Case summary
Background: A 74-year-old male patient complaining of crescendo TIAs, hypertension and hyperlipidaemia.
Investigation: Duplex ultrasound scan MR angiography.
Diagnosis: Pseudoaneurysm stemming from the ICA.
Treatment: An open or endovascular procedure for the right pECCA repair, or a left CEA or CAS.
How should I treat?
Presentation of the case
A 74-year-old male patient complaining of crescendo (defined as two or more episodes in 24 h, with complete recovery after each episode) transient ischaemic attacks (TIAs) was referred to our institution. The patient had a medical history of hypertension and hyperlipidaemia. A duplex ultrasound scan (DUS) revealed a high-grade stenoses at the origin of both internal carotid arteries (ICA). An MR angiography with a T2-weighted brain imaging confirmed the duplex findings and showed a bovine aortic arch configuration (Figure 1) and small bilateral infarctions in the deep white matter. One week later the patient underwent a standard right carotid endarterectomy (CEA) with a bovine pericardial patch angioplasty. The operation was performed under endotracheal general anaesthesia and a Javid shunt was inserted due to the insufficiency of an intracranial collateralisation revealed at the transcranial Doppler (TCD) sonography. The patient was discharged two days after this successful procedure and placed on dual antiplatelet therapy. A contralateral CEA was also scheduled within one month’s time. Unfortunately, the patient returned to our institution 14 days later presenting a large pulsatile neck mass, dysphagia and mild dyspnea. He was also afebrile and had no evidence of local erythema, draining sinus nor leukocytosis. Moreover, C-reactive protein was <1 mg/dL.
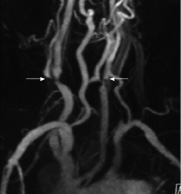
Figure 1. MR-angiography showing a bovine aortic arch variant and severe stenosis at the origin of both internal carotid arteries (white arrows).
A DUS showed a 27 x 43 mm pseudoaneurysm stemming from the ICA at the lateral site of patch implantation and a CTA confirmed the presence of the pseudoaneurysm and highlighted tracheal deviation (Figures 2 a, b). As the acute onset of the right post-endarterectomy carotid artery pseudoaneurysm (pECCA) caused our patient difficulty in breathing and dysphagia combined with contralateral symptomatic stenosis, we were faced with the choice of a first line course of treatment: either an open or endovascular procedure for the right pECCA repair, or a left CEA or CAS. The secondary course of treatment was a simultaneous open or endovascular bilateral approach. These alternative choices in therapy were discussed with the patient’s family who were informed about the success rates and risks accompanying each of these procedures for pECCA and contralateral ICA stenosis therapy.
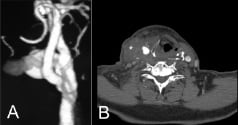
Figure 2. Preoperative contrast-enhanced CT scan. (A) 3D view of the right carotid axis demonstrating the large pseudoaneurysm. (B) Axial view showing right neck mass (asterisk) displacing the trachea to the left. A tight stenosis of the left internal carotid artery is also visible (white arrow).
How could I treat?
The Invited Experts’ opinion
The author has no conflict of interest to declare.
Extracranial carotid artery aneurysms are uncommon lesions. Only a few larger series have been reported since Sir Astley Cooper published the first case report in 1836.1
A precise definition for carotid artery aneurysm is difficult to establish because of the physiological dilatation of the carotid bulb. The incidence of carotid artery aneurysm in the literature is reported in the range of 0.1% to 2% of all carotid artery procedures.2,3
The aetiology of carotid artery aneurysm is variable. The majority of causes is atherosclerosis, other causes can be fibromuscular dysplasia, Marfan’s disease, traumatic/post-operative, Takayasu’s arteritis and mycotic. Treatment options vary with the different aetiologies.
In this case the authors describe a large pseudoaneurysm after carotid endarterectomy two weeks earlier. A complicating factor is the contralateral symptomatic high grade stenosis.
Despite the risk of life-threatening haemorrhage from the pseudoaneurysm, it is preferred to treat the contralateral symptomatic high grade stenosis first. Treatment options are a carotid endarterectomy or carotid artery stent procedure. There are no guidelines, nor specific literature to help us with a decision, therefore, the choice of treatment modality is subjective. My personal preference would be a carotid artery stent procedure.
The aetiology of the pseudoaneurysm should be discussed. Most prominent causes of such a specific lesion are infection of the patch material, a small tear in the patch or partial failure of the suture.
The authors describe a pulsatile neck mass, without local erythema and a C-Reactive Protein of <1 mg/dL. Therefore an infection is highly unlikely. It is important to rule out an infected patch, because in that case replacement of the prosthetic patch by a venous patch is obligatory.
To treat a non-infected pseudoaneurysm there are two treatment options.
First option is treatment with a covered stent-graft.4 This technique is mainly employed in traumatic lesions and in cases of difficult access of the pseudoaneurysm. Preliminary results suggest that placement of stent-grafts is a safe and effective method of treating ICA traumatic pseudoaneurysms. The immediate results are satisfactory when the procedure takes place with appropriate anticoagulation therapy. The periprocedural morbidity and mortality and the early patency are acceptable. The drawback of this technique is the acute occlusion of the external carotid artery and the increased risk of an acute stent-graft thrombosis.
The second option is treatment by open surgical procedure. Considering the fact that the initial operation was performed only two weeks earlier, an easy exploration is anticipated. Most likely an additional suture can be placed without clamping (comparable to groin pseudoaneurysms). In case of patch rupture a new patch should be implanted. Additional advantage of an open procedure is the immediate removal of the mass causing dysphagia and mild dyspnea.
Pseudoaneurysms of the common femoral artery are generally treated by ultrasound guided compression or thrombin injection.5 There is no literature to support the use of these techniques for carotid artery pseudoaneurysms.
How could I treat?
The Invited Experts’ opinion
The author has no conflict of interest to declare.
The authors report a clinical case of a 74-year-old male with acute neurologic presentation and severe bilateral carotid stenosis. One week after presentation the patient undergoes right carotid endarterectomy (CEA) and implantation of a bovine pericardial patch under general anaesthesia. During intervention, the implantation of a Javid shunt was necessary due to insufficient intracranial collateralisation. The patient was readmitted two weeks later because of a large pseudoaneurysm of the right ICA causing tracheal and oesophagic compression and “contralateral symptoms”.
This is an example of a delicate case that turns into a very delicate case.
Before addressing the therapeutic alternatives, there are some understated issues that need reappraisal.
1. Neurologic symptoms and treatment target. The authors talk of “crescendo TIAs” but no clinical information is given as to what is meant as neurologic deficits of certain ischaemic cause; then, one of the two severely stenotic carotid arteries is treated. Unequivocal clinical data, supported by dedicated neuro-imaging as well as details of the carotid plaque composition, are essential elements to guide an appropriate therapeutic decision.
2. Timing of intervention. Despite the dramatic clinical presentation, the patient was treated one week after admission. This suggests that it was not an “ACS” (acute carotid syndrome) any longer, likely due to the effects of medical treatment. Therefore, a decision could reasonably be taken after complete diagnostic assessment in almost elective conditions.
3. Strategy. Severe bilateral carotid stenosis is often a good indication for stenting if performed by expert operators and if the vascular anatomy and plaque characteristics are favourable1. Surgery in this setting is high-risk2, as confirmed by the need to install a shunt to obviate for the insufficient intracranial collateralisation. In such precarious conditions, the surgeon may have performed a sub-optimal procedure urged by the need of minimising the clamping time. The subsequent development of the pseudoaneurysm with leaking from the patch, being a very infrequent complication, can be the consequence of the unfavourable surgical setting3.
4. Last, but not least, a 74-year-old male, with antecedents of hypertension and dyslipidaemia, showing severe bilateral carotid stenosis has a high “a priori” likelihood of having a significant coronary artery disease. A cardiac screening before undergoing CEA should therefore be performed4.
5. At the time of re-admission, other than the compressive symptoms, the authors refer to “contralateral symptomatic stenosis”. This suggests that the contralateral carotid artery become symptomatic as well, likely because of an impairment of the flow through the right ICA and the pseudoaneurysm. Again, the neurological clinical presentation is of paramount importance in this context and should be well documented and weighted before planning a treatment strategy.
Treating this patient for the first time, stenting of the symptomatic artery should have been the preferred option5. Being a cardiologist myself, I believe that a selective coronary angiogram performed immediately before carotid stenting would have added valuable information at the cost of a few millilitres of contrast media. A second stent, three to four weeks after discharge would have been the treatment of choice for the contralateral carotid lesion (if indicated), taking into account the coronary status6.
Regarding the management of the surgical complication, exclusion of the pseudoaneurysm with a covered stent is a good alternative to re-intervention3. Despite the fact that a covered stent at the bifurcation of the ICA-ECA would cause total occlusion of the ECA, this is generally an uneventful situation7. Surgery in this setting has all the known risks of a bilateral carotid disease, further burdened by the longer surgical time needed for the toilette and repair of the pseudo-aneurysm, plus the apparent symptoms of the contralateral artery. All this together goes against repeated surgery in this case.
The real need for the immediate treatment of the contralateral stenosis should be evaluated after the successful treatment of the pseudoaneurysm. In stable conditions, this should be deferred three to four weeks. In this specific case, I would not dare to propose surgery to this patient again, and therefore, another stent would be my preferred option8.
How did I treat?
Actual treatment and management of the case
An urgent percutaneous procedure was performed via a right femoral artery access to treat both the left ICA stenosis and the right pECCA. A bolus of 5,000 units of heparin was administered intravenously. After canulation of the left common carotid artery (CCA) with a long (90 cm) 6 Fr sheath (Shuttle, Cook, Bloomington, IN, USA), a lesion predilatation was carried out with a 3.0 x 20 mm Sterling™ balloon (Boston Scientific, Natick, MA, USA) and a 9 x 30 mm monorail Carotid WALLSTENT® (Boston Scientific, Natick, MA, USA) was successfully used to treat the left ICA stenosis. Neuroprotection was established by distal filter (Filter Wire EZ™, Boston Scientific, Natick, MA, USA). Once this was done the 5 Fr long sheath was exchanged for a short 10 Fr sheath and a 5 Fr JB1 (Cook, Bloomington, IN, USA) diagnostic catheter was gently advanced into the right ICA over a 0,035” hydrophilic guidewire (Radifocus Glidewire®, Terumo Medical, Somerset, NJ, USA). An Amplatz Super Stiff™ 0,035” guidewire (Boston Scientific, Natick, MA, USA) was positioned in the right ICA and a 8x40 mm Fluency® stent graft (Bard, Tempe, AZ, USA) was advanced and deployed at the pECCA site. Post dilatation with a 7x20 mm Sterling™ balloon (Boston Scientific, Natick, MA, USA) was well tolerated by the patient. A final angiogram demonstrated complete pECCA exclusion and a patent right ICA (Figure 3a,b).
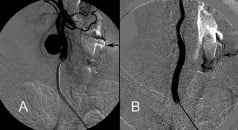
Figure 3. Digital subtraction angiography. (A) Right common carotid injection showing a large pseudoaneurysm stemming from the internal carotid at the lateral site of patch implantation. (B) Poststenting (8x40 mm Fluency® stent graft (Bard, Tempe, AZ, USA)) right carotid angiogram demonstrating complete exclusion of the pseudoaneurysm. Left 9x30 mm monorail Carotid WALLSTENT® (Boston Scientific, Natick, MA, USA), previously implanted, is noted in both images (black arrows).
The patient had an uneventful postoperative course with immediate improvement of his respiratory function and was discharged on clopidogrel 75 mg and ASA 100 mg on postoperative day five. A physical examination one week later showed a marked reduction in right anterior neck swelling. A DUS performed on the first day after the procedure and at 6-month intervals showed no evidence of pECCA perfusion or left ICA restenosis. The patient is alive with no procedure-related complications two years later.
Discussion
In many patients the traditional surgical approach to carotid artery disease is being supplanted by a rapid evolution in endovascular techniques. Recently, encouraging results in the treatment of post-endarterectomy carotid artery pseudoaneurysm (pECCA)1-3 with endoluminal repair have also been reported. Herein we describe the urgent simultaneous endovascular treatment of an unusual case of an early pECCA associated with a contralateral symptomatic carotid stenosis. pECCA is an uncommon complication with an incidence rate of 0.3%-0.7%4-6. Its formation is reported to be more common following a patch angioplasty rather than at primary closure7, particularly when a Dacron or venous patch is used5,6. To-date, only a few cases of bovine pericardial pECCA8-10 have been reported, with no cases of related aneurysmal dilatation or rupture in large series11,12. Mechanisms of pECCA development are: suture failure, degeneration of the arterial wall or patch material and infection. In our case (one out of 567 patients submitted to a CEA with a bovine pericardial patch angioplasty during a 15-year period), the indication of the initial right CEA was based on the severity grade of the stenosis and the patient’s low surgical risk. Because of a contralateral symptomatic carotid lesion, dual antiplatelet therapy was prescribed on discharge13. Due to the lack of other complications including patch infection, we presumed that minimal suture line bleeding might have played a role in the early pECCA development.
The favoured treatment for pECCA is surgical excision followed by autologous grafting, with a reported perioperative major stroke and death rate of 10.8% and a cranial nerve injury rate of 23%14. In a more recent study of 29 surgical patients, adverse neurologic events were noted in three patients (10.3%) with no perioperative deaths5. In past years endoluminal management via stent-graft deployment has emerged as a feasible and effective alternative to open surgical repair with relatively low procedure-related morbidity and mortality rates3.
Due to the fact that repair of both the right pECCA and the left ICA stenosis required urgent attention, a simultaneous endovascular treatment was successfully carried out. In the operating theatre we decided to treat the left ICA stenosis first in order to avoid shortage of cerebral perfusion during prolonged contralateral stent graft ballooning. The advantages of this approach include the use of local anaesthesia, no tracheal intubation nor intensive care requirement, and a shortened hospital stay.
In conclusion, notwithstanding the lack of specific guidelines and published literature on this unconventional therapeutic approach, the employment of a simultaneous endovascular treatment appears to have been a viable therapeutic alternative to conventional two-stage surgery or alternative endovascular procedures however further investigation and longer follow-up periods are required before this simultaneous approach can be recommended for this challenging clinical presentation.

