
The autonomic control of the heart rate is well known: the frequency of sinus nodal discharges is accelerated by sympathetic releases of noradrenalin and decelerated by parasympathetic releases of acetylcholine. Numerous reflexes contribute to the autonomic maintenance of homeostasis of the organism making the heart rate respond to a variety of internal and external stimuli. Under normal circumstances, the vagal influence prevails making the resting heart rate some 30 to 40 beats per minute lower compared to the intrinsic heart rate revealed when all autonomic influences are removed1.
Broadly speaking, increased sympathetic tone accompanies responses of the organism to challenges. Sympathetic drive is therefore present during a variety of pathological conditions and if the sinus node responds to autonomic regulation it is reflected by heart rate increases. In heart failure, the sympathetically elevated heart rate also increases the myocardial metabolic demands, further enhancing the failure. Increased sympathetic tone might also damage natural antiarrhythmic defences2. It is therefore not surprising that elevated heart rates have been repeatedly found to predict poorer outcomes, not only in cardiac patients3,4 but also in other conditions and in the general population5,6.
The conditions in which increased heart rates were found to signify adverse outcomes include aortic stenosis7. It is therefore somewhat unexpected that O’Sullivan et al8 report that, in their patients undergoing transcatheter aortic valve implantation (TAVI), an increased heart rate was not an independent predictor of all-cause mortality during a two-year follow-up. O’Sullivan et al provide a detailed interesting discussion of their findings and present a broad review of the studies that linked elevated heart rates to an increased risk. Still, the question of why O’Sullivan et al have not observed the standard model of autonomic risk prediction in their TAVI patients remains unanswered and may deserve further comments. Three aspects which might contribute to such comments come to mind.
Studying the report by O’Sullivan et al, we firstly note that the investigated patients were, on average, older than 80 years. This is not surprising considering the patient selection, especially at the start of the TAVI programmes during the previous decade. Advanced age is known to reduce autonomic reflexes substantially1,9, which might weaken the mechanisms linking sympathetic drive to outcome prediction. Indeed, the vast majority of previous studies that confirmed the predictive value of heart rate increases included, on average, younger subjects and, in some cases, excluded octogenarians if not using an even lower upper age limit.
An elevated heart rate, especially if combined with advanced age, is also not the strongest indicator of an increased sympathetic drive. The distinction between vagal and sympathetic influences on the sinus node is possible by investigations of heart rate variability10. Risk prediction in cardiac patients can be particularly powerful when applying the heart rate variability method of deceleration capacity11 which quantifies vagal withdrawal. Whether the application of such technologies would have been useful in the population studied by O’Sullivan et al remains an open question since it appears that longer ECG recordings needed for the analysis were not available.
Finally, the observation shown by O’Sullivan et al in their Figure3 is at least as surprising as the lack of the independent predictive value of increased heart rates. The Figure shows that those patients who had a higher heart rate before the TAVI procedure lowered their rate after the procedure whilst patients who initially had a lower heart rate increased it afterwards. The authors suggest possible mechanisms that might have led to the post-TAVI heart rate decreases. Indeed, psychological stress and a fear of impending intervention might easily have caused transient pre-procedure heart rate increases. Nevertheless, the opposite rate changes in patients who initially had a lower heart rate are surely unexpected and puzzling. Among other possibilities, this might have been contributed to by procedures involved in the heart rate assessment.
Even at an advanced age, the heart rate is not stable throughout the day, increasing and decreasing in response to multiple external factors. O’Sullivan et al provide little detail on how the ECGs of their study were obtained and how the heart rates were measured by the independent cardiologists. Nonetheless, in standard settings of active clinical units, it is frequently difficult to eliminate external influences on the heart rate, e.g., by recording ECGs only after the patients were kept not only in a strict supine position but also in a noise-free shielded environment for several minutes or by averaging heart rates repeatedly measured over a longer period of time. The pollution of singular ECG measurements of the heart rate by considerable random variability has been observed in a number of clinical studies12. There is no reason to state firmly that the heart rate data reported by O’Sullivan et al were polluted by random variations but, if this were the case, it might not only explain the “regression to the mean” pattern seen in their Figure3 but also contribute to the lack of the predictive power of an increased heart rate.
To document this possibility, we have used Holter recordings made in the EMIAT trial, i.e., in patients surviving acute myocardial infarction with left ventricular ejection fraction (LVEF) not exceeding 40%13. These patients (n=1,258, 189 women) were much younger than those reported by O’Sullivan et al (mean age 60.4±9.4 years) but they also represented seriously ill cardiac patients (mean LVEF of 30.1±7.2%) and their follow-up also spanned two years. The EMIAT patients were randomised to placebo and amiodarone but, since this had no impact on all-cause mortality, we merged both study arms together. Before randomisation, nominal 24-hour Holter recordings were made. From these Holters, we calculated the heart rate averaged over the complete recording duration and, to model short-term rate assessment, also from 10-second ECG segments starting five and nine hours after the Holter start (since the majority of the Holters started in the morning hours, these 10-second heart rates were measured in the afternoon). For all three heart rate measurements, we assessed their power to predict all-cause mortality during follow-up by comparing one third of the patients with the highest heart rate with the remaining two thirds of the population (closely corresponding to the group proportions investigated by O’Sullivan et al). To compare the predictive power of heart rate with that of more advanced autonomic indices, we also calculated the deceleration capacity in all the Holters and performed corresponding survival analyses. The results of this experiment are shown in Figure 1. Consistent with previous reports14, we found that the heart rates assessed from the complete Holters predicted all-cause mortality strongly, although less strongly compared to deceleration capacity. On the contrary, the short-term 10-second assessment showed only non-significant trends of increased follow-up mortality associated with increased rates. Moreover, although the short-term measurements were only four hours apart, they were influenced by random variations, and their comparison showed a clear “regression to the mean” pattern, reminiscent of that presented by O’Sullivan et al.
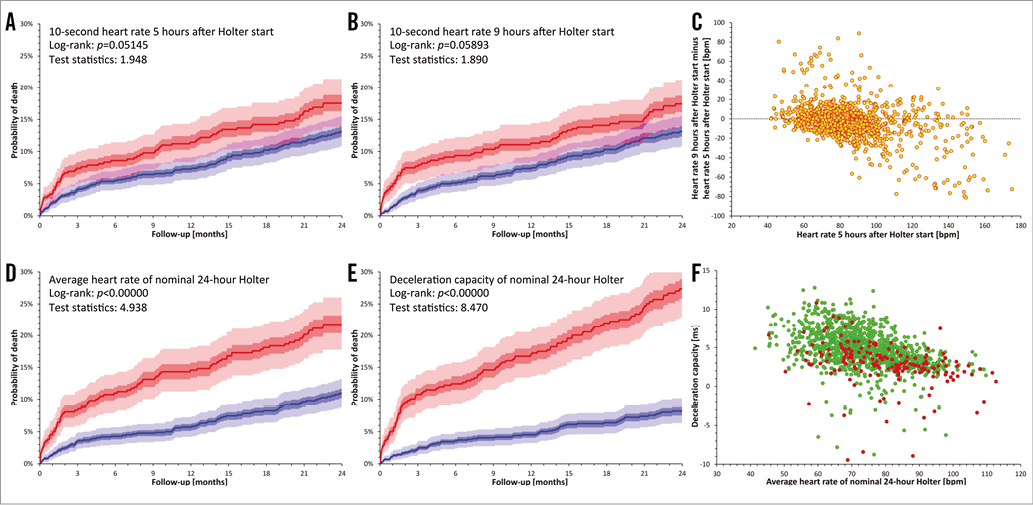
Figure 1. Mortality prediction in the EMIAT population. Comparisons of death probabilities are shown based on stratifying the patients according to 10-second heart rate five hours after the start of Holter recording (A), 10-second heart rate nine hours after the start of Holter recording (B), averaged heart rate of complete nominal 24-hour Holter (D), and deceleration capacity assessed in the complete nominal 24-hour Holter (E). In all these panels, the red lines correspond to one third of the patients with highest heart rate (A, B, D) or lowest deceleration capacity (E) while the blue lines correspond to the remaining two thirds of the patients. The light shaded and dark shaded areas show 90% confidence intervals and interquartile ranges of the survival curves, respectively (obtained by bootstrap recalculations). The violet shaded areas show regions where the confidence intervals of the red and blue survival curves overlap. Panel C shows the comparison of the 10-second heart rates measured at five and nine hours after the Holter start (note the obvious “regression to the mean” pattern). Panel F shows a scatter diagram of nominal 24-hour heart rate averages vs. nominal 24-hour values of deceleration capacity. Green and red marks correspond to patients who did and did not survive the two-year follow-up, respectively. Note that non-survivors are clustered at low deceleration capacity values more densely than at high heart rates.
As already stated, this data experiment does not prove that similar random variations were also present in the data analysed by O’Sullivan et al. Nevertheless, some aspects of the experiment, including the comparison of the predictive powers of heart rate and deceleration capacity, may suggest that the issue of autonomic-related risk prediction after TAVI procedures should not be considered closed. More detailed investigations involving more comprehensive assessment of cardiac autonomic status are needed in TAVI patients before firm conclusions can be drawn.
Conflict of interest statement
The authors have no conflicts of interest to declare.

