of coronary arterial blood flow mechanics in an attempt to speed up processing. On the other hand, clinical acceptance will be governed by robust validation studies, a well-defined position amongst pre-existing functional tests and an analysis of the health economic implications that FFRct may entail. These considerations aside, the initiative to improve the diagnostic accuracy of coronary CTA is a welcome one and the efforts of those involved in the development of FFRct are commendable. The scientific community will eagerly await the forthcoming results of studies validating this technology in a variety of different patient cohorts.
Conflict of interest statement
The authors have no conflicts of interest to declare.

