Abstract
Over the last decade, steady progress has been made in the ability to assess coronary stenosis relevance by merging computerised analyses of angiograms with fluid dynamic modelling. The new field of functional coronary angiography (FCA) has attracted the attention of both clinical and interventional cardiologists as it anticipates a new era of facilitated physiological assessment of coronary artery disease, without the need for intracoronary instrumentation or vasodilator drug administration, and an increased adoption of ischaemia-driven revascularisation. This state-of-the-art review performs a deep dive into the foundations and rationale behind FCA indices derived from either invasive or computed angiograms. We discuss the currently available FCA systems, the evidence supporting their use, and the specific clinical scenarios in which FCA might facilitate patient management. Finally, the rapidly growing application of FCA to the diagnosis of coronary microvascular dysfunction is discussed. Overall, we aim to provide a state-of-the-art review not only to digest the achievements made so far in FCA, but also to enable the reader to follow the many publications and developments in this field that will likely take place in years to come.
Introduction
More than half a century after revolutionising clinical practice, coronary angiography remains the cornerstone for the evaluation and treatment of coronary artery disease (CAD). Notwithstanding its relevance as a diagnostic tool, coronary angiography is fraught with major limitations as a method to ascertain the functional relevance of coronary stenoses, particularly in lesions of intermediate angiographic severity1.
Attempts to bridge the gap between the angiographic morphology and functional relevance of coronary stenoses were led in the 1970s by Lance Gould and other investigators. These authors used stenosis geometry data, objectively assessed with the then newly developed quantitative coronary angiography (QCA) methods, and incorporated these data into fluid dynamic equations used to predict translesional pressure loss over a range of flow values and resistances, ultimately founding the basis for the development of coronary physiology assessment2.
However, the transition of this angiographic tool from the realm of research into clinical practice was hindered, on the one hand, by the lack of correlation with ischaemic and clinical outcomes3 and, on the other, by the development of intracoronary physiology, particularly fractional flow reserve (FFR), which initiated the era of catheter guidewires for evaluation of ischaemia4.
The additive benefit of FFR in clinical decision-making regarding revascularisation in patients with coronary stenoses has been clearly demonstrated in numerous dedicated randomised clinical trials567. Nevertheless, although this ultimately led to FFR being incorporated into international clinical practice guidelines over the following decade, coronary physiological interrogation remained largely underused in cardiac catheterisation laboratories8.
Explanations for the low penetrance in clinical practice are twofold: inertial (i.e., the operator’s apprehension about angiographic data and/or mistrust in coronary physiology) and technical (perceived complexity of adenosine infusion, procedural time and costs)9. The realisation that the adoption of invasive physiology was somewhat hampered by the requirement for vasodilatory drugs and coronary instrumentation has sparked two new developments in the last decade: 1) the introduction of non-hyperaemic indices, greatly simplifying the procedure by not mandating pharmacological hyperaemia induction; and, more recently, 2) the development of functional angiographic systems that can provide physiological information without either pressure guidewires or hyperaemic drugs (Figure 1).
In this review, we revisit the physiological principles and technical aspects of the different modalities of wireless functional coronary angiography (FCA), focusing on invasive coronary angiography (ICA) and coronary computed tomography angiography (CCTA). We will also evaluate the existing evidence and discuss the clinical applicability of wireless FCA in specific clinical scenarios. Finally, we provide our vision for future prospects and how FCA will change current clinical practice.
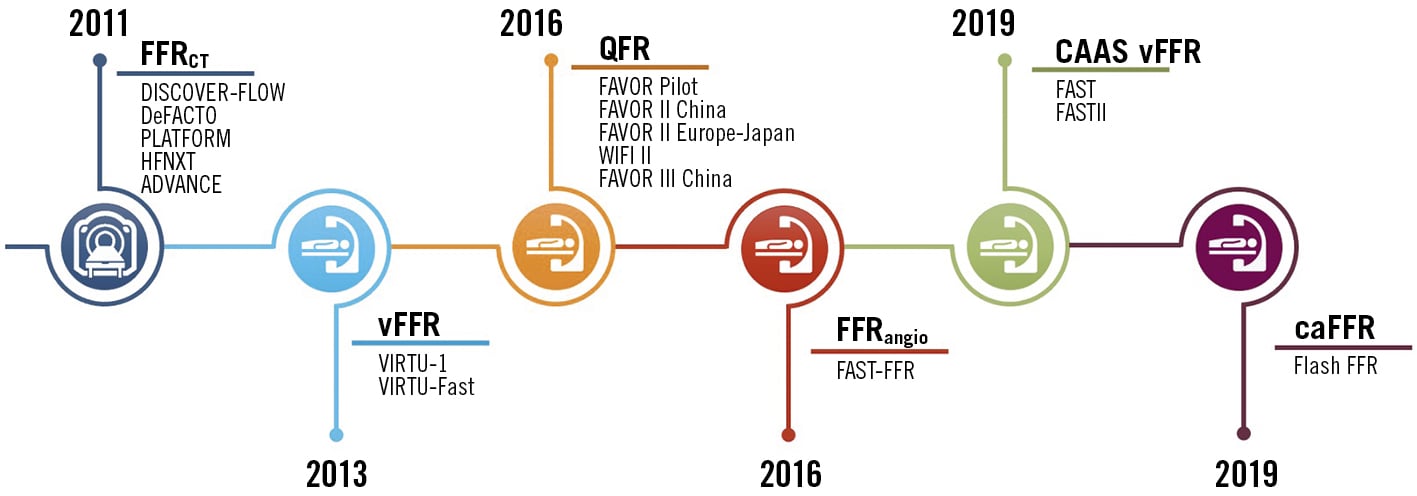
Figure 1. Timeline of the evolution of non-invasive coronary physiology and the respective landmark studies. CAAS vFFR: vessel fractional flow reserve; caFFR: computational pressure-flow dynamics derived FFR; FFR: fractional flow reserve; FFRangio: angiography-derived FFR; FFRCT: computed tomography-derived FFR; QFR: quantitative flow ratio; vFFR: virtual fractional flow reserve
Key principles of functional coronary imaging
Basic concepts and caveats of invasive coronary physiology
To fully understand the developments made in FCA, it is important to revisit some key concepts of coronary physiology.
FFR is the most widely used pressure-derived index of functional coronary stenosis severity; it expresses the percentage reduction in myocardial blood supply attributable to the interrogated stenosis. A translesional pressure ratio of 0.75 obtained during maximal hyperaemia indicates an impairment in myocardial blood supply of 25%, compared to the supply in the hypothetical absence of that same stenosis.
The cornerstone of FFR, which enables the use of pressure as a surrogate of flow, is the linearity of the pressure/flow relationship during hyperaemia. Under hyperaemic conditions, the fall in coronary pressure caused by a stenosis is proportional to the fall in maximal blood supply to the myocardium.
In the epicardial coronary arteries, flow is driven by the myocardial perfusion pressure (MPP) and is equivalent to the difference between the aortic pressure (Pa) and the central venous pressure (Pv)10. Whenever there is an epicardial stenosis, the MPP is equivalent to the difference between the poststenotic distal pressure (Pd) and the Pv . Therefore, FFR can be estimated from pressure measurements as follows: Pd − Pv/Pa − Pv or ≈Pd/Pa, as the effect of central venous pressure is usually negligible. Uniquely, FFR has a constant value of 1.0 in every normal coronary artery and is not influenced by variations in blood pressure, myocardial contractility, or heart rate10.
The pressure loss across a coronary stenosis (ΔP) is dependent upon the severity of the narrowing and also on the magnitude of flow (Q) that goes through it11. Pressure loss across a stenosis is due to: 1) viscous friction (f) and 2) flow separation due to acceleration through the stenosis (t), which leads to blood swirling and reverse currents. The expression ΔP = fQ2 + tQ2 explains why there is a quadratic increment in pressure loss through a stenosis with an increase in coronary flow12.
Assessing functional stenosis relevance from coronary angiograms
To derive patient-specific estimations of blood flow and pressure in coronary arteries from coronary angiography, three fundamental steps must be followed: 1) selection of a fluid equation solver (computational fluid dynamics or simplified fluid dynamics equations), 2) reconstruction of a three-dimensional (3D) model of the coronary arteries, and 3) definition of boundary conditions (Figure 2)13.
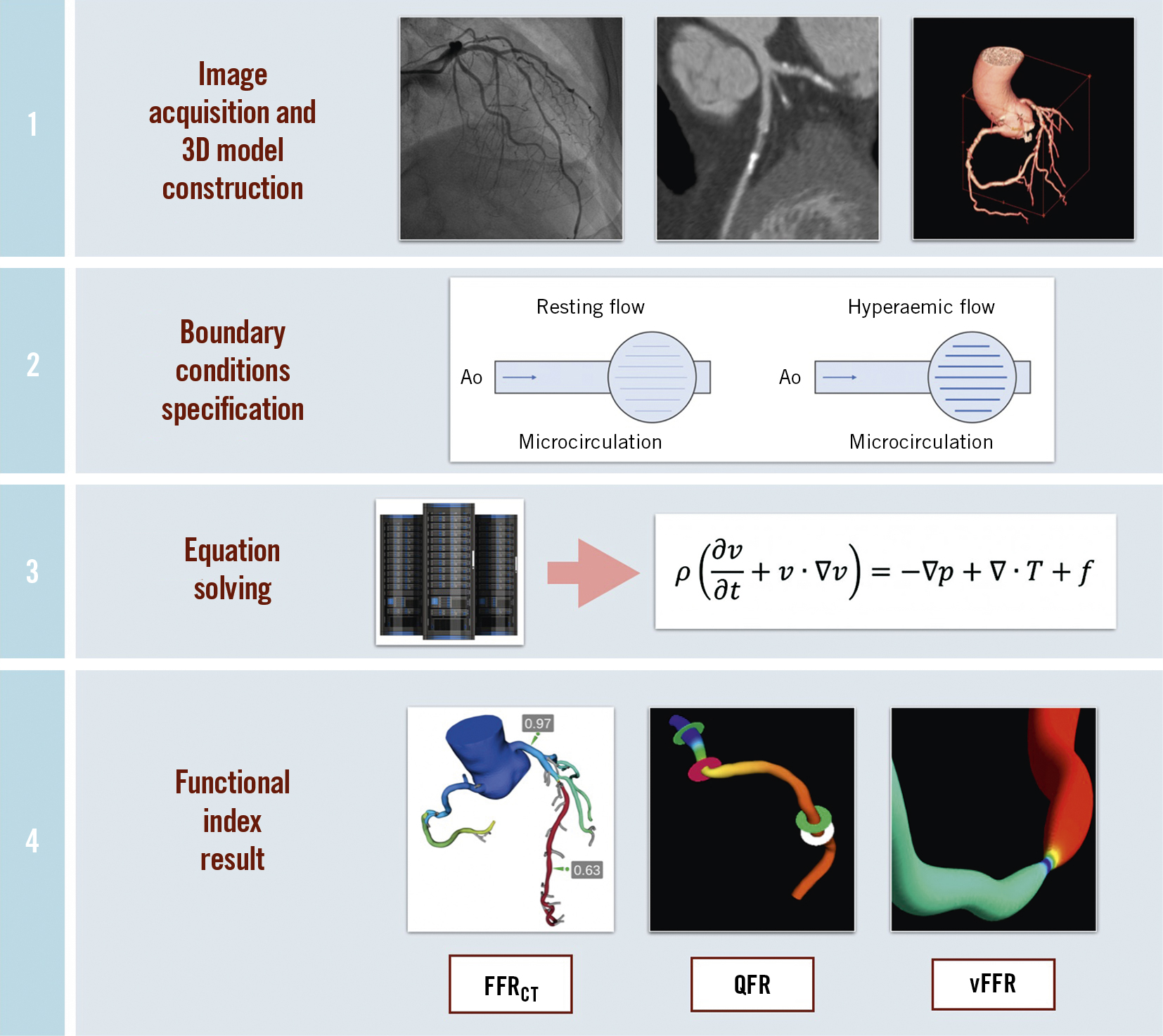
Figure 2. Steps for obtaining a functional coronary imaging index. 1) Standard invasive coronary angiography or computed tomography angiography data are obtained. A quantitative 3-dimensional anatomical model is generated. 2) A physiological model of the coronary microcirculation is obtained from patient-specific data following specific principles: the resting coronary flow is proportional to the subtended myocardial mass; the microvascular resistance is inversely correlated with vessel size; the microvascular resistance is reduced during maximal hyperaemia. 3) Physical laws of fluid dynamics are applied to compute coronary blood flow, solving the Navier-Stokes equations or simplified equations. 4) The chosen functional index is calculated throughout the coronary artery. FFRCT: computed tomography-derived fractional flow reserve; QFR: quantitative flow ratio; vFFR: virtual fractional flow reserve.
Solving fluid equations
Most image-based FFR techniques utilise computational fluid dynamics (CFD), a generic term used for all the mathematical engineering that is required to describe and analyse fluid flow. Through computational processing, the governing equations of fluid dynamics, i.e., Navier-Stokes equations, can be solved for the unknown coronary pressure and blood velocity that vary in position and time14. Blood density and viscosity are usually assumed when solving these equations, as blood, despite its complex rheological properties, can be managed as a Newtonian fluid with constant viscosity, particularly in large arteries15.
In order to reduce the computational power and time required to complete a full CFD analysis, and thus make it feasible for online vessel assessment in the catheterisation laboratory, a simplified version of CFD, using simpler mathematical coefficients, is frequently used in commercially available systems16. These approaches benefit from using actual blood flow velocity by using TIMI (Thrombolysis in Myocardial Infarction) frame count and aortic pressure values, which are directly accessible for online study, instead of standardised values17.
Coronary geometric models
A 3D reconstruction of the vessel lumen, which is used by most FCA systems, can be extracted from computed tomography (CT) images or from orthogonal invasive angiographic projections. Most systems integrate into the calculated metadata embedded in the DICOM image format, containing information on relevant parameters such as table/image intensifier height and frame rates. The obtained geometric model can then be divided into smaller entities, i.e., finite elements, forming the blocks of a virtual mesh, over which the equational unknowns are calculated14. It is important to keep in mind that friction losses causing flow limitation may be due to microhaemorrheological disturbances caused by diffuse disease. Capturing the haemodynamic effect of minute but extensive vessel irregularities with imaging techniques relies on a very accurate reconstruction of the arterial lumen, which may not be possible because of limits in angiographic resolution.
Boundary flow conditions
The haemodynamics of epicardial vessels are strongly influenced by phenomena such as the cardiac cycle, intrinsic microvascular resistance, and extravascular compression. Boundary conditions are the mathematical limits (inlet and outlet) upon which haemodynamic models are applied to the entrance and exit of the reconstructed vessel segments, to simulate the influence of such factors15. Although the inlet boundary is usually easily obtainable (through directly measuring aortic pressures or by proving a population average model) the same is not true for the outlet boundary, which includes terms such as microcirculatory resistance and central venous pressure. Preset conditions are frequently assumed, although, as previously discussed, the actual values of aortic pressure and flow velocity can be incorporated into the calculations to provide a more realistic estimate of boundary conditions15.
Functional analysis of invasive coronary angiograms
By not requiring invasive measurements, pharmacological hyperaemia induction, or incurring additional costs, angiography-based techniques provide a promising solution for performing either online or offline functional evaluations of coronary stenosis in the catheterisation laboratory (Central illustration). A number of different software approaches that estimate FFR from angiography have been developed with reported validation studies (Supplementary Table 1, Table 1). Of note, only four of them have supporting evidence derived from prospective multicentric studies.

Central illustration. Current commercially available functional indices based on invasive coronary angiography. The columns from left to right show user interface display after index calculation; number of angiographic projections needed; need for mean aortic pressure input; capacity to provide microcirculatory resistance evaluation; simultaneous side branch physiological interrogation; and the quality and quantity of published evidence. Colour code: green=advantage; yellow: amenable; red=disadvantage. CAAS vFFR: vessel fractional flow reserve; caFFR: computational pressure-flow dynamics derived FFR; FFR: fractional flow reserve; FFRangio: angiography-derived FFR; QFR: quantitative flow ratio; μQFR: Murray law-based QFR;
Table 1. Technical aspects of the different FCA indices and respective validation studies.
| Technique | Imaging modality | Inlet | Outlet | Flow | Pattern | Equation solution | Other | Studies (Ref) | Year | Design | Vessels | Sensitivity | Specificity | PPV | NPV | Corr. with invasive FFR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COMMERCIALLY AVAILABLE | QFR | 3D-QCA | Patient- averaged aortic pressure | Free | Fixed hyperaemic | Steady | Simplified equation | fQFR | FAVOR Pilot17 | 2016 | Prospective offline | 84 | 0.67 | 0.86 | 0.69 | 0.85 | 0.69 |
| TIMI frame count | Steady | Simplified equation | cQFR | 0.74 | 0.91 | 0.80 | 0.88 | 0.77 | |||||||||
| Adenosine-induced | Steady | Simplified equation | aQFR (hyperaemia) | 0.78 | 0.91 | 0.81 | 0.90 | 0.72 | |||||||||
| - | Free | TIMI frame count | Steady | Simplified equation | cQFR | FAVOR II China16 | 2017 | Prospective online | 328 | 0.95 | 0.92 | 0.86 | 0.97 | 0.86 | |||
| Yazaki et al19 | 2017 | Retrospective offline | 151 | 0.89 | 0.89 | 0.77 | 0.95 | 0.80 | |||||||||
| FAVOR II Europe-Japan18 | 2018 | Prospective online | 317 | 0.87 | 0.87 | 0.76 | 0.93 | 0.83 | |||||||||
| FAVOR III China41 | 2021 | RCT | 3,825 | Lower events at 1-year follow-up: difference –3.0% [95% CI: –4.7 to –1.4]; hazard ratio 0.65 [95% CI: 0.51 to 0.83] | |||||||||||||
| WIFI II39 | 2018 | Prospective offline | 240 | 0.77 | 0.86 | 0.75 | 0.87 | 0.70 | |||||||||
| Free | Fixed hyperaemic | Steady | Simplified equation | fQFR | Ties et al21 | 2018 | Retrospective offline | 151 | 0.57 | 0.93 | 0.67 | 0.89 | 0.71 | ||||
| TIMI frame count | Steady | Simplified equation | cQFR | 0.67 | 0.96 | 0.82 | 0.92 | 0.70 | |||||||||
| Free | Fixed hyperaemic | Steady | Simplified equation | fQFR | Koltowski et al20 | 2018 | Retrospective offline | 306 | 0.90 | 0.63 | 0.64 | 0.89 | 0.72 | ||||
| TIMI frame count | Steady | Simplified equation | vQFR (vessel cQFR) | 0.91 | 0.70 | 0.69 | 0.91 | 0.78 | |||||||||
| iQFR (lesion cQFR) | 0.49 | 0.97 | 0.91 | 0.72 | 0.70 | ||||||||||||
| iQFR (index cQFR) | 0.84 | 0.87 | 0.82 | 0.88 | 0.85 | ||||||||||||
| Free | TIMI frame count | Steady | Simplified equation | cQFR | Mejía-Rentería et al22 | 2018 | Retrospective offline | 300 | 0.89 | 0.87 | 0.85 | 0.91 | 0.83 | ||||
| Free | Fixed hyperaemic | Steady | Simplified equation | fQFR | Emori et al25* | 2018 | Retrospective offline | 75 | 0.94 | 0.62 | 0.69 | 0.92 | 0.84 | ||||
| TIMI frame count | Steady | Simplified equation | cQFR | 0.92 | 0.82 | 0.83 | 0.91 | 0.88 | |||||||||
| Free | TIMI frame count | Steady | Simplified equation | cQFR | Emori et al23 | 2018 | Retrospective offline | 100 | 0.97 | 0.87 | 0.94 | 0.93 | 0.89 | ||||
| Spitaleri et al36** | 2018 | Prospective offline | 45 | 0.88 | 0.97 | 0.94 | 0.94 | 0.90 | |||||||||
| Smit et al29*** | 2018 | Retrospective offline | 82 | 0.71 | 0.95 | 0.85 | 0.89 | 0.74 | |||||||||
| Free | TIMI frame count | Steady | Simplified equation | cQFR | Smit et al24 | 2019 | Retrospective offline | 334 | 0.70 | 0.92 | 0.77 | 0.89 | 0.81 | ||||
| Stähli et al26 | 2019 | Prospective offline | 516 | 0.75 | 0.98 | 0.89 | 0.94 | 0.82 | |||||||||
| Hwang et al27 | 2019 | Retrospective offline | 358 | 0.92 | 0.90 | 0.86 | 0.95 | 0.86 | |||||||||
| Tanigaki et al37 | 2019 | Retrospective offline | 233 | 0.90 | 0.82 | 0.81 | 0.90 | 0.78 | |||||||||
| Lauri et al31 | 2019 | Retrospective offline | 159 | 0.86 | 0.80 | 0.78 | 0.87 | 0.75 | |||||||||
| Asano et al35 | 2019 | Retrospective offline | 310 | 0.74 | 0.74 | 0.86 | 0.57 | - | |||||||||
| Rubimura et al30**** | 2019 | Retrospective offline | 93 | - | - | - | - | 0.79 | |||||||||
| COMMERCIALLY AVAILABLE | FFRangio | ICA (2 views) | Measured mean aortic pressure | Resistance lumped model | Derived from healthy artery cross section + heart rate | Steady | CFD | - | Kornowski et al45 | 2016 | Prospective offline | 101 | 0.97 | 0.93 | 0.88 | 0.95 | 0.97 |
| Tröbs et al57 | 2016 | Retrospective offline | 113 | 0.79 | 0.94 | 0.85 | 0.92 | 0.85 | |||||||||
| ICA (3 views) | Mean aortic pressure measured at hyperaemia | Resistance lumped model | Derived from 3D geometry and scaling | Steady | Fluid equation | - | Pellicano et al46 | 2017 | Prospective offline | 203 | 0.91 | 0.93 | 0.88 | 0.95 | 0.96 | ||
| FAST-FFR47 | 2019 | Retrospective offline | 319 | 0.94 | 0.91 | 0.89 | 0.95 | 0.80 | |||||||||
| Omori et al58 | 2019 | Prospective online | 118 | 0.92 | 0.92 | - | - | 0.83 | |||||||||
| vFFR | Rotational ICA + 3D reconstruction | Measured aortic pulsatile waveform | Resistance lumped model | - | Pulsatile | CFD | vFFR transient CFD | VIRTU-155 | 2013 | Prospective offline | 35 | 0.86 | 1 | 1 | 0.97 | 0.84 | |
| Measured aortic pulsatile waveform | Resistance lumped model | - | Pulsatile | CFD + fluid equation | vFFR pseudotransient steady state | VIRTU-Fast56 | 2017 | Prospective offline | 73 | 1 | 1 | 1 | 1 | 1 | |||
| Measured mean aortic pressure | Resistance lumped model | - | Steady | CFD + fluid equation | vFFR steady state | 1 | 1 | 1 | 1 | 1 | |||||||
| CAAS vFFR | ICA 3D-QCA | Measured mean aortic pressure at hyperaemia | Free | Derived from patient aortic pressure + 3D sizing | Steady | Fluid equation | - | FAST49 | 2019 | Retrospective offline | 319 | - | - | - | - | 0.89 | |
| FASTII50 | 2021 | Prospective observational | 330 | - | - | - | - | - | |||||||||
| FFRCT | CTA | Lumped heart model | Resistance lumped model | - | Pulsatile/steady | CFD/simplified equations | - | DISCOVER-FLOW64 | 2011 | Prospective offsite | 159 | 0.88 | 0.82 | 0.74 | 0.92 | 0.72 | |
| DeFACTO65 | 2012 | Prospective offsite | 407 | 0.90 | 0.54 | 0.67 | 0.84 | 0.63 | |||||||||
| HFNXT66 | 2014 | Prospective onsite | 484 | 0.84 | 0.86 | 0.61 | 0.95 | 0.82 | |||||||||
| NOT COMMERCIALLY AVAILABLE | caFFR | ICA (2 views) | Measured mean aortic pressure | Free | TIMI frame count | Steady | CFD | - | Flash FFR51 | 2019 | Prospective online | 328 | 0.90 | 0.99 | 0.97 | 0.95 | 0.89 |
| vFAI | ICA 3D-QCA | Average human aortic pressure (100 mmHg) | Free | Fixed | Steady | CFD | Derived from DP-flow curve | Papafaklis et al54 | 2014 | Retrospective offline | 139 | 0.90 | 0.86 | 0.80 | 0.94 | 0.78 | |
| FFRQCA | ICA 3D-QCA | Measured mean aortic pressure | Free | TIMI frame count | Steady | CFD | Need of hyperaemia | Tu et al59 | 2014 | Retrospective offline | 80 | 0.78 | 0.93 | 0.92 | 0.91 | 0.81 | |
| *Postmyocardial infarction patients. **Patients with ST-elevation myocardial infarction from ARYOSTO. ***Patients with diabetes. ****Pre-percutaneous coronary intervention. 3D: three-dimensional; aQFR: adenosine-flow QFR; CAAS vFFR: vessel FFR; caFFR: computational pressure-flow dynamics derived FFR; CFD: computational fluid dynamics; cQFR: contrast-flow QFR; CTA: computed tomography angiography; DP: differential pressure; FFR: fractional flow reserve; FFRangio: angiography-derived FFR; FFRCT: coronary computed tomography-derived FFR; fixed-flow QFR; ICA: invasive coronary angiography; NPV: negative predictive value; PPV: positive predictive value; ps-trans: vFFR computed with the pseudotransient steady-state method; QCA: quantitative coronary analysis; QFR: quantitative flow ratio; TIMI: Thrombolysis in Myocardial Infarction; trans: vFFR computed with full transient CFD; vFAI: virtual functional assessment index; vFFR: virtual FFR | |||||||||||||||||
Quantitative flow ratio (QFR)
QFR (QAngio XA 3D; Medis Medical Imaging Systems, and AngioPlus; Pulse Medical Imaging Technology) is an FFR computational method derived from 3D-QCA based on two orthogonal angiographic projections of the coronary vessel, usually separated by at least 25 degrees17. Once the vessel is reconstructed into a 3D model, the haemodynamic pressure drops are calculated at consecutive segments according to derived friction and turbulence pressure coefficients, and subsequently integrated into the whole analysed segment (Figure 3). According to the type of flow value used for its calculation, QFR can be expressed in three different modes: 1) fixed-flow (fQFR) that uses a fixed value of population-averaged hyperaemic flow of 0.35 m/s, 2) contrast-flow (cQFR) that estimates flow velocity based on TIMI frame count, or 3) adenosine-flow (aQFR) that measures flow from hyperaemic coronary angiograms obtained during adenosine administration18. Most studies validating QFR utilised the cQFR method, which offers the best balance between diagnostic yield and technical ease of use17.
Prior studies have shown a good correlation between QFR and FFR values in angiographically intermediate lesions, with a good diagnostic accuracy of QFR for assessing functional stenosis severity192021222324252627282930313233343536373839 (Table 1). Two prospective, multicentre studies (Angiographic quantitative flow ratio-guided coronary intervention - FAVOR II China and FAVOR II Europe-Japan) have reported good diagnostic accuracies of QFR both at patient and vessel levels16 and better sensitivity and specificity than 2D-QCA in assessing functional stenosis relevance18. In a patient-level meta-analysis of 16 high-quality studies comparing FFR and QFR, QFR demonstrated good positive and excellent negative predictive values in ascertaining the functional relevance of coronary stenoses with a cut-off FFR of ≤0.8040.
Of note, QFR is the only functional angiographic system in which the clinical value has been tested in the setting of a randomised clinical trial. The FAVOR III China Study randomised 3,825 patients with acute and chronic coronary syndromes, with at least one lesion with a diameter stenosis of 50-90% on angiography, to a QFR-guided strategy (PCI indicated if QFR ≤0.80) or a standard angiography-guided strategy.
After 1 year of follow-up, patients randomised to the QFR-guided strategy had fewer myocardial infarctions and ischaemia-driven revascularisations (hazard ratio [HR] 0.65, 95% confidence interval [CI]: 0.51-0.83; p=0.0004)41. Moreover, the recently published 2-year follow-up analysis showed that QFR-guided patients had better outcomes in terms of major adverse cardiovascular events (MACE; HR 0.66, 95% CI: 0.54-0.81; p<0.0001)42. The FAVOR III Europe Japan Study (ClinicalTrials.gov: NCT03729739) will assess 1-year clinical outcomes comparing a QFR-based strategy to a strategy of pressure wire-based FFR.
Several studies have shown that QFR values correlate better with FFR than with non-hyperaemic pressure ratios (NHPR)43. This may be due to the fact that, compared with FFR, NHPR are more sensitive to friction losses of intracoronary pressure44 which, as discussed above, may be more difficult to identify with FCA. As a matter of fact, a significant difference in the correlation between both invasive indices and QFR has been shown to occur only in vessels with a diffuse pattern of disease, as assessed with guidewire-based longitudinal vessel interrogation43.
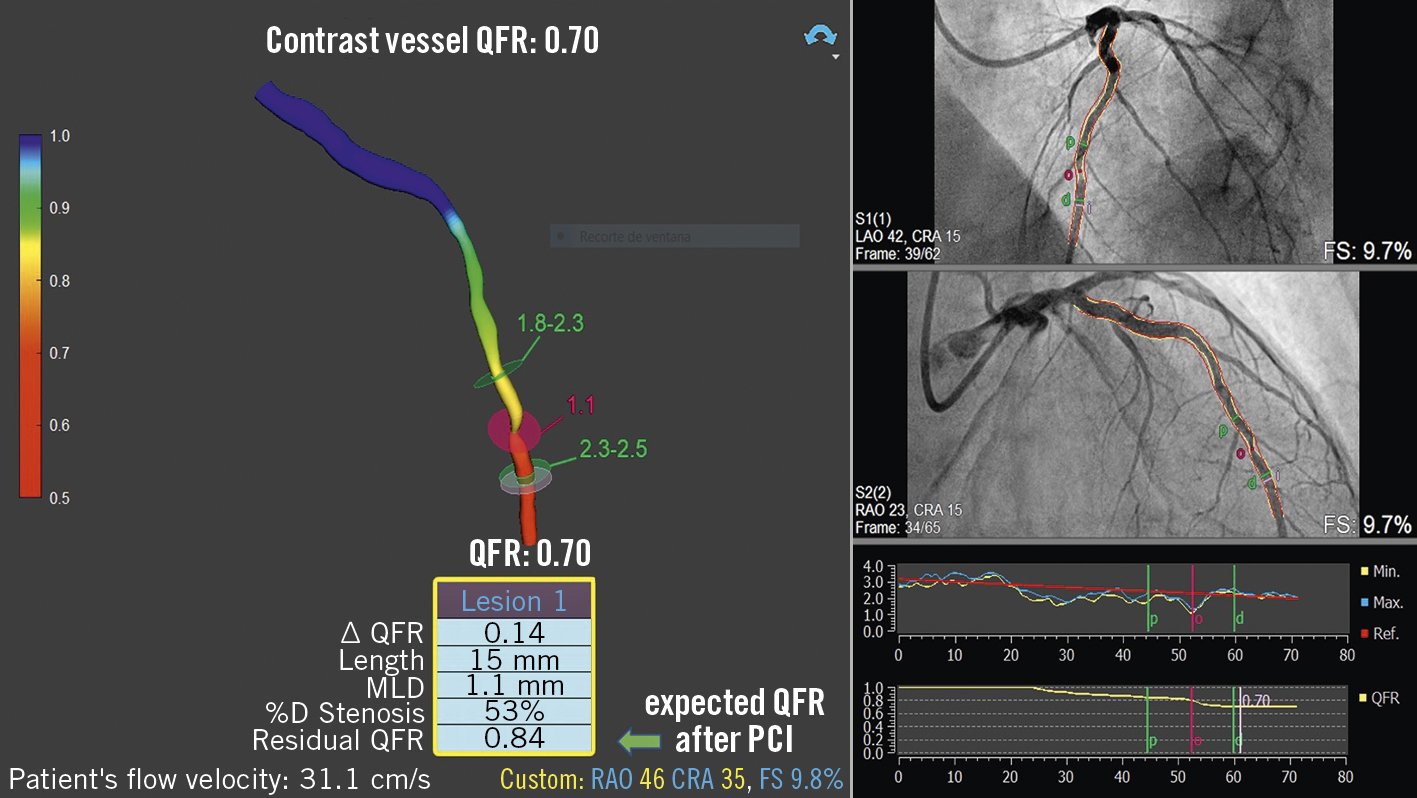
Figure 3. Contrast QFR for the left anterior descending artery. Coronary angiography showed an intermediate lesion in mid-left anterior descending artery. Contrast QFR in the same vessel was positive for significant functional ischaemia (0.70). Virtual angioplasty in a small segment (between the green markers) showed an expected final QFR of 0.84 after stenting. MLD: minimum lumen diameter; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; %D: % diameter
Coronary angiography-derived FFR
Coronary angiography-derived FFR (FFRangio; FFRangio System; CathWorks Ltd.) involves the creation of a 3D representation of the entire coronary arterial tree, using at least three single-plane angiographic projections, and estimates flow through a stenosis by solving simplified fluid equations4546. The microcirculatory resistance is estimated based on scaling formulas. The expected hyperaemic flow is estimated through the calculated microcirculation resistance and the total volume and length of the reconstructed coronary arterial system. The FFRangio is then obtained by dividing the expected hyperaemic flow rate in the stenotic artery with the hyperaemic flow rate calculated for a healthy artery (Supplementary Figure 1). Some advantages of FFRangio, compared with other invasive physiological systems, include the possibility of assessing the entire coronary vessel tree, including side branches, in a single analysis, without the need of intravenous nitrate administration.
After smaller prospective offline studies validated and reported a good diagnostic accuracy of FFRangio4546, the multicentre FFRangio Accuracy vs. Standard FFR (FAST-FFR) Trial prospectively enrolled 301 patients and reported an excellent per-vessel sensitivity (94%) and specificity (91%), with invasive FFR as the reference. Interestingly, even for “grey zone” FFR values (0.75-0.85), the diagnostic accuracy was high (87%)47.
The clinical value of FFRangio has recently been announced in a cohort of 518 patients. The cumulative incidence of death, myocardial infarction and repeat revascularisation after 2 years of follow-up was 3.6% in patients with an FFRangio-guided deferred strategy and 6.7% in patients treated with PCI48.
Vessel fractional flow reserve
Vessel fractional flow reserve (CAAS vFFR; Pie Medical Imaging) is calculated using 3D-QCA and simplified fluid equations. The inlet is determined by the aortic root pressure in hyperaemia, and flow velocity is derived by applying the measured pressure to the reconstructed 3D coronary geometry (Supplementary Figure 2). Based on the good linear correlation with wire-derived FFR in the Fast Assessment of STenosis severity (FAST) study49, the CAAS vFFR system was the first invasive angiography-based physiology system to obtain U.S. Food and Drug Administration market clearance. The recently reported FASTII trial results showed that CAAS vFFR has a high diagnostic accuracy to detect FFR ≤0.8050. The ongoing FAST III trial (ClinicalTrials.gov: NCT04931771) will address its prognostic impact compared with FFR.
Computational pressure-flow dynamics derived FFR
Computational pressure-flow dynamics derived FFR (caFFR; FlashAngio caFFR System; RainMed Ltd.) uses 3D angiographic reconstructions based on two orthogonal views, which are subsequently merged with computational fluid dynamics (Supplementary Figure 3). Patient-specific aortic pressure, applied at the inlet boundary, and resting flow velocities, determined by the TIMI frame count method, are integrated into the calculations to solve the Navier-Stokes equations51. In the Accuracy of Computational Pressure-Fluid Dynamics applied to Coronary Angiography to Derive Fractional Flow Reserve (Flash FFR) Study, caFFR demonstrated good diagnostic accuracy for the identification of functionally significant lesions, with wire-based FFR as the reference51.
Murray law-based QFR
Recently, a new approach to quantify functional stenosis severity from a single angiographic view has been proposed (Supplementary Figure 4). With the support of artificial intelligence algorithms, the Murray law-based QFR (μQFR) enables FFR estimation with a single, good quality angiographic projection, adjusting both the reference vessel diameter and the outgoing flow through side branches according to fractal geometry rules. After reporting an excellent agreement between μQFR and FFR in a post hoc analysis of the FAVOR II China study52, Tu et al demonstrated an almost perfect agreement with 3D-QFR in a small cohort53.
Other systems for functional assessment of coronary stenoses based on invasive angiography
In addition to the commercially available indices discussed above, other systems have also been reported.
The Virtual Functional Assessment Index (vFAI) uses 3D-QCA technology and CFD equations54. Average aortic pressure is applied at the inlet boundary. Blood flow is calculated by simulating pressures at 1 and 3 ml/s (average flow at rest and in hyperaemic conditions in human coronary arteries). Unlike the other systems described previously, virtual FFR (vFFR; Philips) is based upon rotational coronary angiography (RoCA; Philips)55. The system uses a CFD analysis in which aortic pressure waveforms are measured and applied at the inlet boundary, and the outlet boundary is derived by coupling a model of microcirculation. A simplified version of vFFR using steady-state CFD and simplified fluid dynamics equations has been described56.
Angiographic FFR (AngioFFR) uses CFD Simulation software (Siemens) and performs calculations using two angiographic projections of the coronary vessel, patient-specific heart rate and blood pressure; it is supported by artificial intelligence algorithms57. In a study including 253 lesions (200 patients), AngioFFR resulted in a good overall sensitivity and specificity, with a shorter processing time than invasive FFR58.
Tu et al developed an angiographic index from 3D quantitative coronary angiography and TIMI frame count (FFRQCA) with an overall good discrimination capacity to predict FFR ≤0.8059.
Limitations of angiography-derived FFR
Technical issues
Current versions of most angiography-derived techniques are highly operator dependent and require multiple steps (manual indication of landmarks, correction of vessel contours and indication of the target vessel start and endpoints, which account for significant interobserver variation in non-trial settings40. As accuracy depends directly on the quality of images and optimal projections, overlapped or highly tortuous vessels cannot be correctly evaluated.
In systems in which TIMI frame count is used as an estimate of coronary flow, the quality of contrast injection might influence the accuracy of estimations. The lack of standardisation of contrast injections might account for the differences in the reported correlations with invasive FFR20. The adoption of systems requiring rotational coronary angiography is limited by the availability of angiographic hardware with this feature. Since rotational angiography provides fewer images per projection, the number of end diastolic frames available for analysis is reduced55.
Angiography-derived FFR using simplified fluid equations is reportedly less time-consuming when compared with wire-based FFR. However, the same reports did not account for the time needed and spent in acquiring the prespecified images to make the analysis possible, which is often challenging20.
Issues related to reported evidence
Most published studies had a retrospective offline design, with a limited number of patients and were not powered for hard clinical endpoints (Table 1). Currently, only QFR have published data demonstrating improved outcomes from randomised controlled trials.
As with most clinical trials and validation studies, the number of exclusion criteria applied resulted in a highly selected population whose representability in real-world practice is somewhat hindered. This results in the exclusion of approximately 15% of screened patients (for the following reasons: aorto-ostial lesions, bifurcation lesions, presence of coronary bypass grafts, left main disease, chronic total occlusions, stent restenosis or diffuse disease) (Supplementary Table 2).
Confounding factors
Important discrepancies between FFR and QFR are found in patients with chronic kidney disease and diabetes, which might be related to the increased prevalence of microvascular dysfunction60. A recent study from MejÃa-RenterÃa et al demonstrated that microcirculatory dysfunction, defined by an index of microvascular resistance (IMR) ≥23, decreased the diagnostic performance of QFR to detect functionally significant lesions as determined by FFR. Nevertheless, QFR was still superior to angiography alone in ascertaining functional stenosis severity22. Hwang et al demonstrated discordance between QFR and instantaneous wave-free ratio (iFR) when considering abnormal coronary flow reserve (CFR) values27. Additionally, Westra et al demonstrated that resting Pd/Pa and FFR functional classification disagreement is associated with a reduced diagnostic accuracy of QFR61.
Functional angiography based on coronary computed tomography
Merging CCTA with FFR by using advanced computational analysis allows a complete haemodynamic and anatomical evaluation of a coronary lesion in one single non-invasive test, improving clinical outcomes, although without significantly reducing costs62.
Coronary computed tomography-derived fractional flow reserve
In the last decade, CCTA has been shown to be a highly accurate and non-invasive method for the evaluation of CAD and the assessment of plaque. Coronary computed tomography-derived fractional flow reserve (FFRCT; HeartFlow FFRCT Analysis; HeartFlow) is based on the application of CFD methods to solve the Navier-Stokes equations to simulate flow, pressure and velocity during rest and hyperaemia (Figure 4).
Contrary to FCA based on invasive angiograms, a full CFD analysis is performed using a supercomputer. The coronary 3D modelling is obtained by conventional CCTA and the patient-specific boundary conditions are determined as lumped models for the heart (time varying elastance model – inlet) and coronary microcirculation (outlet). Recently, reduced-order and steady-state models have been introduced to compensate for the limitations of supercomputer dependence and offsite calculation, averaging the Navier-Stokes equations over vessel cross-sections. Machine learning models have also recently materialised utilising artificial intelligence algorithms to calculate stenosis severity based on a multilayer neural network architecture and offline training63.
A number of prospective studies have been conducted to validate FFRCT using wire-based FFR as the gold standard reference. The Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) study found good correlation between FFRCT and FFR and a higher diagnostic performance of FFRCT compared to CCTA alone64. Similar results were found in the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study65.
The Analysis of Coronary Blood Flow Using CT Angiography (HTNXT) study scheduled high-quality CCTA (beta blocker and nitroglycerine administration) prior to ICA, and used a refined FFRCT algorithm which resulted in considerable enhancement of diagnostic accuracy on a per-patient and per-lesion basis for FFRCT, compared with CCTA alone, and subsequently higher specificity with comparable sensitivity66.
The Prospective LongitudinAl Trial of FFTCT: Outcome and Resource Impacts (PLATFORM) Study prospectively randomised 584 patients with a planned ICA to receive usual care testing or CCTA/FFRCT. The results showed that 61% of ICA procedures were deferred after receiving the CCTA/FFRCT results, with low rates of clinical events at 90 days in both arms67. The 1-year outcome data from this trial showed that care guided by the CCTA/FFRCT was associated with lower costs but equivalent quality of life and clinical outcomes68.
Finally, the Assessing Diagnostic Value of Non-invasive FFRCT in Coronary CarE (ADVANCE) registry prospectively enrolled 5,083 patients with coronary atherosclerosis identified on CCTA to evaluate the clinical significance of functionally significant stenosis with FFRCT. Revascularisation and MACE events occurred more frequently in patients with an FFRCT ≤0.80, than with patients with an FFRCT>0.80 (risk ratio [RR] 6.87; 95% CI: 5.59-8.45; p<0.001 and RR: 1.81; 95% CI: 0.96-3.43; p=0.06, respectively)69.
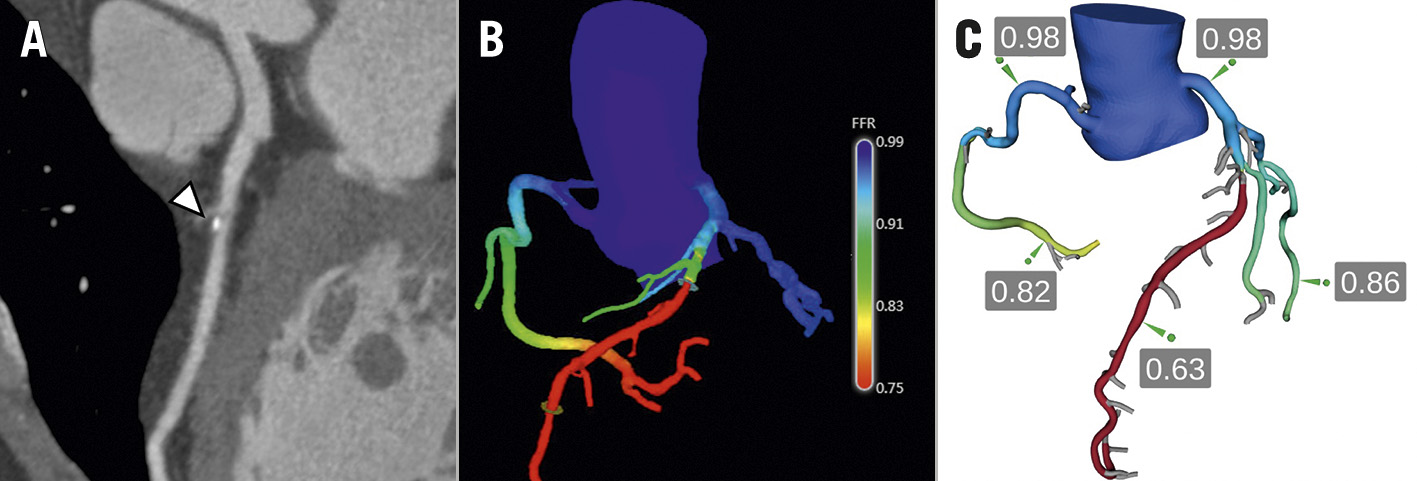
Figure 4. Coronary computed tomography-derived fractional flow reserve. A) CCTA of the LAD artery is shown. Arrowhead indicates a calcified plaque in the middle segment with 50% diameter stenosis. B) A quantitative 3D colour-coded anatomical model is generated. C) FFRCT is calculated throughout the coronary tree. The FFRCT value of 0.63 in the distal LAD artery suggests the presence of a functionally significant stenosis after the emergence of the first diagonal branch. Courtesy of Dr Joo Myung Lee. CCTA: coronary computed tomography angiography; FFR: fractional flow reserve; FFRCT: coronary computed tomography-derived FFR; LAD: left anterior descending
Limitations of computed tomography-derived FFR
Technical issues
Currently, FFRCT is time-consuming and analysed offsite. Furthermore, the accuracy of FFRCT is dependent upon a high-quality CCTA, which requires new-generation CT scans that are not always readily available. The analysis is also strictly dependent on the absence of artefacts (motion, intracoronary stents, pacemakers, internal defibrillators, prosthetic heart valves, excessive coronary calcification, uncontrolled atrial arrhythmias or frequent ventricular ectopic beats), resulting in significant dataset rejection in the validation studies (11-33%) due to poor image quality646566. Limited information is available on whether FFRCT is cost-effective.
Issues related to reported evidence
As previously described for the angiography-derived FFR techniques, the application of FFRCT analysis is not available for all patients. According to the proprietary company specifications, FFRCT is currently not recommended for patients with acute coronary syndromes (ACS) or recent myocardial infarction (MI), prior coronary artery bypass grafting (CABG), complex congenital heart disease, body mass index >35 kg/m2 or haemodynamic instability.
In the ADVANCE registry, baseline medications were not reported and optimised medical therapy was not considered in the core lab recommendations. Therefore, the primary outcome results do not reflect current recommended practice by international guidelines69. Furthermore, based on the presented studies, FFRCT was validated in a particular low-risk subgroup of patients and the performance of FFRCT in intermediate- or high-risk populations is currently unknown70. Importantly, neither core lab readers nor site investigators were blinded to the study aims, creating an opportunity for potential information bias on post-CCTA management by both entities69.
Clinical scenarios for functional coronary angiography
In Figure 5, we propose a decision-making algorithm for the use of FCA in everyday practice. It should be kept in mind that most of the validation studies discussed have been performed in patients with chronic coronary syndromes and intermediate coronary stenoses located in a major coronary branch. However, many researchers have explored the value of FCA in other relevant clinical and anatomical scenarios. These are discussed in greater detail below.
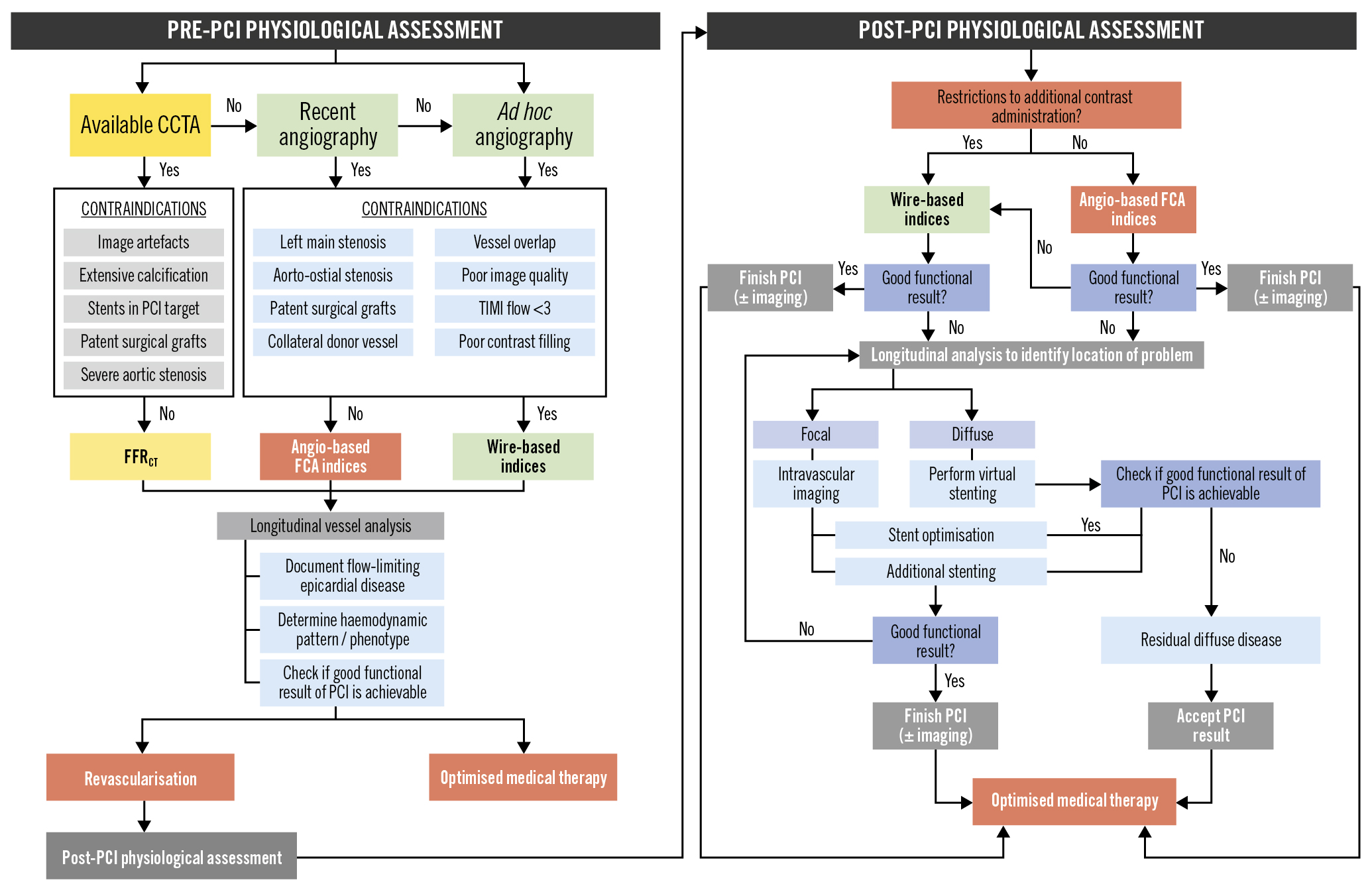
Figure 5. Utilisation of FCA in clinical practice. Decision-making algorithm for the usage of FCA in pre- and post-PCI settings. CCTA: coronary computed tomography angiography; FCA: functional coronary angiography; FFRCT: computed tomography-derived fractional flow reserve; LM: left main; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction
Non-culprit vessel evaluation in acute coronary syndromes
Approximately half of patients presenting with ST-segment elevation myocardial infarction (STEMI) have multivessel disease with non-culprit lesions (NCL)71. Repeated invasive tests are associated with increased risk, cost, and time, especially in non-functionally significant NCL. The ability to estimate the functional relevance of NCL offline after primary percutaneous coronary intervention (PCI) could greatly facilitate the management of these patients and optimise the catheterisation laboratory workflow.
Overall, the available evidence is concordant and supports the value of QFR for this indication. Lauri et al published a retrospective, observational, multicentre study demonstrating that QFR has excellent diagnostic accuracy when assessing the functional relevance of NCL during primary PCI31. Sejr-Hanse et al performed a post hoc analysis of the Intravascular Ultrasound Guided PCI in STEMI (iSTEMI) study and demonstrated that acute phase QFR has a very good diagnostic performance with staged FFR as reference33. Finally, Spitaleri et al, in a proof-of-concept study performed in patients with STEMI with NCL, found excellent correlation and agreement between QFR and FFR values in acute and subacute procedures. They also found that a QFR value ≤0.80 was associated with a higher risk of adverse events (HR 2.3, 95% CI: 1.2-4.5; p=0.01)36.
Choi et al, in a multicentre retrospective registry, identified that QFR consistently showed high diagnostic performance in predicting the functional significance of epicardial coronary stenosis, regardless of vessel location, lesion length, or various clinical presentations including ACS with NCL (n=153), previous MI (n=30), or diabetes mellitus (n=190)38.
Erbay et al studied the prognostic impact of QFR analysis in postinterventional culprit and non-culprit vessels in 792 patients with ACS. The results showed that an abnormal QFR is an independent predictor of 2-year MACE in both non-culprit arteries (odds ratio [OR] 3.78, 95% CI: 2.21-6.45; p<0.001) and post-PCI culprit arteries (OR 3.60, 95% CI: 2.09-6.20; p<0.001)72.
Duguay et al retrospectively investigated the prognostic value of FFRCT based on machine learning in patients with ACS and multivessel disease in 48 patients. Receiver operating characteristics including the framing risk score (FRS), the Coronary Artery Disease-Reporting and Data System (CAD-RADS) classification and FFRCT showed a discriminatory power superior to the FRS alone for the prediction of MACE (area under the curve [AUC] 0.66; p=0.032)73.
Thus, for the time being, FCA has the potential to deliver equivalent measurements to FFR in non-culprit lesions in the context of ACS. It has to be kept in mind that the value of FFR in this context is a matter of current debate, with discordant results in trials74. The existence of time-dependent changes in microcirculatory status, or higher prevalence of vulnerable lesions, have been proposed to explain why recent trials failed in demonstrating the value of FFR in decision-making in this context75. Further research will show whether in this context QFR mirrors FFR-linked outcomes or, alternatively, if FCA has any advantages.
In the setting of non-functionally significant lesions, intravascular imaging such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) is the gold standard for the detection of vulnerable plaques. However, by measuring CT attenuation in Hounsfield units, CCTA can detect plaque composition with similar accuracy. Plaques with high CT attenuation correspond to calcified lesions, whilst low-attenuation plaques correspond to the existence of time-dependent changes in high-risk lipid-rich lesions on virtual histography IVUS76. In addition, the pattern of CT attenuation can also be used to detect plaque vulnerability. The presence of the “napkin-ring” sign in CCTA is associated with thin-cap fibroatheromas in OCT. Furthermore, Otsuka et al demonstrated this sign is an independent strong predictor of future ischaemic events at 2-year follow-up, including cardiac death, myocardial infarction and unstable angina (HR 5.55, 95% CI: 2.10-14.7; p<0.001)77.
Hybrid approach of QFR/FFR in clinical decision-making
A strategy based on primary QFR-FFR, using FFR only when QFR values are in the “grey zone” could reduce procedural time, associated costs, and complications. Lauri et al reported that using a hybrid QFR-FFR approach − primarily using QFR if values are <0.75 or>0.85, and FFR only when QFR values were within these boundaries − would result in an overall classification agreement of 96.7% in post-ACS NCL31. Compared to the iFR/FFR strategy, validated in the ADVISE II registry78, the main advantage of the reported QFR/FFR approach is the avoidance of unnecessary pressure wire evaluation, avoiding repeated diagnostic procedures in 58.5% of patients31.
Planning percutaneous coronary revascularisation
In addition to identifying flow-limiting disease, FFRCT might prove useful in guiding and planning coronary revascularisation. Jensen et al recently confirmed an increase in the PCI/ICA ratio in the high-risk population, with more ICA cancelled on the basis of FFRCT79. This study introduces the possibility of decision-making during PCI without the need to confirm ischaemia invasively, because of a high concordance between invasive and non-invasive measures of ischaemia. Van Belle et al also demonstrated that adding FFRCT to the strategy algorithm changes the treatment decision in one-third of lesions80. Another important aspect in PCI planning is the ability to simulate the post-PCI coronary artery, allowing the calculation of post-PCI FFRCT (Figure 6). In the original feasibility study, virtual post-PCI FFRCT demonstrated good overall correlation with invasive post-PCI FFR81. This method still has limited clinical utility, as the current computational requirements do not allow for a timely evaluation. With future developments in computation, this method could be performed online by onsite physicians, further aiding revascularisation decision-making.
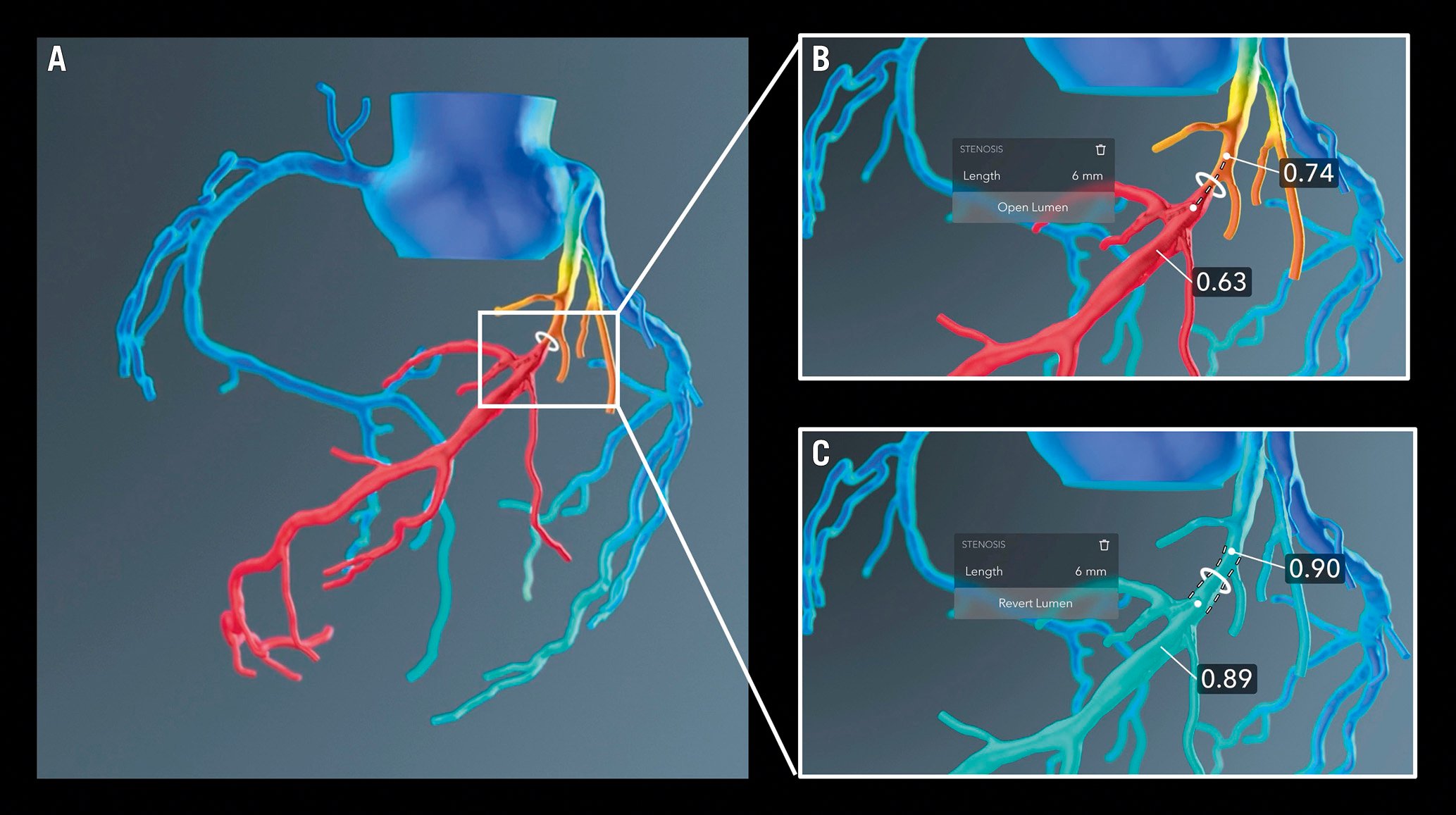
Figure 6. Simulation of PCI results using FFRCT. A) CT-based functional coronary angiography showing a flow-limiting stenosis in the mid-segment of the left anterior descending artery. B) Simulation of PCI results after choosing the target segment to treat and C) expected haemodynamic results after achieving revascularisation. FFRCT: coronary computed tomography derived fractional flow reserve; PCI: percutaneous coronary intervention
The value of FCA in planning interventions extends to Heart Team decisions on revascularisation of multivessel disease
By being able to provide a non-invasive functional Synergy Between Percutaneous Coronary Intervention With Taxus And Cardiac Surgery (SYNTAX) score, FFRCT offers significant potential to reduce the need of ICA or invasive adjudication of ischaemia for Heart Team decisions.
The incremental value of a functional assessment of CAD, beyond solely anatomical ICA imaging, was highlighted in a study published by the Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention (FAME) study investigators, showing that adding FFR into the classic anatomical SYNTAX score reclassifies over 30% of patients82. The advantages of state-of-the art PCI guided by hybrid assessment with iFR and FFR in improving clinical outcomes in patients with de novo 3-vessel disease were also demonstrated in the SYNTAX II trial83.
Asano et al derived a functional SYNTAX score based on QFR (fSSQFR) by retrospectively screening and analysing all lesions interrogated by iFR/FFR in the SYNTAX II trial. According to the 2-year patient-oriented composite endpoint (all-cause death, myocardial infarction, or any revascularisation), fSSQFR reclassified 26.1% of patients into the low-risk group (net reclassification improvement 0.32; p<0.001), and its accuracy to predict this composite endpoint was higher than that of the classic anatomical SYNTAX score (0.68 vs 0.56; p=0.002)35.
The calculation of the SYNTAX score derived from coronary CCTA (CT SYNTAX score) has been shown to have similar accuracy when compared to the one derived from ICA84. By being able to provide a non-invasive fSS, FFRCT offers significant potential to reduce the need of ICA or invasive adjudication of ischaemia. The SYNTAX III trial, which randomised patients to non-invasive and invasive adjudication of ischaemia, identified that in patients with left main or 3-vessel CAD, agreement concerning treatment choices (PCI or CABG) between coronary CCTA and ICA was high (Cohen’s kappa 0.82, 95% CI: 0.74-0.91). FFRCT also changed the treatment decision in 7% of patients85.
Despite the potential role of FCA in this setting, evidence of a clear benefit in clinical practice is lacking. In addition, the results of the FAME 3 trial showed that FFR-guided PCI was inferior to CABG in patients with 3-vessel CAD regarding death, myocardial infarction, stroke or repeat revascularisations at 1 year86. Given that all the FCA indices were validated against FFR, we do not recommend using FCA to decide the revascularisation strategy (PCI vs CABG) until further evidence is available.
In-stent restenosis
The performance of QFR in in-stent restenosis (ISR) was evaluated retrospectively in a multicentre, international, blinded study by Liontou et al, showing a reasonably high classification agreement between FFR and QFR (0.83), comparable to those reported with de novo lesions. Additionally, the reported performance of QFR to establish ISR relevance, with wire-based FFR as reference, was high (AUC 0.90, 95% CI: 0.83-0.97)87.
To date, there is no available evidence regarding the use of FFRCT in evaluating ISR. Recently published studies highlighted relevant limitations of CCTA imaging in the presence of coronary stents due to artificial lumen narrowing, ranging between 10 and 60%, being more pronounced in small diameter stents (<3 mm)88. Despite having a very high negative predictive value (NPV) for the exclusion of ISR, its positive predictive value (PPV) is markedly worse89. With the recent introduction of dual source CT scanners, there has been a significant improvement in the reported performances.
Functional evaluation in the presence of aortic stenosis
Concomitant CABG surgery is performed in approximately 40% of patients undergoing surgical aortic valve replacement and the prevalence of significant CAD in patients undergoing transcatheter aortic valve implantation (TAVI) is greater than in surgical series, with a reported prevalence of over 60%90. However, despite being recommended by international guidelines, the safety and benefit of revascularisation in this group of patients is yet to be proven.
The prognostic impact of CAD in this setting is still unclear and PCI before TAVI has not been associated with improved hard endpoints or symptoms during follow-up91. The increased imposed afterload in this setting is responsible for a complex array of macro- and microcellular transformations that culminate in left ventricular hypertrophy, increased diastolic pressures, interstitial fibrosis, and microvascular dysfunction. These maladaptive modifications ultimately compromise the microcirculatory response to pharmacological vasodilation and, therefore, might explain the underestimation of intermediate stenosis functional significance with FFR92. Interestingly, the PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION (ACTIVATION) trial showed that coronary revascularisation in patients without angina and undergoing TAVI is not beneficial93. The role of image-based physiology will be assessed in the Quantitative Flow Ratio Guided Revascularization Strategy for Patients Undergoing Primary Valve Surgery With Comorbid Coronary Artery Disease (FAVOR4-QVAS) trial testing a QFR-guided revascularisation strategy in patients undergoing aortic valve replacement. Nevertheless, the use of FFRCT is of particular interest, since currently all patients undergoing TAVI require CT prior to evaluating their suitability and procedural planning.
Functional ischaemia after PCI
Clinical outcomes regarding clinical decision-making based on non-intracoronary physiology remain to be published. Nevertheless, the prognostic value of QFR in successfully revascularised patients was investigated prospectively by Biscaglia et al in the Angio-based Fractional Flow Reserve to Predict Adverse Events After Stent Implantation (HAWKEYE) study. In this trial, a 3-fold increase in the vessel-oriented composite endpoint was identified (cardiovascular death, myocardial infarction or ischaemia-driven target vessel revascularisation) in vessels with offline post-PCI QFR ≤0.89 (HR 2.91, 95% CI: 1.63-5.19; p<0.001), with significant differences in all three composites94. Most importantly, owing to the integrated pullback trace, QFR was able to discriminate the mechanism underlying the suboptimal functional result (Figure 7). The relevance of this field is likely to increase based on reported studies using post-PCI invasive functional assessment, which have shown worse prognosis if functionally significant obstructive disease is left untreated, even after angiographically successful PCI95.
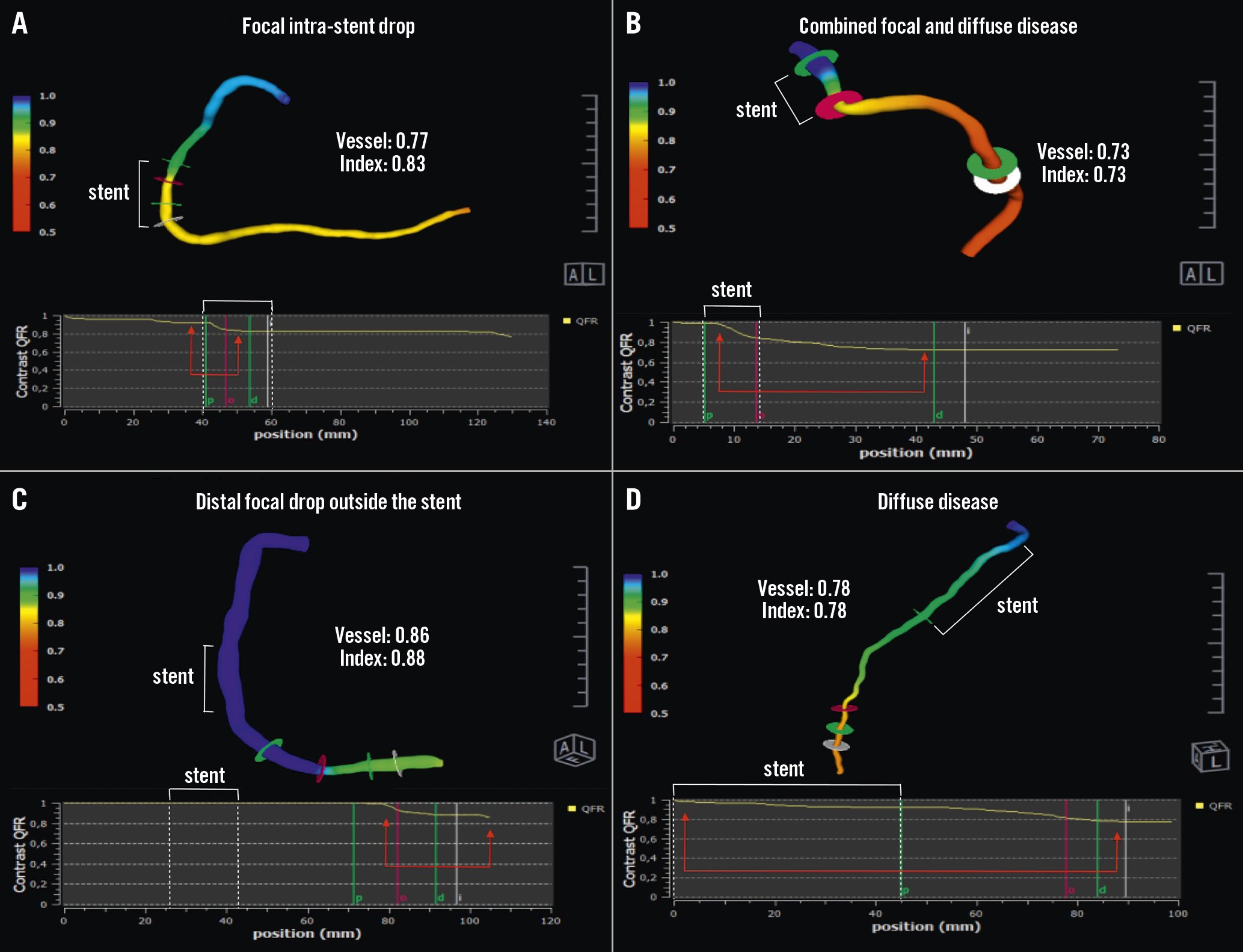
Figure 7. Establishing the cause of suboptimal functional PCI results with QFR. The white brackets indicate the stented segment. The red arrows point to the major QFR drop during pullback. A) Focal intra-stent drop. B) Combined intra-stent focal drop and distal diffuse disease. C) Distal focal drop outside the stent. D) Diffuse disease. Courtesy of Dr Simone Biscaglia. PCI: percutaneous coronary intervention; QFR: quantitative flow ratio
Microcirculatory coronary resistance
Recently, two proof-of-concept studies were conducted to test angiography-derived indices of microcirculatory resistance. De Maria et al developed and validated an angiography-derived index of microcirculatory resistance (IMRangio) in STEMI, demonstrating good correlation between conventional IMR and IMRangio measured immediately before stenting in primary PCI96.
Tebaldi et al validated another angio-based IMR (A-IMR), using IMR as a gold standard, in patients with chronic coronary syndrome and an intermediate left anterior descending (LAD) artery lesion, showing good correlation between the two indices97.
Recently, MejÃa-RenterÃa et al developed a wire- and adenosine-free IMR (angio-IMR) that exhibited good correlation with invasive IMR and its feasibility in interpreting functional stenosis assessed by QFR (Figure 8)98.
Finally, Choi et al confirmed the prognostic value of an elevated angio-IMR calculated using computational flow and pressure simulations in two cohorts of STEMI patients that were followed up for 10 years, demonstrating that patients with an angio-IMR >40 had a significantly increased risk of cardiac death and hospitalisation for heart failure99.
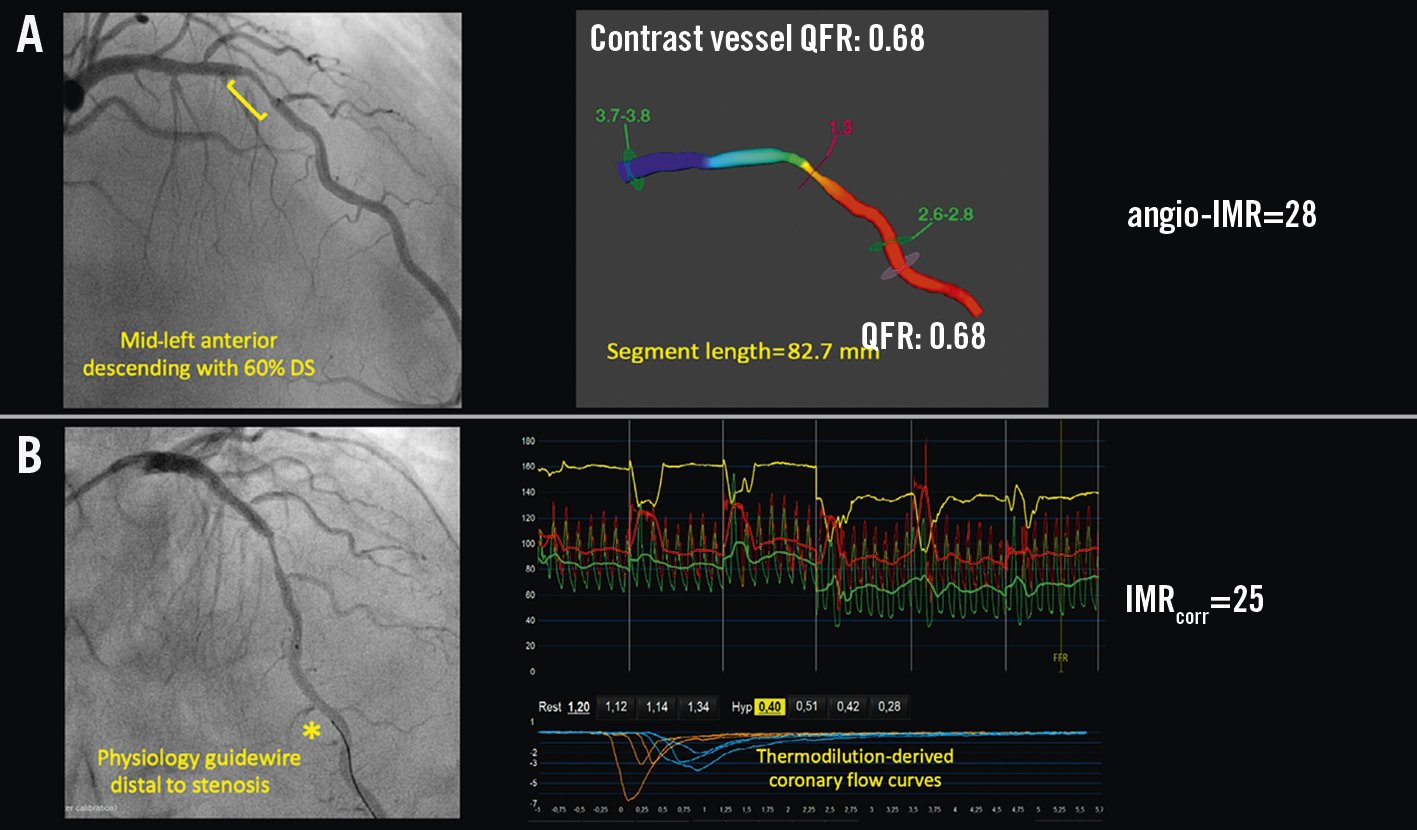
Figure 8. Derivation of coronary microvascular resistance from FCA. A) Steps and formulas for calculating the hyperaemic index of coronary microvascular resistance without the need for guidewires or hyperaemic drug administration. B) Invasive assessment of IMR by thermodilution-derived coronary flow curves. angio-IMR: wire- and adenosine- free IMR; DS: diameter stenosis; FCA: functional coronary angiography; IMR: index of microvascular resistance; IMRcorr: IMR adjusted by Young’s formula; QFR: quantitative flow ratio
Conclusions
In recent years, major technical developments in the field of coronary imaging (invasive angiography and CCTA) have made it possible to obtain functional information from a primary anatomical examination. FCA and CCTA now allow a wireless-based evaluation, with preliminary studies demonstrating good correlation with invasive FFR and overall good reproducibility and feasibility. The developments in both modalities and their clinical adoption worldwide could move FCA from the research field to clinical practice in the coming years. Issues regarding the time burden and costs, particularly with CTFFR, might hinder their broad applicability in routine clinical practice. The significant number of predetermined assumptions when computing flow by FCA are reflected in a wide “grey zone” of measurements that will still require confirmation by invasive physiology. Studies and trials are required to assess major clinical endpoints and the cost-effectiveness of FCA.
Conflict of interest statement
H.Mejía-Rentería has received speaker fees from Abbott Vascular, Boston Scientific, and Philips Healthcare. J.M.Lee received aresearch grant from Abbott Vascular and Philips/Volcano. H.Matsuo has received speaker fees from Philips, Boston Scientific, Abbott Vascular, and Zeon Medical. S.Baptista has received personal fees from Abbott, Boston Scientific, and HeartFlow. N.Gonzalo has received consultancy and speaker fees from Abbott Vascular, Boston Scientific, Philips/Volcano, and Shockwave Medical. J.Escaned has received speaker and/or advisory board member fees from Abbott Vascular, Boston Scientific, Medis, and Philips. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

