
CASE SUMMARY
BACKGROUND: An 88-year-old male with a symptomatic severe low-flow low-gradient aortic stenosis was referred for catheter-based treatment (STS 7.7%).
INVESTIGATION: Transthoracic echocardiography showed a severe low-flow low-gradient aortic stenosis (peak velocity of 2.9 m/s, AVA 0.6 cm², LVEF 30%). CT during work-up revealed an unexpected finding of a large fusiform infrarenal AAA with a diameter of 59 mm, a mural thrombus and dissection.
DIAGNOSIS: Severe low-flow low-gradient aortic stenosis and a large fusiform infrarenal AAA with mural thrombus and dissection.
MANAGEMENT: The strategy was to treat both conditions in the same setting (“one-stop shop”) using a complete percutaneous approach under local anaesthesia. Immediately after transfemoral implantation of an Edwards S3 29 mm, a Medtronic Endurant II endograft was implanted in the abdominal aorta.
KEYWORDS: abdominal aortic aneurysm, aortic stenosis, incidentaloma, multislice computed tomography, transcatheter aortic valve implantation
PRESENTATION OF THE CASE
An 88-year-old male (173 cm, 78 kg) with progressive symptoms of dyspnoea (NYHA Class III) was referred for catheter-based treatment of aortic stenosis. Except for mild chronic kidney disease (GFR 58 ml/min) there were no antecedents. Physical examination showed him to be a vital, independently living, elderly patient (ADL 0/6, IADL 2/14, MMSE 30/30). There were no signs of cardiac failure. The ECG revealed a sinus rhythm (93 bpm) with non-specific repolarisation disturbances. Cardiac enzymes were normal, NT-proBNP was 1,027 pmol/L. Transthoracic echocardiography showed a severely calcified tricuspid aortic valve (Figure 1A), with a peak velocity of 2.9 m/sec over the aortic valve (valve area 0.6 cm²) in the presence of an impaired LV function (LVEF 30%) and grade 1 mitral regurgitation. On coronary angiography, one-vessel disease (stenosis in the proximal and mid segments of the right coronary artery) was seen.
First, the patient was discussed by the Heart Team before performing MSCT. A decision was taken first to perform PCI that did not affect the patient’s symptoms. A decision was then taken to perform MSCT in preparation for TAVR. This confirmed the presence of a severely calcified aortic valve (Figure 1B) with an annulus area of 590 mm², perimeter of 88 mm and diameter of 27.4 mm. The diameter of both common femoral arteries was 8 mm, without significant calcification or tortuosity. However, there was a large fusiform infrarenal abdominal aortic aneurysm (AAA) with a diameter of 59 mm, a mural thrombus and dissection (Figure 2).
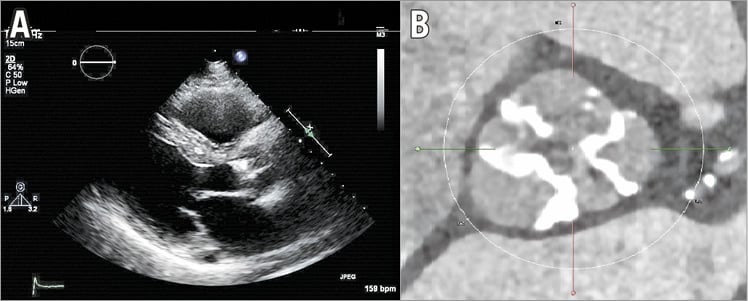
Figure 1. Imaging during work-up. A) Transthoracic echocardiography showing a severely calcified aortic valve. B) Severely calcified aortic valve on multislice computed tomography.
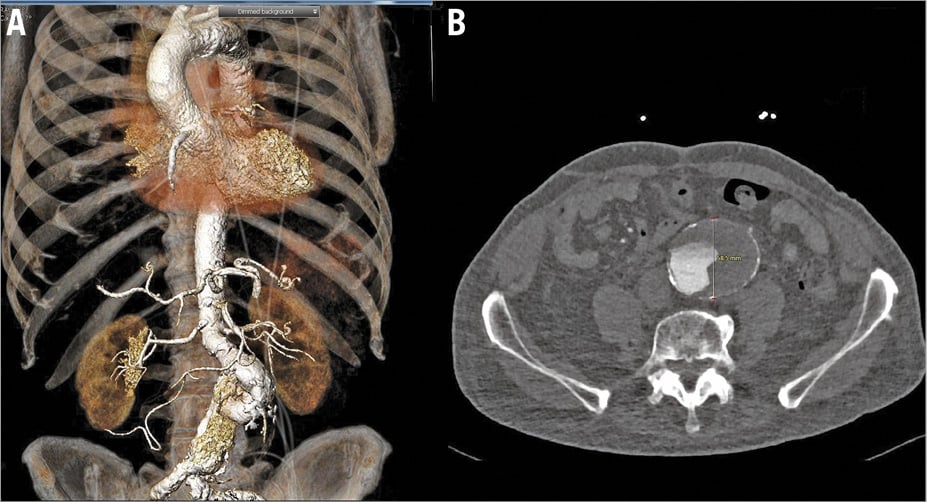
Figure 2. Imaging during work-up. A) A three-dimensional CT reconstruction of the fusiform infrarenal abdominal aortic aneurysm. B) Axial view of the fusiform abdominal aortic aneurysm.
The patient was rejected for surgical aortic valve replacement (SAVR) because of age, risk (STS score 7.7) and the unexpected finding of the abdominal aneurysm on MSCT. Transfemoral TAVR was technically feasible but not preferred given the need for crossing the aneurysms with wires and catheters. A subclavian approach was considered but was not possible since the annulus required the implantation of a SAPIEN 3, 29 mm valve (Edwards Lifesciences, Irvine, CA, USA), the sheath size of which exceeded the diameter of the subclavian artery. A transapical TAVR was possible but not considered ideal given the impaired LV function. In addition, there was the aortic aneurysm with mural thrombus that would also determine the prognosis. What would the recommendation be for planning and executing an invasive treatment, including the anaesthesiologic management of this elderly but otherwise vital and independently living patient?
How would I treat?
THE INVITED EXPERTS’ OPINION

The present case is challenging for several reasons: an 88-year-old patient with severe symptomatic aortic stenosis, a high procedural risk (STS score 7.7) and impaired left ventricular function (LVEF 30%) at first sight seems to be a straightforward TAVR candidate. However, in the presence of a low-flow low-gradient aortic stenosis (LFLG-AS) and an incidental abdominal aortic aneurysm (AAA), decision making becomes more complex. Co-existing one-vessel coronary artery disease is considered to be haemodynamically irrelevant, and other potential causes of the symptoms have been ruled out. Given the complexity and various possible strategies, we would discuss this case in the Heart Team with cardiologists, cardiac and vascular surgeons, and anaesthetists regarding the following issues:
1) LFLG-AS is quite common with almost 12% of all patients undergoing TAVR suffering from LFLG-AS and even more from paradoxical LFLG-AS1. In order to evaluate whether the patient has true severe AS and to assess left ventricular flow reserve, a low-dose dobutamine stress echocardiography is recommended2,3. In our opinion, the present patient’s symptoms and clinical findings (including the MSCT) indicate a true AS. Consequently, the patient should benefit from AVR. Considering the patient’s age and risk, TAVR is the procedure of choice.
2) Due to the large diameter of the AAA (59 mm) and according to guidelines, there is an indication for thoracic endovascular aortic repair (TEVAR) as well4. One might argue whether treatment of AAA should be performed prior to, simultaneously with or after TAVR. There are several reports of successful, simultaneous TAVR and TEVAR, but in this very aged patient a staged procedure may be favoured with respect to the patient’s convalescence.
3) Regarding the TAVR access, the authors are sceptical about crossing the AAA with wires and catheters during transfemoral TAVR. However, in our opinion this should be feasible without significantly increased procedural risk. Nevertheless, as we would opt for a staged procedure, we would favour transapical TAVR, thus minimising crossing of the AAA and performing TEVAR as a second step. The authors’ concerns regarding transapical access in patients with reduced EF are quite common, but recent studies suggest that there is no impact of the apical approach on global LV function5. An alternative option would be transfemoral TAVR, which would have the advantage of the procedure being able to be performed without general anaesthesia and TEVAR being performed simultaneously in case of uneventful TAVR. Of note, patients with reduced LVEF tolerate new-onset aortic regurgitation (AR) quite badly, thus preoperative planning, including sizing and valve selection, as well as the procedure itself should be optimised to reduce paravalvular leaks.
4) The only remaining concern for this patient is the known unfavourable midterm outcome for patients with LFLG-AS undergoing TAVR. However, this specific vital and independently living patient with symptomatic AS needs treatment to retain his quality of life.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION

The combination of a severe symptomatic aortic stenosis (AS) and a significant aneurysm of the abdominal aorta (AAA) is not rare in daily practice. Based on the patient’s age and comorbidities, a percutaneous approach was logically selected by the local Heart Team. The patient’s symptoms are mainly related to the aortic stenosis but TAVI cannot be performed alone. In my opinion, both AAA and AS must be treated during the same procedure. Indeed, given the large diameter of the AAA, any post-procedural increase in systolic blood pressure after TAVI could apply excessive strain within the aortic wall and potentialise the risk of rupture. However, we must anticipate several risks inherent to a combined approach: cholesterol embolisation, stroke and contrast-induced nephropathy. Among commercially available transfemoral TAVI devices, the Edwards SAPIEN 3 (S3) 29 mm is the only one suitable given the patient’s aortic annulus diameter (27.4 mm). The Medtronic Evolut™ R (Medtronic, Dublin, Ireland) 34 mm, recently approved by the Food and Drug Administration in the USA, is not yet available in Europe. As the S3 must be relocated onto the carrier balloon within the abdominal aorta, the manoeuvre is likely to be performed within the aneurysm, increasing the risk of stroke or even aortic perforation. The S3 should therefore be protected, during its course across the abdominal aorta, by an external conduit. The anatomy of the AA appears suitable for a percutaneous procedure owing to the presence of a well-defined infrarenal collar and preserved integrity of the common iliac arteries. A large thrombus burden is easily identifiable on MSCT.
My strategy would be first to proceed with the placement of a Claret Sentinel™, cerebral protection device (Claret Medical, Santa Rosa, CA, USA) via the right radial artery, to limit the risk of stroke. Heparin should be provided beforehand aiming at an ACT above 250 sec. The second step would be to perform the endovascular treatment of the AAA. Finally, across the AAA endograft, a 16 Fr eSheath™ (Edwards Lifesciences) or a 22 Fr re-collapsible SoloPath™ sheath (Terumo Corp., Tokyo, Japan) could then be advanced easily and the transfemoral TAVI procedure carried out in a conventional way. If needed, at the end of the procedure, the AAA endograft collar and legs could be re-expanded using appropriate post-dilatation balloons. As an identified risk, contrast-induced nephropathy should be prevented by preprocedural proper hydration and keeping the contrast total volume below fourfold the creatinine clearance (232 ml) during the procedure6.
Several reports have illustrated the feasibility and safety of a concomitant percutaneous treatment of AS and AAA. This could be the default strategy in such scenarios7.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
This patient has a severe low-flow low-gradient aortic stenosis and a fusiform infrarenal AAA (59 mm) that determine the prognosis when left untreated. Given the patient’s vital status and age, a decision was taken to treat them both in the same setting using a complete percutaneous approach, although a sequential treatment consisting of TAVI followed by (percutaneous or surgical) AAA correction was considered but rejected given the preference to offer a “one-stop shop” to minimise the number of hospitalisations.
In addition, sequential treatment exposes the patient to an increased risk of AAA rupture in case one would first treat the aortic stenosis due to the eventual increase in systolic blood pressure after TAVI, acknowledging that a fusiform aneurysm is less prone to rupture than a saccular aneurysm8-10. Vice versa, if one were first to correct the AAA, the patient would be exposed to a fivefold increase in perioperative mortality and non-fatal myocardial infarction11.
In order also to minimise the length of stay, we chose local anaesthesia since this has been shown to be associated with a shorter hospital stay12. Since 2006 we have had a default strategy of echographically guided vascular access during TAVI13. This technique was used for the infiltration of the region of the common femoral arteries using 2x20 ml of a combination of lidocaine 2 mg/kg and bupivacaine 1 mg/kg. A 16 Fr eSheath (Edwards Lifesciences) was then introduced into the right femoral artery and a 9 Fr sheath into the left femoral artery after the application of two Proglide® devices (Abbott Vascular, Santa Clara, CA, USA) at each site. An Edwards S3 29 mm was implanted under fluoroscopic guidance using rapid pacing at 180 bpm for 20 seconds. The valve was well deployed (Figure 3). There was minimal (grade 0-1) residual aortic regurgitation, and absence of a gradient and conduction disorders.
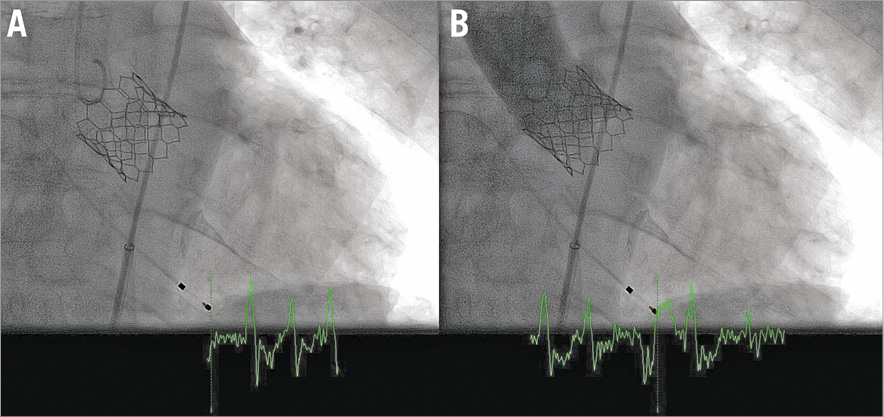
Figure 3. Intraprocedural imaging. Angiography without (A) and with (B) contrast immediately after implantation of an S3 29 mm valve.
Immediately after TAVI, the vascular surgeons implanted a Medtronic Endurant II endograft under fluoroscopic guidance (ETBF 2516C166EE right side and ETLW 1616C82EE and ETLW 1620C124EE left side). Angiography confirmed the correct position and deployment of the prosthesis just below the ostium of the renal arteries and no type 1a or 1b endoleak (Figure 4A). Complete haemostasis was achieved with the Proglide closure devices.
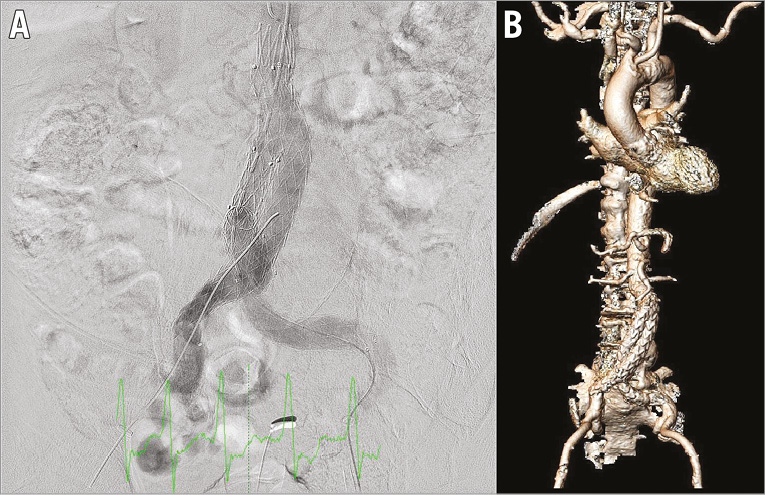
Figure 4. Intraprocedural and post-procedural imaging. Abdominal angiography immediately after the implantation of the aortic prosthesis just below the origin of the renal arteries (A) and three-dimensional CT reconstruction post EVAR (B).
Transthoracic echocardiography before discharge revealed a peak velocity of 1 m/sec (peak gradient 4 mmHg) and a grade 0-1 aortic regurgitation. CTA before discharge showed that the prosthesis was well positioned (Figure 4B); however, there was a type 2 endoleak with unchanged diameter of the aneurysm sac. The patient was discharged 10 days after the procedure.
Several reports have described similar cases in which simultaneous or sequenced transfemoral TAVI and EVAR were performed, but so far this is the only case report in which both procedures have been simultaneously performed under locoregional anaesthesia.
Conflict of interest statement
The authors have no conflicts of interest to declare.

