
CASE SUMMARY
BACKGROUND: A 64-year-old male with Budd-Chiari syndrome (BCS) due to inferior vena cava (IVC) occlusion after liver transplant presented with massive ascites and lower extremity oedema.
INVESTIGATION: A computed tomography venogram (CTV) of the abdomen and pelvis was performed after work-up including venous ultrasound could not identify the aetiology of the oedema.
DIAGNOSIS: The patient was found to have chronic total occlusion (CTO) of the suprahepatic IVC with thrombosis in the hepatic, renal, and iliac veins and the infrahepatic IVC.
MANAGEMENT: Venography of the IVC along with catheter directed thrombolysis were performed on the first day. Subsequently, a transseptal needle was used to transverse the occlusion. A snare was used from the IVC to retract a guidewire cranially through the tract. The lesion in the IVC was then dilated and stented with the help of IVUS.
KEYWORDS: Budd-Chiari syndrome, inferior vena cava occlusion, inferior vena cava stent placement, liver transplant, pedal oedema
PRESENTATION OF THE CASE
A 64-year-old African-American male, nine years post orthotopic liver transplantation (OLT) who had IVC occlusion and Budd-Chiari syndrome (BCS) with chronic pedal oedema and ascites, worsening over a three-month period, was referred to Interventional Cardiology at the Ochsner Medical Center for evaluation and treatment.
The patient had hepatitis C with cirrhosis and underwent a liver transplant in 2003. He developed massive ascites, abdominal distention, and lower extremity oedema over three months leading to debilitation, becoming bedbound. He had gained approximately 60 pounds. Hepatic ultrasound showed a patent main portal vein with partially occlusive thrombus in the IVC and renal veins bilaterally, hepatomegaly, and a large volume of ascites. Along with diuretics, he was put on anticoagulation as an outpatient. A venogram showed suprahepatic IVC occlusion, and attempts by several operators to cross the occlusion were unsuccessful. He was then referred to the Vascular Surgery Department but no surgical intervention was possible because the suprahepatic portion of his IVC had multiple collaterals to the superior vena cava and right atrium (RA). He was then referred for interventional cardiology given his severe symptoms.
Inferior vena cava occlusion complicating liver transplantation can cause severe morbidity in patients including portal hypertension leading to abdominal fullness, weakness, abdominal pain, lower extremity oedema, hepatomegaly, splenomegaly, ascites, distended abdominal vein, leg ulcers and, more rarely, gastrointestinal bleeding, jaundice and hepatic encephalopathy1.
Initial management of symptoms from IVC occlusion is medical therapy with diuretics, anticoagulants and, in rare acute cases, fibrinolytic therapy. IVC occlusion post liver transplantation has been reported to be infrequent at 1-6% (especially with piggyback anastomosis), but can lead to portal hypertension, renal failure, pedal oedema, graft loss and even death depending on the level of occlusion2-5. Surgical portosystemic shunt placement and liver transplantation are treatment options. Balloon dilation of IVC occlusion is rarely successful because of rupture of new anastomosis, acute re-occlusion, and restenosis. IVC stent placement has been used for CTO in patients with BCS whose symptoms were refractory to conservative management1-3,6-9.
On presentation to the cardiology clinic the patient weighed 281 pounds. He had no jugular venous distention. His abdomen was markedly distended with fluid wave, ascites, marked chronic pitting oedema involving his entire lower extremity, back and abdomen up to his chest wall. He also had venous stasis dermatitis of his lower extremity. A computed tomography venogram (CTV) of his abdomen was performed (Figure 1A, Figure 1B). The anterior-posterior and lateral CT venogram imaging showed ascites, right pleural effusion, and suprahepatic IVC stenosis 1 cm in diameter axially and 7 mm in length. There were multiple areas of large clots in the infrahepatic IVC with thrombus extending into the left renal vein and at the bifurcation of the iliac veins (Figure 1A, Figure 1B). His cardiac medications on presentation were losartan 50 mg daily, felodipine 10 mg daily, furosemide 40 mg twice a day.
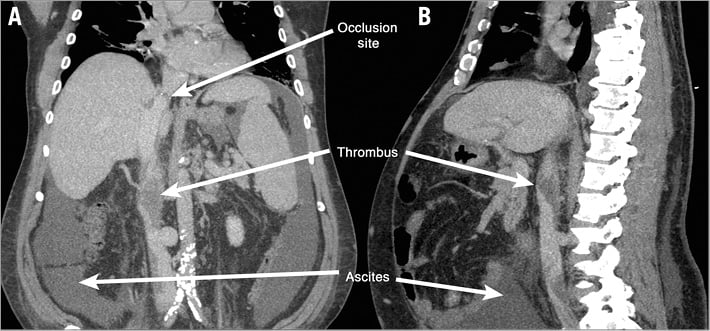
Figure 1. Inferior vena cava occlusion below the hepatic transplant with multiple areas of non-occlusive thrombus is present on the computed tomography venogram. Ascites can be visualised as well. Additionally, heavy calcification of the iliac arteries is present. A) Anterior-posterior projection of the computed tomography venogram showing infrahepatic occlusive thrombus. B) Lateral projection of the computed tomography venogram showing infrahepatic occlusive thrombus.
Given his severe morbidity with the disease, he was referred to interventional cardiology for treatment and re-attempt at canalisation of his infrahepatic IVC.
How would I treat?
THE INVITED EXPERTS’ OPINION

This case report is about a BCS patient who underwent liver transplantation and presented with severe symptoms of lower extremity oedema and ascites due to a persistent suprahepatic inferior vena cava (IVC) chronic occlusion refractory to multiple prior recanalisation attempts, which were very difficult to cross with conventional endovascular techniques. A CT scan showed an extensive thrombosis in the IVC proximal to the occlusion.
As a first step we would plan a double venous access by means of a 6 Fr sheath in the jugular vein and a 12 Fr sheath in the femoral vein.
We would then advance a Solent Omni AngioJet™ catheter (Boston Scientific, Marlborough, MA, USA) from below, via a femoral vein access. Such a device has been reported to be successful in managing thrombus within veins in different situations10. As reduction of thrombus burden is essential in order to avoid late stent thrombosis and embolisation following recanalisation, several steps (at least three) are advisable to obtain an acceptable debulking result. The embolic risk related to thrombectomy is minimised by the downstream IVC occlusion, hence the use of a filter is considered unnecessary in this particular situation11.
The suprahepatic occlusion, as shown by CT scan, is very short and, considering the several failed recanalisation attempts by different operators, it probably features a very resistant, completely occlusive septum12. We would introduce a transseptal needle via the femoral vein facing the occlusion. A 0.035” wire advanced from the jugular access until the occlusion level will provide a reference marker to align the puncture towards the vein axis. Under wire-aided transoesophageal guidance, we would puncture the occlusion in order to recanalise it. Such a strategy is meant to minimise the risk of IVC perforation, which may be very dangerous in the presence of a coagulation disorder, as expected in a liver transplanted patient. After a successful puncture, a stiff Back-up Meier™ 0.035” wire (Boston Scientific) would be advanced in the inferior vena cava. A very gentle predilation should then be performed with an undersized balloon to obtain a channel into the occlusion allowing a Covered Mounted CP Stent™ (NuMED, Hopkinton, NY, USA) to pass through it. The stent deployment should be gradual to avoid massive vein rupture. To achieve a durable patency result, antiplatelet treatment has to be carefully dosed and anticoagulation status constantly monitored in consideration of the patient’s comorbidity13.
In conclusion, a transoesophageal guided antegrade puncture with (retrograde) marker wire might be a reasonable treatment strategy for the recanalisation of this specific and challenging refractory IVC occlusion complicated by thrombus.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

The case describes a 64-year-old African-American male nine years after orthotopic liver transplantation (OLT) due to cirrhosis, presenting with ascites, abdominal distention, and lower extremity oedema. The original venogram showed occlusion; a subsequent CT venogram showed suprahepatic inferior vena cava (IVC) stenosis with multiple thrombi in the infrahepatic IVC extending to the iliac bifurcation.
This appears to be a case of primary BCS and a vascular problem, not one due to external compression (secondary BCS). Causes of BCS after transplant are often time-dependent. Early after transplantation, causes include twisting of the hepatic vein/venous anastomosis due to sliding of a graft, compression by an oversized graft, and overtightened suture14. Later causes include intimal hyperplasia and fibrotic changes at the anastomosis site15,16, as well as thrombotic changes17. From the text, this appears to be a late presentation at nine years from the original transplant.
Some tests are often performed in non-liver-transplant patients with BCS that may be worth considering here. The first is a test for prothrombotic conditions in patients18. The second would be to rule out concomitant hepatic stenosis which can be determined by ultrasound. CT shows no hepatic vein outflow obstruction but this should be evaluated in all phases (arterial, portal venous, and delayed)19.
Medical therapy is the first step. The patient is on a diuretic regimen and has received a course of anticoagulant therapy without benefit. Current anticoagulation status is unclear; non-liver-transplant patient literature shows the benefit of long-term anticoagulation for three to 12 months with warfarin in chronic IVC thrombosis20 and balloon angioplasty by relieving thrombus burden21.
Intervention urgency should be calculated with the hepatologist. Some scores exist that may help with this process, such as the Rotterdam score of III, Clichy >6.6, model for end-stage liver disease (MELD) >20, and Child-Pugh class C. When compared, the Rotterdam score was found to be especially valuable in determining treatment urgency22.
The decision for surgical or percutaneous management is dependent on the length of occlusion of the segment as well as the technical feasibility of percutaneous approaches. Patients with focal or segmental obstruction or stenosis (such as our patient) are good candidates for recanalisation18. Other approaches include transjugular intrahepatic portosystemic shunt (TIPS, which is rarely performed post liver transplant), caval homograft, or surgical thrombectomy23,24.
The thrombus appears to be chronic, but may still have mixed features. Angiography would show chronic thrombus (consider angioplasty with possible stenting) or acute features (start with transcatheter thrombolysis).
Transcatheter thrombolysis consists of a 4 Fr femoral venous sheath and advancement of a 4 Fr Multipurpose catheter (Cook Medical, Bloomington, IN, USA) to just below the infrahepatic IVC thrombus over a 0.018” ControlWire™ (Boston Scientific, Marlborough, MA, USA) placed in the superior vena cava. Alteplase 0.5-1 mg per hour would be used with monitoring of fibrinogen, prothrombin time, and activated partial thromboplastin time every six hours. After 24 hours, repeat angiography would help dictate the course. If there was no change in thrombus, then the catheter position would be assessed and readjusted if necessary; thrombolysis would continue for 24 hours. If further thrombolysis was needed for this reason, low-dose heparin would be given starting at 48 hours.
Angioplasty is the next step. The femoral venous sheath would be upsized to a 9 Fr venous sheath. Subsequent balloon dilations from 10 mm to 25 mm balloon diameter would be performed at the level of the thrombus and the stenosis.
Placement of a stent above the intrahepatic inferior vena cava increases the difficulty of repeat liver transplantation, where anastomosis of the donor and host IVC is required; this decision should be made with the hepatologist and transplant surgeon. A stent can be considered if luminal diameter retraction is greater than 50%, if the pressure gradient is higher than 15 mmHg, or if there is evidence of IVC dissection. However, our recommendation would be to hold off on stenting unless there is clinical evidence of restenosis with associated signs and symptoms in the follow-up period.
After treatment, the patient should be on low-molecular weight heparin as a bridge until warfarin reaches therapeutic levels. Warfarin should be continued for 12-24 months with an international normalised ratio (INR) of 2-3. Follow-up should be performed at one, three, six and 12 months and yearly thereafter or whenever symptoms present. Tests should include clinical history and physical, laboratory tests (coagulation, liver, kidney function), and colour Doppler ultrasound of the liver and IVC.
Percutaneous recanalisation can be technically successful in experienced centres; data for this come from patients mostly without prior transplant. One study25 with 168 patients showed success rates of 95% with a complication rate of 5%. At one, five, and ten years, the rates were 95%, 77% and 58% for primary patency and 96%, 83%, and 73% for secondary patency, respectively. This paper included a 30% only PTA rate and a 70% stent rate. Another study26 with 133 patients of endovascular treatment for BCS complicated by IVC thrombosis had a 98.5% success rate with a 4.5% rate of complications. At one, five, and 10 years, the primary patency rate was 96.3%, 84.0% and 64.6%, respectively. During the average follow-up period of 46 months, only two patients died. Re-obstruction may be more likely in patients with primary angioplasty alone, HV stenting, and lack of anticoagulation therapy for at least six months prior to angioplasty18.
Conflict of interest statement
S. Gafoor’s, J. Franke’s, P. Matic’s, M. Reinartz’s, S. Bertog’s, L. Vaskelyte’s, I. Hofmann’s, and H. Sievert’s institution has ownership interest in or has received consulting fees, travel expenses or study honoraria from the following companies: Abbott, Access Closure, AGA, Angiomed, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Guided Delivery Systems, Inc., InSeal Medical, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm medical, Rox Medical, Sadra, SJM, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Velocimed, Veryan. The other author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
A literature review showed that patients with BCS who underwent IVC stent placement post liver transplant have improvement in venous outflow with a clinical success rate of 86% and patency rate of 96.7% at 58-month follow-up1,2,9. IVC stenting with catheter-directed thrombolysis has been shown to have added benefit9,27,28. Rare procedural complications include pericardial effusion, myocardial infarction, renal failure, malpositioning, stent angulation, stent migration and mortality1,4,7,27,28. Fourteen percent of the patients who had undergone IVC stent placement needed to have another stent placement due to problems with liver function test, positioning, angulation or migration of the first stent4,5. Patients who were not on anticoagulants for six months post procedure were more likely to have re-occlusion with recurrence of symptoms1.
Day 1. On 9 January 2013, an IVC venogram via a 6 Fr sheath showed complete occlusion of the hepatic IVC at the liver transplant anastomosis site, partially thrombosed bilateral common iliac veins and infrarenal IVC, and subtotally occluded left renal vein (Figure 2A). A Cragg-McNamara® Valved Infusion catheter (ev3/Covidien, Plymouth, MN, USA) was inserted into the left renal vein, and r-tPA was infused via the catheter and sheath at 1 mg/hour. The 6 Fr sheath was sutured in place and low-dose heparin was also infused intravenously. The patient was transferred to the cardiac intensive care unit.
Day 2. Repeat venography showed persistent suprahepatic IVC occlusion, persistent thrombus in the infrahepatic IVC and left renal vein thrombosis. After failed attempts to traverse the IVC occlusion, a 6 Fr sheath was inserted into the right internal jugular (IJ) vein followed by a Multipurpose catheter (Cook Medical, Bloomington, IN, USA) positioned just above the IVC occlusion (Figure 2B).
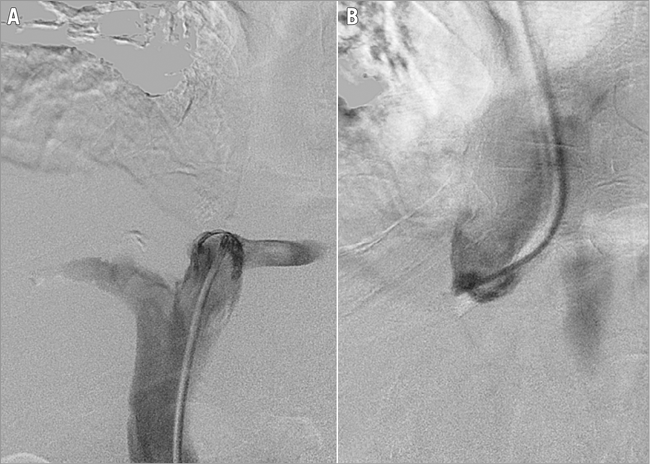
Figure 2. Inferior vena cava occlusion. This is demonstrated caudally from a transfemoral approach with a Multipurpose catheter (A) and cranially from a transjugular approach with a Multipurpose catheter (B).
The femoral sheath was exchanged for an 8 Fr 55 cm Flexor® Raabe Sheath guiding sheath (Cook Medical), followed by insertion of an 8 Fr Mullins Sheath (Bard Inc., Billerica, MA, USA). A transseptal needle introduced via the Flexor Raabe sheath traversed the suprahepatic IVC occlusion under fluoroscopic guidance of the target proximally marked by the IJ catheter (Figure 3). The guidewire was snared into the RA with a 10 mm Needle’s Eye Snare (Cook Medical) through the tract. The diameter of the IVC was assessed via intravascular ultrasound prior to dilation and stenting. A Mustang 6.0×40 mm balloon (Boston Scientific) was inflated at 10.0 atm followed by deployment of a 10×29 mm Palmaz stent on a 20 mm×4 cm BIB balloon (both Cordis Corporation, Bridgewater, NJ, USA) in the IVC occlusion (Figure 4). Post intervention, the IVC was completely patent with brisk flow (Figure 5).
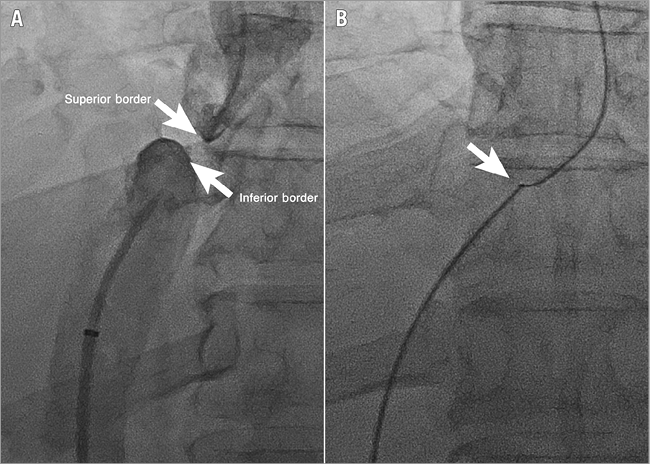
Figure 3. Inferior vena cava occlusion. This is demonstrated cranially and caudally by dual injection from a transjugular approach marking the superior border and a transfemoral approach marking the inferior border simultaneously (A). After traversing with a transseptal needle, a snare is used to externalise the guidewire (B).
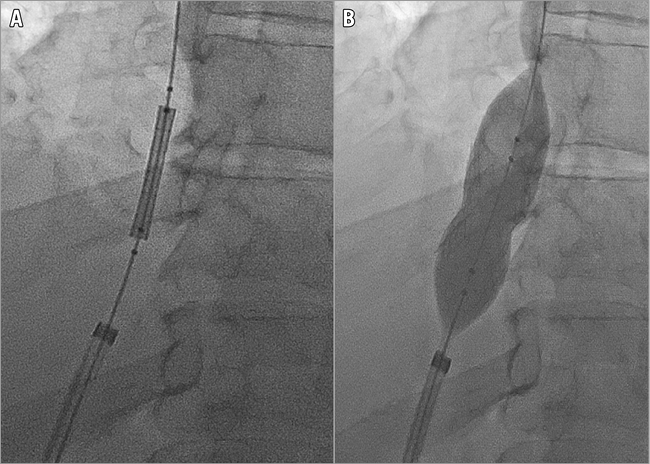
Figure 4. Alignment of the 10×29 mm Palmaz stent on a 20 mm×4 cm BIB balloon (Cordis). Alignment across the inferior vena cava occlusion (A), followed by inflation at 10.0 atm (B).
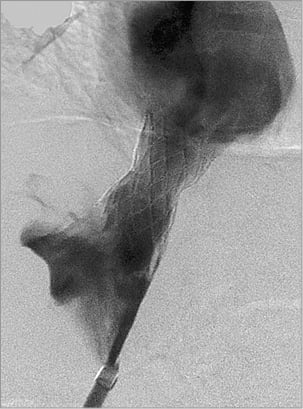
Figure 5. Brisk flow through the deployed stent in the inferior vena cava.
After the successful recanalisation of the suprahepatic IVC without complications, the patient was placed on aspirin, clopidogrel for one month, and warfarin. The patient experienced diuresis and lost 40 pounds during the index hospitalisation before his discharge on 14 January 2013. At an outpatient visit two weeks post stenting, he weighed 227 pounds with complete resolution of his ascites and pedal oedema. At one-year follow-up, the patient remained asymptomatic and reported that he was now able to ambulate without any problems.
Conflict of interest statement
The authors have no conflicts of interests to declare.

