
Abstract
Aims: Resting heart rate (HRate) is a modifiable risk factor among patients with cardiovascular disease, including aortic stenosis (AS). However, the effect of resting HRate on clinical outcomes of patients with severe symptomatic AS undergoing transcatheter aortic valve implantation (TAVI) is unknown. Our aim was therefore to assess the effect of resting HRate on clinical outcomes among high-risk patients with symptomatic severe AS in normal sinus rhythm (NSR) undergoing TAVI.
Methods and results: Of 606 consecutive patients undergoing TAVI, 349 (57.6%) with severe AS and a baseline 12-lead electrocardiogram (ECG) showing NSR undergoing TAVI were analysed. Patients were dichotomised into low HRate (LHR; <77 beats per minute [bpm]) and high HRate (HHR; ≥77 bpm) groups. The primary endpoint was all-cause mortality at two years. As compared with baseline LHR, no significant differences in all-cause mortality at two years (adjusted [adj] hazard ratio [HR] 1.23, p=0.40) were observed among patients with baseline HHR. Of 197 patients with available discharge ECGs remaining in NSR, mean HRate significantly increased among LHR patients (∆HRate 8.35, p<0.001) but decreased among HHR patients (∆HRate -4.88, p<0.001). On thirty-day landmark analysis, discharge HHR was significantly associated with two-year all-cause mortality (HR 2.30, 95% CI: 1.16-4.56, p=0.017), but not after extensive adjustment for comorbidities (adj HR 2.01, 95% CI: 0.98-4.09, p=0.056). A significant interaction for two-year mortality (p-interaction 0.021) was observed on landmark analysis for discharge, but not baseline, HHR.
Conclusions: Baseline and discharge resting HRate were not associated with adverse outcomes after TAVI.
Introduction
A high resting heart rate (HRate) is a potentially modifiable cardiovascular risk factor, both in the general population and among patients with cardiovascular disease1-3. Large observational studies have shown a link between increased HRate and all-cause mortality and cardiovascular events in patients with coronary artery disease, hypertension, metabolic syndrome and heart failure4-7. A high resting HRate has recently been shown to be associated with major cardiovascular events and cardiovascular death among patients with asymptomatic aortic stenosis (AS)8. In addition, it was recently demonstrated that sympathetic nervous system (SNS) activity is increased and arterial baroreflex is impaired in patients with AS undergoing transcatheter aortic valve implantation (TAVI)9. A high HRate reflects an increase in SNS activity, which may trigger or exacerbate many of the pathophysiological features associated with AS, such as cardiac hypertrophy and decreased peripheral perfusion9. While there have been several studies published to date assessing the effect of rhythm disturbances (e.g., atrial fibrillation10,11) and conduction abnormalities (e.g., left bundle branch block12,13) on clinical outcomes after TAVI, the majority of patients remain in normal sinus rhythm after TAVI10,11. Heart rate is an easily modifiable risk factor and therefore it is clinically useful to determine whether or not an elevated heart rate may affect clinical outcomes after TAVI. However, to the best of our knowledge, this association has never before been investigated. We therefore aimed to assess the effect of resting HRate on clinical outcomes among high-risk patients with symptomatic severe AS in normal sinus rhythm undergoing TAVI.
Methods
PATIENT POPULATION
This is a retrospective analysis of prospectively collected data from a database that includes all patients with severe native valve AS who underwent TAVI at our institution between August 2007 and December 2012 (n=606). All patients were deemed inoperable or at high surgical risk for conventional surgery by a multidisciplinary team comprising interventional cardiologists and cardiovascular surgeons. Patient flow is shown in Figure 1. Included in this study were all consecutive patients with symptomatic severe native valve AS in sinus rhythm on 12-lead electrocardiogram (ECG) performed ≤30 days prior to TAVI. Exclusion criteria are shown in Figure 1. Patients were dichotomised into low (<77 beats per minute [bpm]) and high (≥77 bpm) HRate groups according to the baseline HRate measured on the pre-procedural 12-lead ECG. Discharge 12-lead ECGs were subsequently analysed after TAVI to assess the discharge HRate and any changes in HRate occurring after TAVI. A cut-off HRate of 77 bpm was chosen based on the results of the randomised Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT), where evidence of a significant treatment effect with ivabradine was noted only in the subgroup of patients with baseline heart rates higher than the median 77 bpm14.
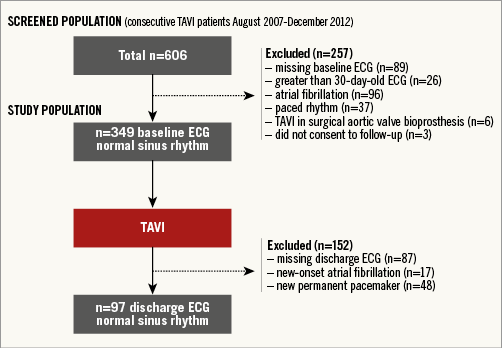
Figure 1. Patient flow for the study population.
The cohort study complies with the Declaration of Helsinki, was approved by the local ethics committee, and all patients provided written informed consent.
ELECTROCARDIOGRAPHY AND ECHOCARDIOGRAPHY
Available 12-lead ECGs were analysed individually by two cardiologists unaware of clinical data. Discrepancies were resolved through consensus. Transthoracic echocardiography was performed in all included patients at baseline as previously described15.
CARDIAC CATHETERISATION
All TAVI patients underwent coronary angiography and 290/349 (83%) had a full invasive evaluation by right and left heart catheterisation prior to TAVI as previously described16.
TRANSCATHETER AORTIC VALVE IMPLANTATION PROCEDURE
TAVI was performed as previously described. Vascular access was transfemoral using the Medtronic CoreValve Revalving System (MCRS) (Medtronic, Minneapolis, MN, USA), Edwards SAPIEN/XT (ESV) (Edwards Lifesciences, Irvine, CA, USA) or transapical for the latter bioprosthesis or the self-expanding Symetis ACURATE TA™ valve (SA) (Symetis Inc., Ecublens, Switzerland) or trans-subclavian using the MCRS16.
CLINICAL FOLLOW-UP
Adverse events were assessed in hospital, and regular clinical follow-up was performed at one, six, 12 and 24 months by means of a clinical visit or standardised telephone interview. All suspected events were adjudicated by an unblinded clinical events committee, comprising cardiac surgeons and interventional cardiologists who were unaware of the heart rate assessments.
STUDY ENDPOINTS
Clinical endpoints were defined according to the criteria proposed by the Valve Academic Research Consortium-2 (VARC-2) consensus document17. The primary endpoint was all-cause mortality at two years. The secondary endpoint was cardiovascular death at two years. All endpoints were assessed for both baseline and discharge HRate.
STATISTICS
Continuous data are presented as means±standard deviations (SD), and categorical variables are shown as percentages and numbers. Categorical variables were compared by means of the χ2 test (or Fisher’s test for two-group comparisons), and continuous variables were compared using the unpaired t-test. Time-to-event data are presented using Kaplan-Meier curves, with incidence rates calculated from life tables at two-year follow-up. Univariate and adjusted Cox proportional hazards models were used to derive hazard ratio estimates of clinical time-to-event comparisons between the two groups. Inverse probability of treatment weighted (IPTW) hazard ratios (HR) and 95% confidence intervals (CI) were derived from Cox regressions. IPTW values for high versus low heart rate at baseline, and again separately for heart rate at discharge, were calculated using the following baseline clinical variables: age, gender, height, body mass index, diabetes, coronary artery disease (CAD), multivessel CAD disease, previous myocardial infarction, cardiac surgery, percutaneous coronary intervention, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), renal failure, moderate or severe mitral regurgitation, New York Heart Association (NYHA) functional Class III or IV (selected if p<0.2 on all-cause death at two years or p<0.2 on high vs. low). Missing data were replaced with single imputation (COPD, NYHA III or IV, mitral regurgitation, n=1, 1, and 32, respectively). The changes in heart rate from baseline pre-TAVI to discharge post-TAVI were analysed with paired t-tests and linear regression. All p-values and 95% CI are two-sided. Two-sided p-values <0.05 were considered statistically significant. All analyses were performed with Stata version 14 (StataCorp, College Station, TX, USA).
Results
BASELINE CHARACTERISTICS
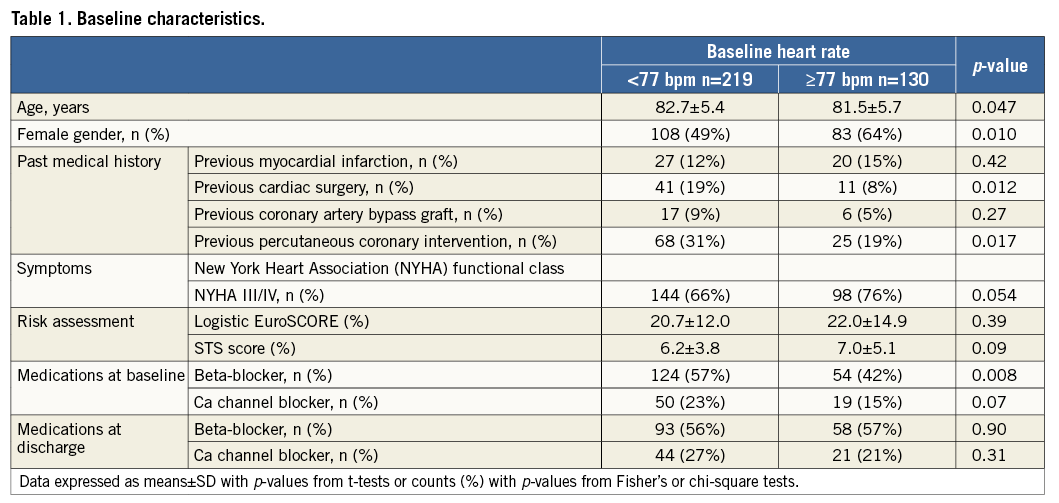
Patient characteristics are shown in Table 1. The present analysis included 349 patients out of 606 consecutive patients (58%) undergoing TAVI between August 2007 and December 2012 in our institution. Of the 349 included patients in sinus rhythm prior to TAVI, 219 (63%) had a low resting HRate (LHR) (defined as <77 bpm) and 130 (37%) had a high resting HRate (HHR) (defined as ≥77 bpm) at baseline before TAVI. As compared with LHR, patients with HHR were significantly younger, more likely to be female, less likely to have undergone previous cardiac surgery and previous percutaneous coronary intervention, and were less likely to be taking beta-blockers. ECGs were performed at a mean±standard deviation of 3.6±5.0 days (range 0-28 days) prior to TAVI. Data on periprocedural changes in medications, including beta-blockers and calcium channel blockers, are shown in Table 1. We found no significant difference in the prevalence of beta-blockers (56% vs. 57%, p=0.90) or calcium channel blockers (27% vs. 21%, p=0.31) prescribed at discharge between low and high heart rate patients.
ELECTROCARDIOGRAPHIC, ECHOCARDIOGRAPHIC AND INVASIVE HAEMODYNAMIC CHARACTERISTICS
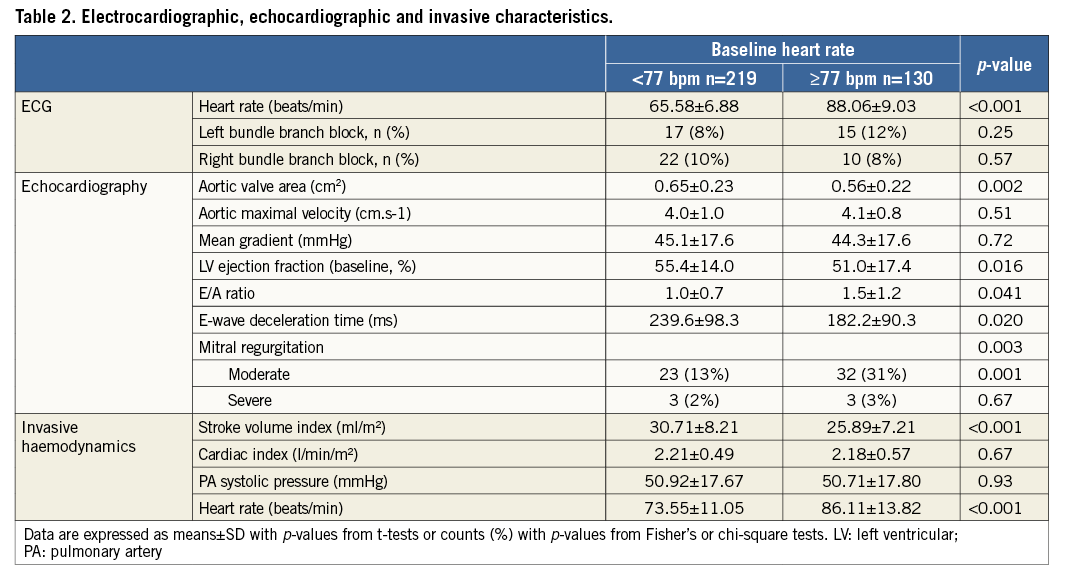
Baseline electrocardiographic, echocardiographic and invasive haemodynamic characteristics are shown in Table 2. As compared with LHR, patients with HHR had smaller aortic valve areas, worse LV systolic and diastolic function and a higher prevalence of moderate to severe mitral regurgitation on baseline echocardiography. Patients with HHR also had a more adverse baseline haemodynamic profile on pre-procedural right and left heart catheterisation. Interestingly, despite significantly lower stroke volume indices among HHR patients, no significant differences in cardiac indices were observed between groups. No significant differences in pulmonary artery systolic pressures were observed between groups. As expected, HRate measured during right heart catheterisation was significantly higher among HHR patients as compared with LHR patients, thereby corroborating the HRate measured on baseline ECG.
PROCEDURAL CHARACTERISTICS
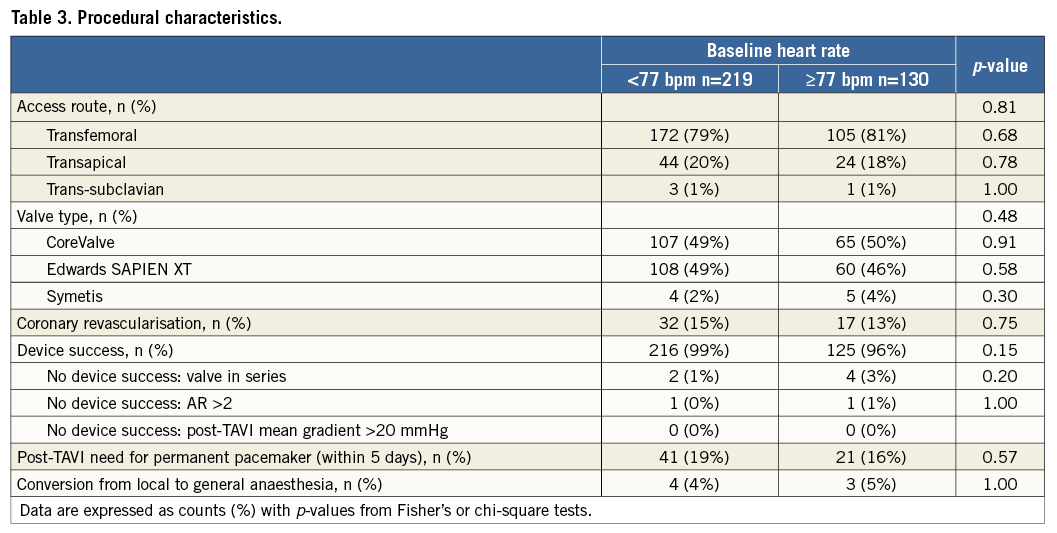
Procedural characteristics are shown in Table 3. The majority of patients included in the present analysis underwent transfemoral TAVI (79%). No significant differences in procedural characteristics were found between groups.
CLINICAL OUTCOMES STRATIFIED ACCORDING TO BASELINE HRate
Clinical follow-up was complete for 338/349 (97%) patients (n=9 lost to follow-up and n=2 refused follow-up). Event rates with adjusted hazard ratios (adj HR) and 30-day landmark analyses for all-cause mortality and cardiovascular death at two years stratified according to baseline HRate are provided in Table 4.
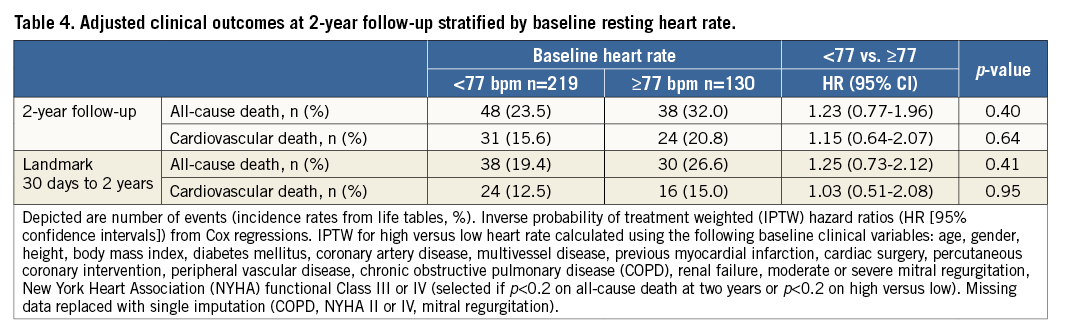
Time-to-event curves for all-cause mortality and cardiovascular death at two-year follow-up are shown in Figure 2A and Figure 2B, respectively. A 30-day landmark analysis for all-cause mortality and cardiovascular death at two years is shown in Figure 2C and Figure 2D, respectively. As compared with baseline LHR, no significant differences in all-cause mortality or cardiovascular death were observed among patients with baseline HHR at two-year follow-up. On 30-day landmark analysis, no significant differences in all-cause mortality or cardiovascular death were observed among patients with baseline HHR on unadjusted or adjusted analysis at two-year follow-up (Table 4, Figure 2C, Figure 2D).
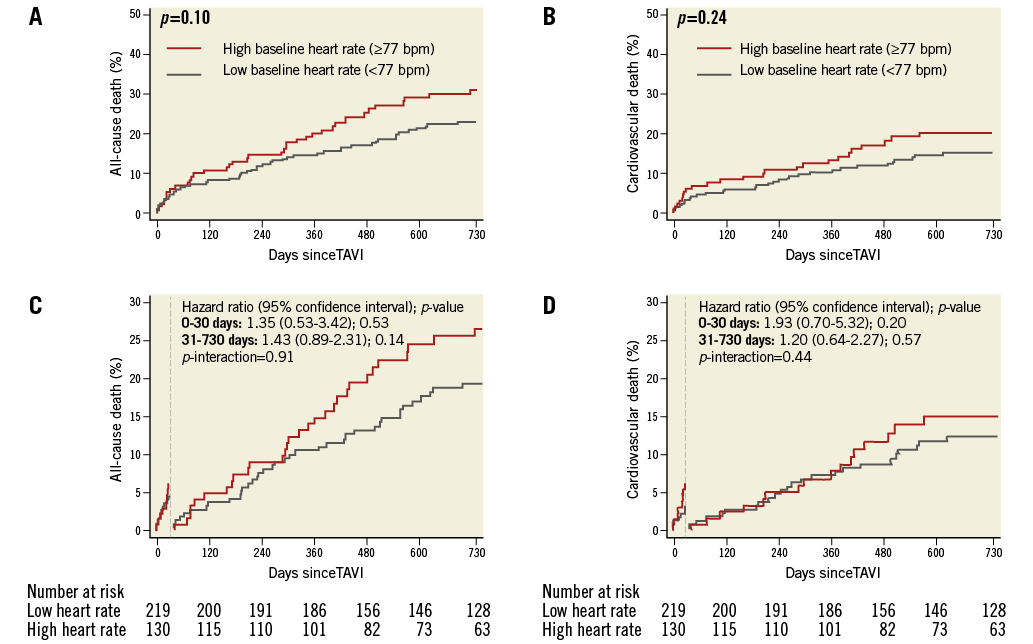
Figure 2. Clinical outcomes according to baseline heart rate. Kaplan-Meier analysis of death (A) and cardiovascular death (B) at two years among patients undergoing transcatheter aortic valve implantation (TAVI) stratified according to low (heart rate <77 beats per minute [bpm]) versus high (heart rate ≥77 bpm) baseline resting heart rate. Thirty-day landmark analysis of death (C) and cardiovascular death (D) at two years among patients undergoing TAVI stratified according to low versus high resting heart rate at baseline.
No significant interaction was observed between overall mortality (p-interaction 0.91) and cardiovascular death (p-interaction 0.44) event rates occurring before and after 30-day follow-up (Figure 2C, Figure 2D).
CHANGES IN HRate AFTER TAVI
Changes in resting HRate measured on 12-lead ECGs before and after TAVI among patients remaining in normal sinus rhythm on discharge ECG (n=197) are shown in Table 5 and Figure 3. Overall, the mean HRate increased after TAVI on discharge ECG. Among LHR patients, the mean HRate significantly increased following TAVI on discharge as compared with baseline ECG. Conversely, among HHR patients, the mean HRate significantly decreased after TAVI on discharge as compared with baseline ECG.
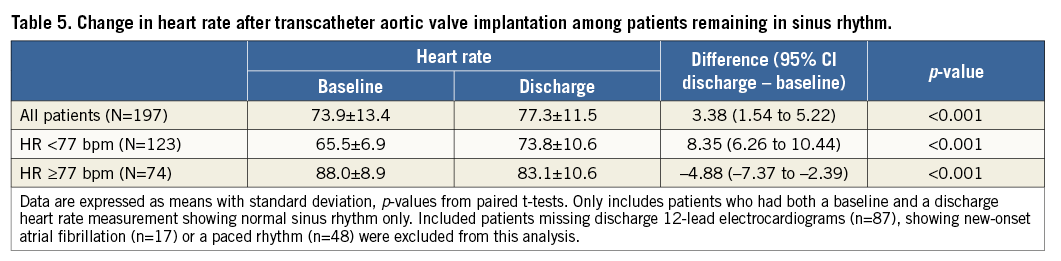
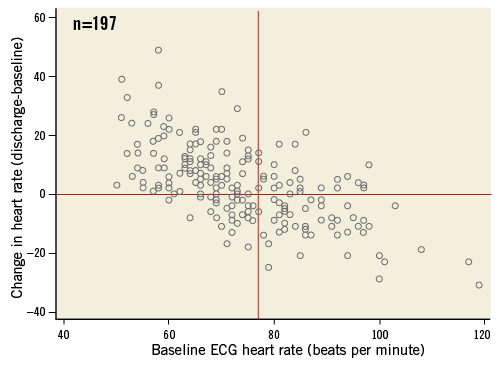
Figure 3. Scatter plot showing the changes in heart rate before and after transcatheter aortic valve implantation (TAVI) among patients remaining in normal sinus rhythm as measured on the baseline and discharge 12-lead electrocardiograms (n=197 patients), respectively. The resting heart rate at baseline on the x-axis is plotted against the change in heart rate (delta heart rate) after TAVI, i.e., discharge heart rate minus baseline heart rate, on the y-axis.
CLINICAL OUTCOMES STRATIFIED ACCORDING TO DISCHARGE HRate
Event rates with adj HRs for all-cause mortality and cardiovascular death and 30-day landmark analyses at two years stratified according to discharge HRate are provided in Table 6. Time-to-event curves for all-cause mortality and cardiovascular death at two-year follow-up stratified according to discharge HRate are shown in Figure 4A and Figure 4B, respectively. A landmark analysis at 30 days for all-cause mortality and cardiovascular death at two years is shown in Figure 4C and Figure 4D, respectively.
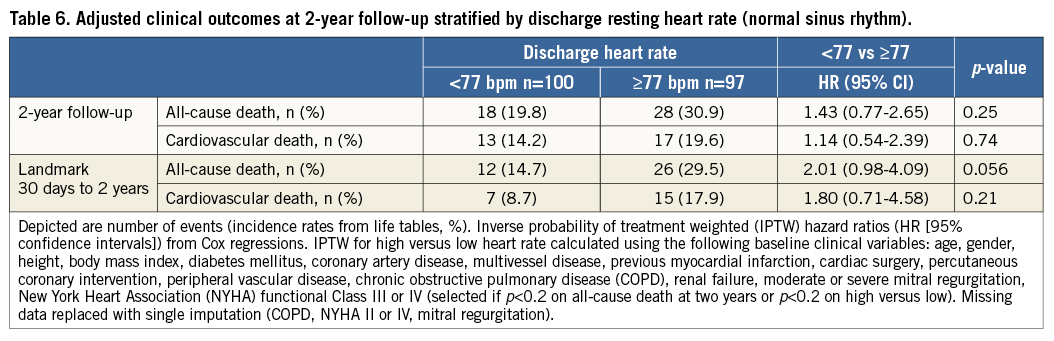
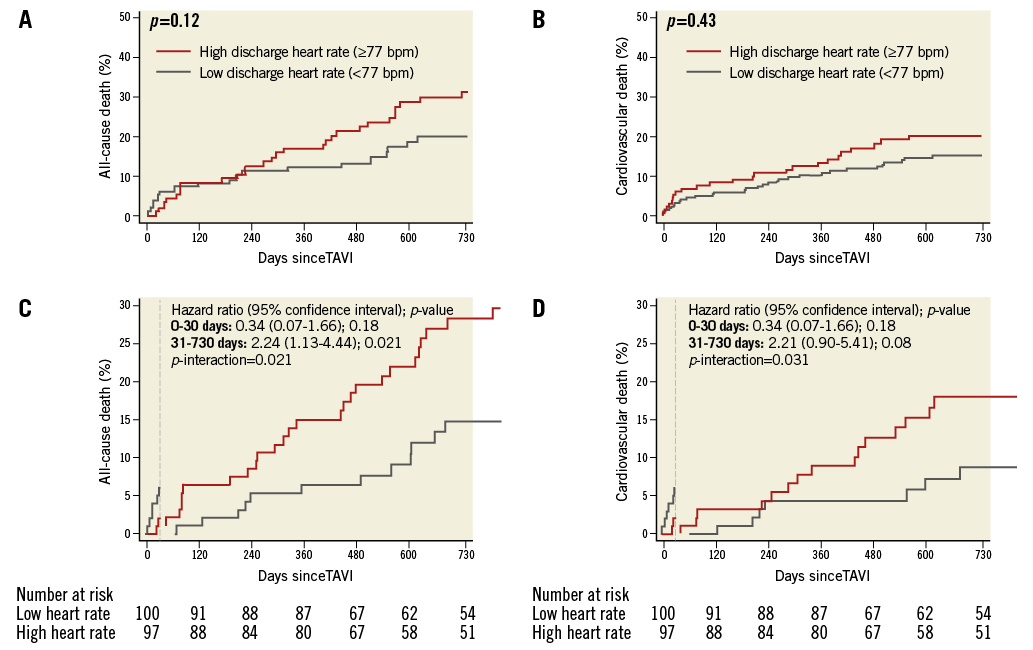
Figure 4. Clinical outcomes according to discharge heart rate. Kaplan-Meier analysis of death (A) and cardiovascular death (B) at two years among patients undergoing transcatheter aortic valve implantation (TAVI) stratified according to low (heart rate <77 beats per minute [bpm]) versus high (heart rate ≥77 bpm) discharge resting heart rate. Thirty-day landmark analysis of death (C) and cardiovascular death (D) at two years among patients undergoing TAVI stratified according to low versus high resting heart rate at discharge.
As compared with discharge LHR, no significant differences in all-cause mortality or cardiovascular mortality among discharge HHR patients were observed. On 30-day landmark analysis, discharge HHR was significantly associated with all-cause mortality at two-year follow-up (HR 2.30, 95% CI: 1.16-4.56, p=0.017) although this significance was lost after extensive adjustment for comorbidities (adj HR 2.01, 95% CI: 0.98-4.09, p=0.056). A statistically significant interaction was observed for overall mortality (p-interaction 0.021) and cardiovascular death (p-interaction 0.031) events occurring before and after 30-day follow-up (Figure 4C, Figure 4D) for discharge HRate.
Discussion
The main finding from the present study was that neither baseline nor discharge HHR was associated with overall or cardiovascular mortality two years after TAVI. Although patients with HHR had worse echocardiographic and invasive haemodynamic profiles at baseline as compared with LHR patients, this did not translate into worse clinical outcomes at two-year follow-up. A novel observation was that TAVI resulted in a significant physiological modification of the resting HRate, i.e., the majority of LHR patients had a significant increase in resting HRate, whereas the majority of HHR patients exhibited a significant decrease in resting HRate after TAVI (Figure 3). The latter observation may explain the lack of effect of baseline resting HRate on clinical outcomes after TAVI. In contrast to baseline HRate, a discharge HHR was significantly associated with overall mortality two years after TAVI on 30-day landmark analysis, although this significance was lost after extensive adjustment (HR 2.01, 95% CI: 0.98-4.09, p=0.056).
RELATIONSHIP BETWEEN RESTING HRate AND OUTCOMES IN CARDIOVASCULAR DISEASE
A significant association between resting HRate and mortality has been reported among patients with a variety of cardiovascular diseases, including acute coronary syndromes18, and hypertension19. Furthermore, HRate was found to be directly related to the risk of death, cardiovascular death, or admission to hospital in patients with heart failure20, and HRate reduction was found to be associated with improved outcomes21. Resting HRate is included in several risk assessment algorithms for patients after acute coronary syndromes, such as the Global Registry of Acute Coronary Events risk prediction (GRACE) score and the Thrombolysis In Myocardial Infarction (TIMI) risk score22,23. Ivabradine is a selective inhibitor of the funny current (If), which results in HRate reduction with no other apparent direct cardiovascular effects24. In the Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL) trial, ivabradine-induced HRate reduction did not affect the primary composite endpoint of cardiovascular death, hospital admission for acute myocardial infarction, and hospital admission for new-onset or worsening heart failure24. However, it did reduce the secondary endpoints of hospital admission for fatal and non-fatal myocardial infarction and coronary revascularisation. Furthermore, a subanalysis of the BEAUTIFUL trial revealed that an HRate of ≥70 bpm was associated with a 34% increased risk of cardiovascular death, a 53% increase in admission to hospital for heart failure, and a 46% increase for admission to hospital for myocardial infarction compared with HRate lower than 70 bpm, even after adjustment for other predictors of outcomes7. In the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT), comprising patients in sinus rhythm with a resting HRate ≥70 bpm (in contrast to the 60 bpm cut-off used in the BEAUTIFUL trial) and LVEF ≤35%, ivabradine was associated with an 18% relative risk reduction in the composite primary endpoint of cardiovascular death or hospital admission for worsening heart failure (p<0.0001)14.
RELATIONSHIP BETWEEN RESTING HRate AND OUTCOMES IN PATIENTS WITH AS
Among patients with asymptomatic AS, recent studies have also shown resting HRate to be an independent predictor of cardiovascular mortality8. In a subanalysis of the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) study, Greve et al assessed the prognostic value of an elevated resting HRate during watchful waiting in asymptomatic AS8. An elevated resting HRate was observed to be independently associated with major cardiovascular events and cardiovascular death during long-term follow-up even after adjusting for other predictors of outcomes8. Dumonteil et al recently demonstrated that TAVI normalises SNS overactivity9. The investigators directly measured muscle sympathetic nerve activity and arterial baroreflex gain in 14 patients with severe symptomatic AS before and a week after TAVI and in 14 control patients without AS9. They found that patients with AS had increased SNS activity associated with a decrease in sympathetic arterial baroreflex gain compared with controls, and that TAVI normalised these parameters9. In the present study, the normalisation of sympathetic overactivity may explain the significant HRate reduction observed among HHR patients after TAVI. This may ultimately explain the lack of differences in clinical outcomes observed between patients with HHR and LHR after TAVI. We observed that HHR patients had significantly lower stroke volumes and stroke volume indices on right heart catheterisation as compared with LHR patients. It is likely that this low flow state leads to activation of the SNS leading to an increase in HRate in an effort to compensate for the low stroke volume. We also observed that patients with LHR had a significant increase in HRate after TAVI. The reason for this is unclear. Further studies correlating changes in heart rate after TAVI with post-procedural changes in cardiac output and stroke volume are required in order to explain this phenomenon.
Limitations
There are a number of limitations that need to be considered when interpreting this study. First, the present study was a single-centre retrospective study. Even though all data were prospectively collected and all events adjudicated by an independent clinical events committee, our results may contain unmeasured bias. Second, the sample size is relatively small (n=349), and only 197 patients had available data for discharge analysis. However, to the best of our knowledge, this is the first study to question the association between resting sinus rhythm HRate and clinical outcomes in TAVI patients. Third, this is not a consecutive patient series, as patients with missing ECGs or those who received ECGs >30 days prior to TAVI were excluded. Furthermore, not all included patients had discharge ECGs available. Finally, there are other factors that can influence resting HRate apart from SNS activity.
Conclusions
Neither baseline nor discharge resting HHR was associated with all-cause mortality or cardiovascular death after TAVI.
| Impact on daily practice Resting sinus rhythm heart rate is known to be a modifiable risk factor for cardiovascular disease, including aortic stenosis. However, there have been no studies to date assessing the effect of baseline and discharge resting sinus rhythm heart rate on clinical outcomes after transcatheter aortic valve implantation (TAVI). We observed that resting sinus rhythm heart rate at baseline and discharge had no effect on two-year mortality rates after TAVI. |
Conflict of interest statement
S. Windecker has received research contracts to the institution from Abbott, Boston Scientific, Biotronik, Medtronic, Edwards Lifesciences and St. Jude. The other authors have no conflicts of interest to declare.

