
Abstract
Aims: The aim of this study was to investigate the total amount, size and heterogeneity of debris captured among different transcatheter valve types and while repositioning.
Methods and results: A total of 328 patients who underwent transcatheter aortic valve implantation (TAVI) with the SENTINEL cerebral embolic protection (CEP) at our centre were eligible. Histopathological and semiquantitative analysis of captured debris was performed and data were entered into our prospective database. TAVI was performed with either the Evolut R/PRO (N=123), SAPIEN 3 (N=113) or Lotus valve (N=92). Capture of debris occurred in 98% of patients. Lotus TAVI resulted in more frequent foreign body material (62% vs 40% vs 47%, p=0.006), endothelium (49% vs 30% vs 16%, p<0.0005), calcified material (33% vs 12% vs 24%, p=0.001) and myocardial tissue (19% vs 11% vs 2%, p<0.0005) compared to SAPIEN 3 or Evolut R/PRO. Native (functional) bicuspid valves (OR 2.91, 95% CI: 1.20-7.03, p=0.02) and Lotus (OR 2.44, 95% CI: 1.14-5.24, p=0.02) were associated with the highest risk for dislodging particles ≥1,000 um. Valve repositioning was independently associated with larger amounts of debris (OR 2.96, 95% CI: 1.42-6.16, p=0.004).
Conclusions: All THV platforms had similar amounts of captured debris. THV repositioning seemed to be associated with a higher risk for dislodging greater amounts of debris to the brain.
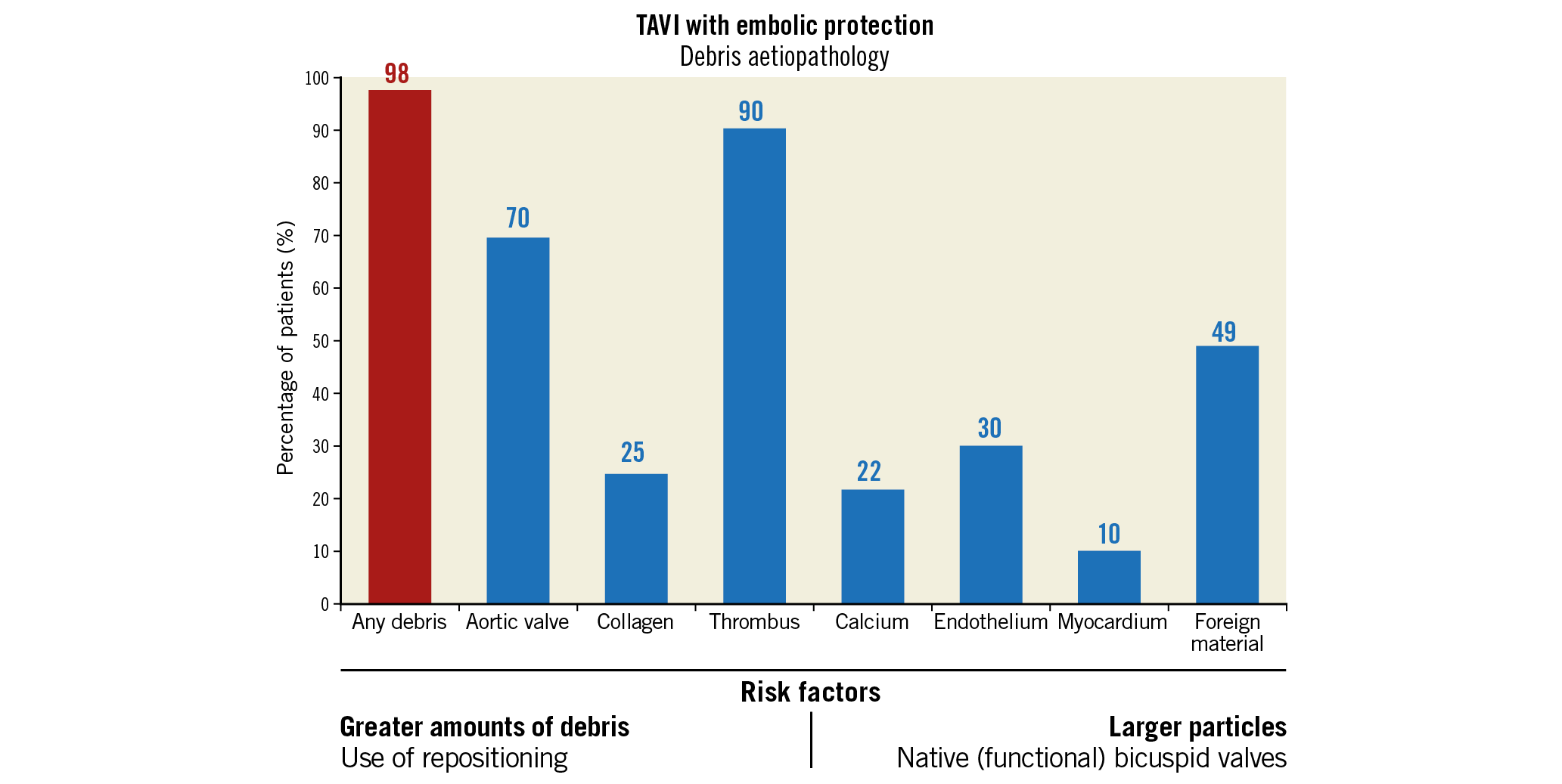
Visual summary. Aetiopathology of debris captured by cerebral embolic protection filters during TAVI, including risk factors for greater amounts or larger particles of debris.
Introduction
Periprocedural stroke typically occurs in the first 24-48 hours after transcatheter aortic valve implantation (TAVI). These early strokes are causally related to periprocedural embolisation1. Patients who suffer a stroke after TAVI are at higher risk of mortality (up to 3.5-fold), morbidity and physical disability2. On top of that, clinically silent cerebral lesions are found in more than 90% of patients after TAVI. These silent lesions may be associated with early neurocognitive decline, dementia and subsequent strokes3. Recent data have suggested that the use of cerebral embolic protection (CEP) was associated with fewer overt strokes, lower total lesion volume and a smaller number of new ischaemic lesions4,5,6. Moreover, two studies with histopathological analysis of captured debris have investigated embolic heterogeneity among different transcatheter heart valve (THV) designs with, however, contradictory results7,8.
The aim of the present study was to investigate the total amount, size and heterogeneity of debris captured by the SENTINEL™ dual-filter Cerebral Protection System (Boston Scientific Corp., Marlborough, MA, USA) among different THV types. Additionally, we studied the size and amount of debris when the repositioning feature (with Evolut™ [Medtronic, Minneapolis, MN, USA] or Lotus [Boston Scientific]) was de facto used in the procedure.
Methods
PATIENT SELECTION
A multidisciplinary Heart Team discussed eligibility for TAVI. Baseline demographics, procedural characteristics, clinical outcomes and histopathology analysis data were prospectively collected and transferred into a database. Every patient provided written informed consent for the TAVI procedure and data analysis for research purposes. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the EMC Institutional Review Board. We included all patients who underwent TAVI with the SENTINEL CEP system at our centre starting from December 2011 (introduction of CEP to our centre) up to January 2019. From 2015 onwards, use of CEP during TAVI was standard for all our patients. This means that all patients who had sufficient diameters of the innominate (9-15 mm) and left carotid arteries (6.5-10 mm) and less then severe calcification of the origin of previously mentioned vessels were eligible and selected for neurological protection during TAVI. On top of this, before 2015, patients needed to be at high risk for periprocedural stroke by decision of the operators (i.e., history of stroke before TAVI, peripheral artery disease and/or severe annular calcifications). We conformed to the advised diameters of the innominate and left carotid arteries for all CEP patients. Exclusion criteria were the absence or unreliability of performed histopathological analysis, the use of non-commercial heart valves and TAVI with a THV with <20 cases.
HISTOPATHOLOGICAL ANALYSIS OF CAPTURED DEBRIS
All filters were fixed in formalin upon retrieval and sent to the Pathology Department. Two expert pathologists performed all analyses. Content of the filters was removed and processed using a Cellient Automated Cell Block System (Hologic, Toronto, Canada). Haematoxylin and eosin stained 3 μm sections were examined routinely for the presence of the following types of material: aortic valvular tissue, collagen, thrombus, calcified material, endothelium, myocardial tissue and foreign body material (Figure 1). Using an eye piece-mounted micrometre, particles were measured and classified into three size categories based on their maximal diameter, i.e., ≥150 μm, ≥500 μm and ≥1,000 μm. The lower cut-off value of 150 μm was used because of the current pore size of the SENTINEL CEP system (140 μm). This analysis, focused on type of debris and size (diameter) of particles, was performed for every patient undergoing protected TAVI. In addition, from April 2014 onwards, through semiquantitative visual assessment, the amount of debris in each filter was classified into five categories: no material, little material, moderate amount of material, lots of material and massive. This was a visual assessment based on the number of particles captured in the filters. Semiquantitative visual assessment was performed by a dedicated pathologist (J. von der Thusen) who was blinded to THV type.
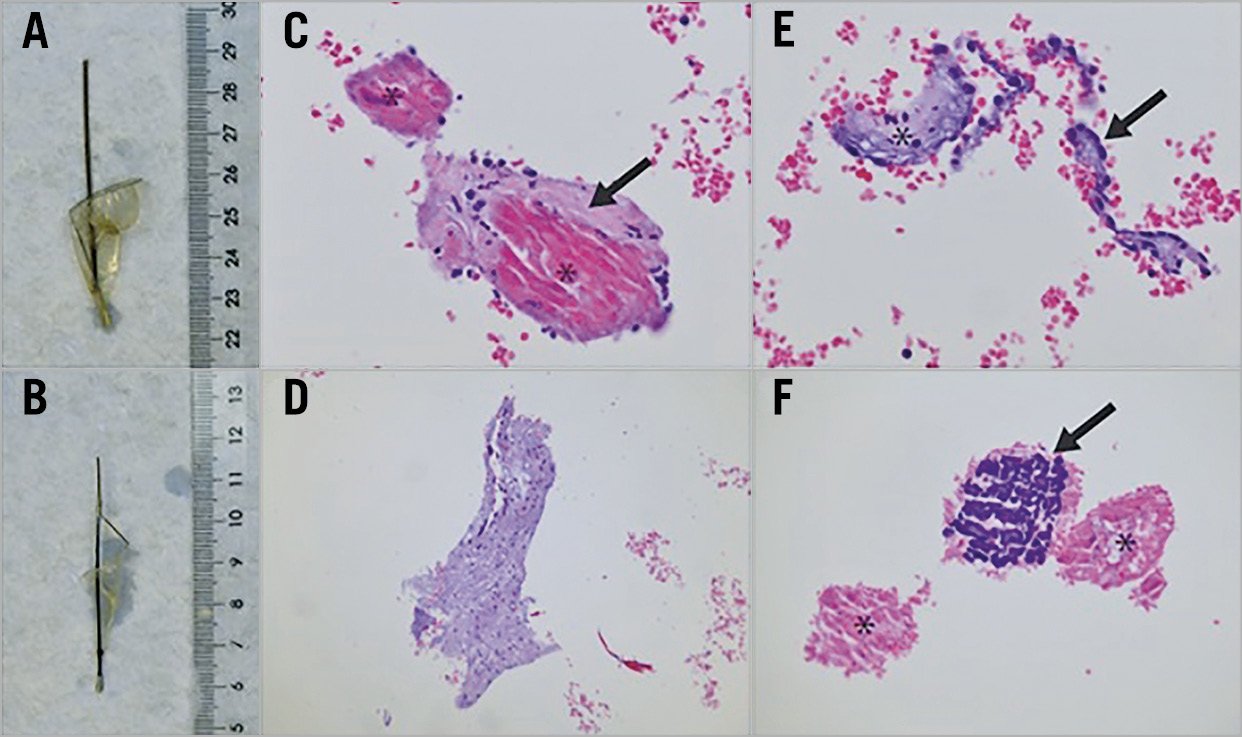
Figure 1. Macroscopy and histology of filters containing captured material following TAVI and representative histological appearance (haematoxylin-eosin [H&E] stain) of tissue types encountered as filter content. A) & B) Proximal and distal filter, respectively, as submitted to the pathology laboratory. C) Myocardium (asterisk) and adjacent subendocardium (arrow), 20x magnification. D) Aortic valve material with myxoid change, 10x magnification. E) Endocardial endothelium (arrow) and subendocardial tissue (asterisk), 20x magnification. F) Necrotic (asterisk) and calcified (arrow) amorphous material, presumably debris from a calcified aortic valve, 20x magnification.
STATISTICAL ANALYSIS
Continuous variables were presented as mean (±SD) or median (interquartile range) and categorical variables as n (%). The distribution of continuous variables was examined for normality through Q-Q plots and normality tests (Shapiro-Wilk). For the comparison of continuous variables among the three different valve types (mechanically expanding, balloon-expandable and self-expanding valves), one-way ANOVA or the non-parametric Kruskal-Wallis test was performed, according to the distribution of the variables. For the comparison of categorical variables, the Pearson χ² test or the Fisher’s exact test was used, as appropriate. For ensuing pairwise comparisons, we applied Bonferroni’s correction to account for multiple testing.
Additionally, we performed multivariable logistic regression to estimate the effect of the three THVs on two pre-specified debris characteristics, namely the presence of particles with the largest diameter ≥1,000 um (1st analysis) and more than moderate amount of debris (2nd analysis) captured in the SENTINEL filters. We selected those baseline covariates that displayed a p-value <0.10 in univariate analysis. In case of limited events, we chose those covariates with a p-value <0.10 and likely to contribute to the size and amount of debris (i.e., use of repositioning, anatomical features such as presence of functional or true bicuspid valves and annular/left ventricular outflow tract [LVOT] calcification levels). When sufficient events were available, we added variables that displayed baseline differences with a p-value <0.10 among the three THVs. To analyse the effect of the actual application of the repositioning feature during the procedure on the amount and size of particles we categorised the balloon-expandable SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) as “never repositioned”. Results are displayed as odds ratios (OR) with a 95% confidence interval (CI). All statistical analyses were performed with SPSS, Version 25.0 (IBM Corp., Armonk, NY, USA), and a two-sided value of p<0.05 was considered statistically significant.
Results
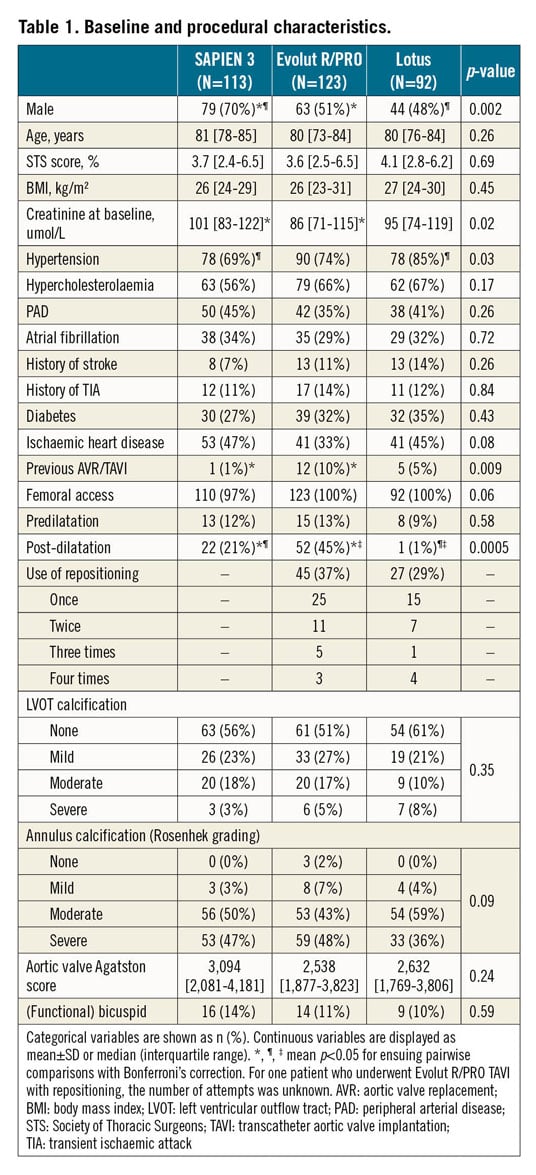
Between December 2011 and January 2019, a total of 956 patients underwent TAVI at our centre, 447 of them with CEP. We excluded all patients with 1) no or unreliable histopathological data (N=17), 2) use of non-contemporary commercially available heart valves (CoreValve®, N=59 [Medtronic], SAPIEN XT, N=23 [Edwards Lifesciences]), and 3) analysable filters in TAVI with a THV with <20 cases (ACURATE, N=17 [Boston Scientific], Portico™, N=3 [Abbott Vascular, Santa Clara, CA, USA]). In three cases we were inclined to use embolic protection but were finally unable to do so: in two patients due to difficulty achieving radial access, and in one patient due to extracranial arterial anatomy which inhibited deployment of the filter device. We experienced difficulty deploying CEP in two patients. In one patient we deployed the device through the brachial artery due to tortuosity of the radial artery. The other patient required three attempts to achieve a satisfactory position and underwent uncomplicated TAVI; however, this patient was excluded from the current analysis because of the use of a contemporary non-commercially available heart valve. A total of 328 protected TAVI patients were included. Patients underwent TAVI with either the Evolut R/PRO (N=123; Medtronic), SAPIEN 3 (N=113, no SAPIEN 3 Ultra cases) or Lotus valve (N=92, one LOTUS Edge™; Boston Scientific). A total of 57% of the patients were male, median 81 (75-85) years old, and patients were treated almost exclusively in a transfemoral fashion (99%). Median Society of Thoracic Surgeons (STS) predicted risk of mortality was 3.7% (2.6-6.4). Data with regard to the captured amount of debris were available for 263 patients (SAPIEN 3, N=91; Evolut R/PRO, N=123; Lotus, N=49) as we started collecting these data from April 2014 onwards. There were no filter-related safety issues in our series and in all cases both filters were deployed successfully. All baseline characteristics are displayed in Table 1.
HISTOPATHOLOGY OF CAPTURED DEBRIS
Patients treated with the SAPIEN 3 valve were more often male (SAPIEN 3 70% vs Evolut R/PRO 51% vs Lotus 48%, p=0.002), had a higher baseline creatinine level compared to patients treated with Evolut R/PRO (101 vs 86 vs 95 umol/L, p=0.02) and less often had hypertension compared to Lotus (69% vs 74% vs 85%, p=0.03). Also, Evolut R/PRO was more often implanted in failed surgical bioprostheses and more often required post-dilatation compared to the other THVs. Debris was captured in 98% of patients (N=322/328). No significant differences were observed in the presence of collagen and thrombus. However, analysis showed that deployment of the Lotus valve resulted in less debris derived from the aortic valve (Lotus 58% vs SAPIEN 3 69% and Evolut R/PRO 81%, p=0.001), but more often calcified material (33% vs 12% and 24%, p=0.001), endothelial (49% vs 30% and 16%, p≤0.0005) and myocardial tissue (19% vs 11% and 2%, p≤0.0005), as displayed in Figure 2. Foreign material was most dominantly present with the mechanically expanding Lotus valve (62% vs 40% and 47%, p=0.006). The presence of calcified material and foreign material was lowest with the SAPIEN 3 valve, while myocardial and endothelial tissue was lowest with use of the self-expanding valves.
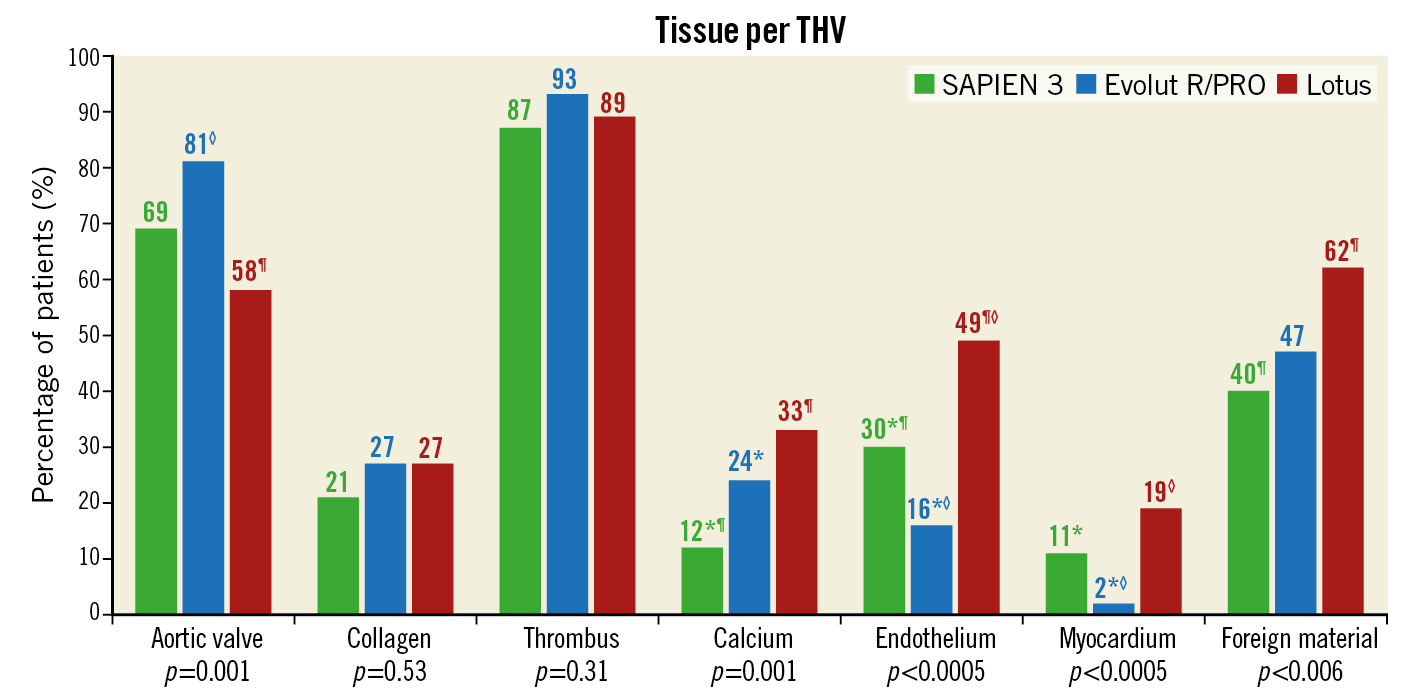
Figure 2. The distribution of tissue types among the three separate valves. Tissue type analysis was available for 328 patients who underwent TAVI. Percentage of affected patients is displayed above each bar. *,¶,◊mean p<0.05 for ensuing pairwise comparisons with Bonferroni’s correction. THV: transcatheter heart valve
QUANTITATIVE ANALYSIS ON AMOUNT AND SIZE OF DEBRIS
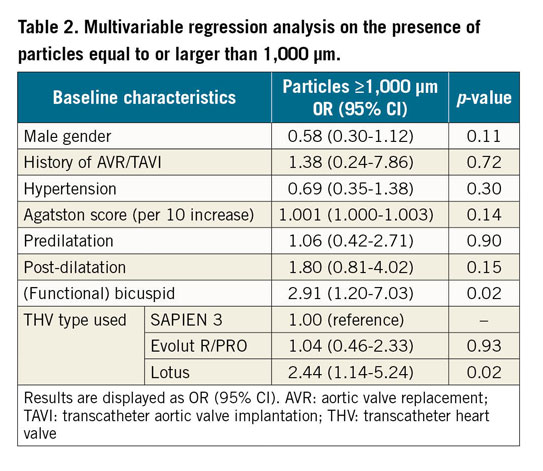
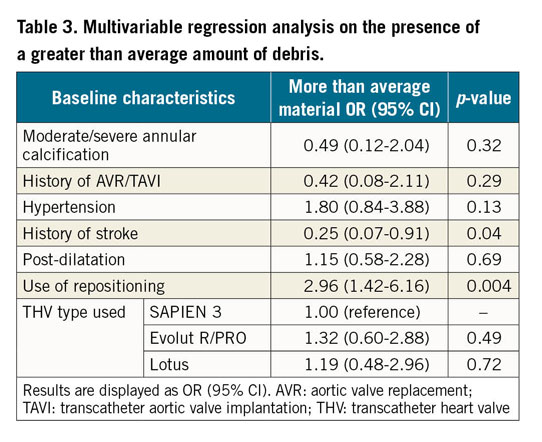
The largest pieces of debris (maximal diameter ≥1,000 μm, N=89/328) were mostly present with the Lotus THV (N=33/92, 36% of patients) and least with the balloon-expandable SAPIEN 3 valve (N=21/113, 19%), as shown in Figure 3. A more than average amount of debris (N=72/263) was numerically most frequent with the Evolut R/PRO THVs (N=40/123, 33%) and less with SAPIEN 3 (N=18/91, 20%), but this did not reach statistical significance (p=0.11). We also performed multivariable regression analysis to estimate the effect of the three valves on size and amount of debris. Univariate analyses are displayed in Supplementary Table 1 and Supplementary Table 2. In multivariable analysis, Lotus TAVI showed the highest risk of dislodging particles ≥1,000 μm to the brain (OR 2.44, 95% CI: 1.14-5.24, p=0.02) while Evolut R/PRO TAVI was comparable (OR 1.04, 95% CI: 0.46-2.33, p=0.93) to SAPIEN 3 TAVI. Also, the presence of a (functional) bicuspid native valve significantly increased the risk for larger particles (OR 2.91, 95% CI: 1.20-7.03, p=0.02), as shown in Table 2. In univariate analysis the Evolut R/PRO valve seemed to result in greater amounts of debris (OR 1.95, 95% CI: 1.03-3.70, p=0.04), but in multivariable analysis the separate valves did not differ significantly (Table 3). Importantly, the actual application of the repositioning feature during the procedure was an independent predictor for greater amounts of debris captured in the SENTINEL filters (OR 2.96, 95% CI: 1.42-6.16, p=0.004), but did not result in larger particles (OR 1.35, 95% CI: 0.77-2.39, p=0.30).
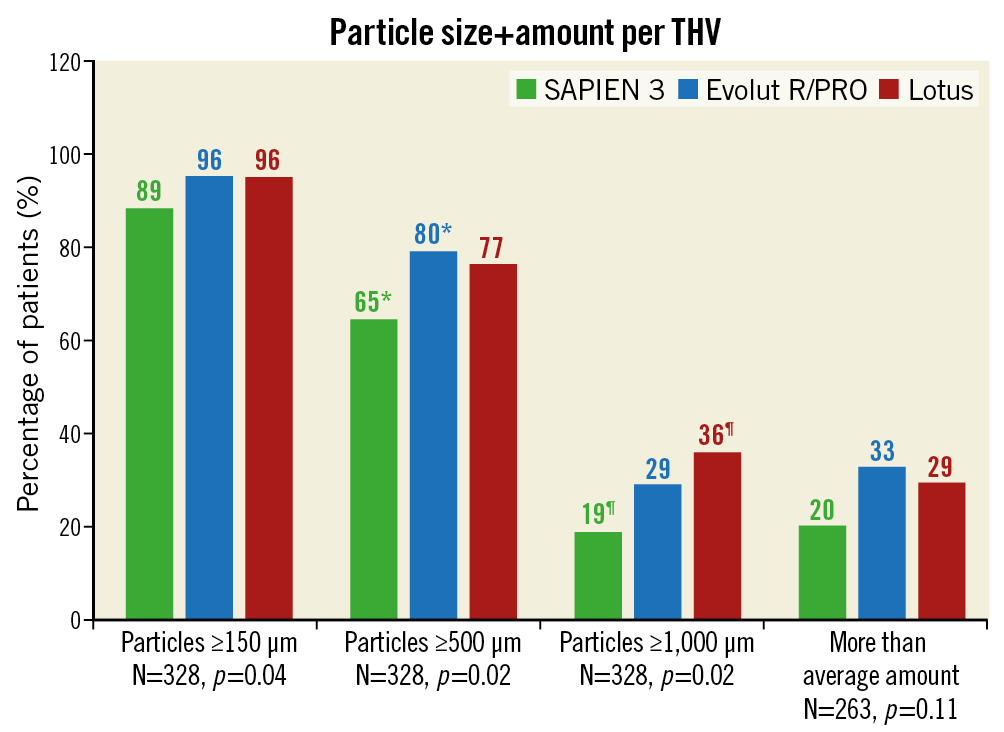
Figure 3. Size and more than average amount of debris for the three separate valves. Debris size was available for 328 patients who underwent TAVI, while the quantification of debris was present for 263 patients. Percentage of affected patients is displayed above each bar. *,¶ mean p<0.05 for ensuing pairwise comparisons with Bonferroni’s correction. THV: transcatheter heart valve
Discussion
The main findings of this single-centre observational study in 328 TAVI patients protected by CEP can be summarised as follows: 1) embolic debris was captured in 98% of TAVI patients, 2) foreign material, as well as endothelium, calcified material and myocardial tissue, was more prevalent with Lotus TAVI, 3) native (functional) bicuspid valves resulted more frequently in capturing particles ≥1,000 μm, and 4) THV repositioning during the procedure resulted in a greater amount of debris, but not larger particles.
Four patients (1.2%) of our reported population suffered a stroke after TAVI, which is comparable to reported stroke rates in protected patients in the current literature4,6,9. Although a downward trend over time is reported, overall stroke rates remain high at around 3-5% of patients who undergo TAVI overall10. Histopathological analysis of debris captured in the filters may provide important information regarding the pathogenesis of stroke during and shortly after TAVI. We reported that debris en route to the brain was captured in 98% of our patients. Previous studies confirmed captured debris in the majority (86-100%) of patients7,8,11,12,13,14. Recently, Seeger et al and Schmidt et al reported on analysed filters per THV type in 100 and 246 patients, respectively7,8. Our findings corroborated the frequent finding of embolised debris consisting of thrombus, aortic valve and foreign material during TAVI. Notably, thrombus remained the most prevalent form of captured debris and seemed unrelated to the actual activated clotting time during the procedure7,8,11,12,13,14. In this present study, foreign body material was captured more frequently with the Lotus compared to other valves. Specific to the (first-generation) Lotus valve is the foreshortening from both ends during the locking of the valve, which results in interaction with myocardial and vascular tissue15. This feature may have improved with the second-generation LOTUS Edge with DepthGuard technology. Other important features of the Lotus valve are the possibility to reposition and recapture the valve, but also the capability to deliver high radial force. These Lotus features may explain the higher amounts of foreign material, myocardial tissue, calcified material and endothelial tissue. In contrast, Evolut R/PRO TAVI resulted in more valvular tissue and less myocardial material. These findings are consistent with the report by Schmidt et al8. Perhaps more important than the type of debris that is captured is the size and amount of debris particles. Seeger et al found that SAPIEN 3 TAVI resulted in larger particles and had equally larger overall amounts of debris as compared to Evolut R TAVI. Conversely, in the report by Schmidt et al, SAPIEN 3 was associated with smaller particles and a lesser amount of debris overall7,8. In our study, the use of the (non-repositionable) balloon-expandable SAPIEN 3 had the lowest risk for large particles. On multivariable analysis, the overall amount of debris was similar with all THV platforms. Of note, the study of Seeger et al was conducted in a relatively small study population (Lotus, N=23; Evolut R, N=35; and SAPIEN 3, N=42), and patients who underwent SAPIEN 3 TAVI numerically more often had peripheral artery disease (14% vs 4% and 6%) and/or carotid artery stenosis (10% vs. 4% and 6%), which could increase the risk for cerebral embolisation7. Two studies investigating other imaging modalities (magnetic resonance imaging and transcranial Doppler) found a lower volume of new lesions and fewer high-intensity transient signals with the SAPIEN 3 compared to self-expanding valves13,16. In contrast, in 81 patients undergoing TAVI with older platforms we found that the use of a balloon-expandable valve was an independent predictor of tissue embolisation14. A transcranial Doppler study found the highest level of high-intensity signals during positioning and subsequent implantation of the valve16. This is important, as both the Lotus and Evolut R/PRO valves feature the (partial) repositioning and recapturing capability. In our multivariable analysis, the actual use of the repositioning feature during the procedure was an independent predictor for dislodging greater amounts of debris to the brain, while valve type did not contribute significantly. Particles ≥1,000 µm were more common with Lotus compared to SAPIEN 3 (OR 2.44, 95% CI: 1.14-5.24, p=0.02), but not versus Evolut R/PRO (OR 1.04, 95% CI: 0.46-2.33, p=0.93). The presence of larger particles was more dependent on THV type and presence of (functional) bicuspid valves than the use of repositioning. All four patients who suffered a stroke post TAVI received the Lotus valve, and all had particles >1,000 µm present in their filters. Importantly, LVOT and annular calcification levels and the use of predilatation or post-dilatation seemed not to contribute significantly to the amount or size of debris dislodged to the brain. Counterintuitively, a history of stroke seemed to be protective against a more than average amount of captured material. However, patients with a history of stroke in our series appeared to have less peripheral artery disease or significant LVOT calcification levels.
Study limitations
This is a single-centre retrospective, observational study and may be subject to inherent bias. Importantly, this is a non-randomised comparison between transcatheter heart valves which may imply selection bias and thus preclude definite conclusions. We cannot completely exclude that filters during deployment and retrieval may have generated debris too. Separate histopathological data for each filter and semiquantitative visual assessment were not available for all patients, because we started to collect these specific data from 2014 onwards; the vast majority of cases were performed from 2014 onwards. All these analyses were performed by the same pathologist to prevent interobserver variability. We did not capture the time needed for filter deployment and therefore could not correlate this parameter with debris. From 2015 onwards, we used CEP in all TAVI patients with anatomical CEP eligibility (in contrast with selected use before), which may have introduced selection bias and may not reflect global standard practice. Procedural execution changed over time, which could possibly have introduced chronological bias that is not completely correctable through statistical methods. Of note, our study consisted mainly of the first-generation Lotus valves without DepthGuard technology. The foreshortening from both ends, specific to the early-generation Lotus THV, may result in more interaction with myocardial and valvular tissue and thus more debris dislodgement compared to the newer generation. Importantly, presence of (functional) bicuspid valves, moderate to severe LVOT/annular calcifications and history of stroke or transient ischaemic attacks, all possible risk factors for embolisation to the brain, did not differ significantly among the three valves. To our knowledge, we report on the largest real-world protected TAVI population to date with regard to histopathological analysis. Larger trials are needed to confirm these findings.
Conclusions
All THV platforms had similar amounts of captured debris. THV repositioning seemed to be associated with a higher risk for dislodging greater amounts of debris to the brain.
|
Impact on daily practice Debris en route to the brain is captured in the majority of patients during transcatheter aortic valve implantation. Mechanical valve expansion seemed to be associated with more frequent embolisation of foreign body material. Transcatheter valve repositioning may increase the risk for larger amounts of embolised debris to the brain. |
Acknowledgements
We would like to thank Thijmen Hokken (from Erasmus University Medical Center) for his valuable help in data acquisition.
Conflict of interest statement
N.M. Van Mieghem has received research grants from Abbott, Daiichi Sankyo, Medtronic, Boston Scientific, Edwards Lifesciences, Abbott, and PulseCath. He is an advisor to Abbott, Boston Scientific, Medtronic and PulseCath. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

