Abstract
Aims: Large intracoronary thrombus in patients with acute coronary syndromes remains a challenge in percutaneous coronary intervention, despite technical advances of manual aspiration catheters and mechanical thrombectomy devices. The Trevo® Pro 4 is a novel self-expanding mechanical thrombus retrieval device, designed for removal of occlusive thrombi in the setting of acute ischaemic stroke. We describe the first use of this novel mechanical thrombus retrieval device in the setting of coronary intervention.
Methods and results: In close collaboration with the interventional radiology department, two patients presenting with an acute coronary syndrome, complicated by refractory large intracoronary thrombus, were treated using the Trevo Pro 4. Both patients were treated successfully, resulting in complete removal of refractory thrombus without the occurrence of adverse events.
Conclusions: The Trevo Pro 4 can be successfully used in the setting of coronary intervention. It is simple to use, does not require complex preparations, and the handling is straightforward. A larger study to assess the safety and efficacy of this device in the setting of coronary interventions is warranted.
Introduction
Large intracoronary thrombus in patients with acute coronary syndromes (ACS) remains a challenge in contemporary percutaneous coronary intervention (PCI), despite technical advances of manual aspiration catheters and mechanical thrombectomy devices. Particularly when manual thrombus aspiration is unsuccessful, few other feasible treatment options exist. For example, the use of rheolytic thrombectomy and filter devices is frequently limited as it is considered more technically challenging, and these devices are not suited for small calibre or very tortuous arteries. In the setting of acute ischaemic stroke (AIS), the use of so-called stent retrievers for thrombus removal and immediate flow restoration is increasing. These devices, named after their resemblance to intracranial stents, provide a practical means for intra-arterial thrombus removal, which yields favourable outcomes in AIS patients1. Potentially, stent retrievers may provide a feasible alternative in the setting of coronary intervention, when manual thrombus aspiration fails and other feasible treatment options are lacking. However, these devices have not been evaluated in the setting of coronary interventions.
The Trevo® Pro 4 (Concentric Medical Inc., Mountain View, CA, USA) is a novel self-expanding mechanical thrombectomy device, designed for removal of occlusive thrombi in the setting of acute ischaemic stroke (AIS), that has shown favourable results in comparison with other mechanical thrombectomy devices2,3. We describe the first-in-man intracoronary application of this novel device in the setting of acute coronary syndrome complicated by large refractory intracoronary thrombus after failure of manual thrombus aspiration.
First-in-man intracoronary application of the Trevo Pro 4 mechanical thrombectomy device
CASE 1
The first patient was a 41-year-old woman with anterior ST-segment elevation myocardial infarction (STEMI). Emergency angiography showed a coronary dissection with a large thrombus in the proximal left anterior descending coronary artery (LAD), comprising 80% of the coronary lumen, and flow-limiting embolisation in the distal LAD (Figure 1A, Moving image 1-Moving image 3). Manual thrombus aspiration (Export; Medtronic, Minneapolis, MN, USA) was attempted repeatedly without success (Moving image 4 and Moving image 5). The procedure was halted at this stage, but ST-segment resolution was limited (Moving image 6 and Moving image 7). Despite intravenous abciximab for 12 hours, ST-segment elevation and symptoms persisted. Repeat angiography was performed, which showed large residual thrombus in the proximal LAD, still comprising 60% of the coronary lumen, and persistent flow-limiting distal embolisation (Figure 1B, Moving image 8). The small calibre of the distal LAD precluded the use of a rheolytic thrombectomy device and motivated consultation with an interventional neuroradiologist. The interventional cardiologist and interventional neuroradiologist together deployed a Trevo Pro 4 in an attempt to treat the distal LAD occlusion (Moving image 9 and Moving image 10). Whereas the distal occlusion persisted, the first pass with the Trevo device (Moving image 11) resulted in retrieval of the large proximal thrombus in its totality (Figure 1C, Moving image 12 and Moving image 13).
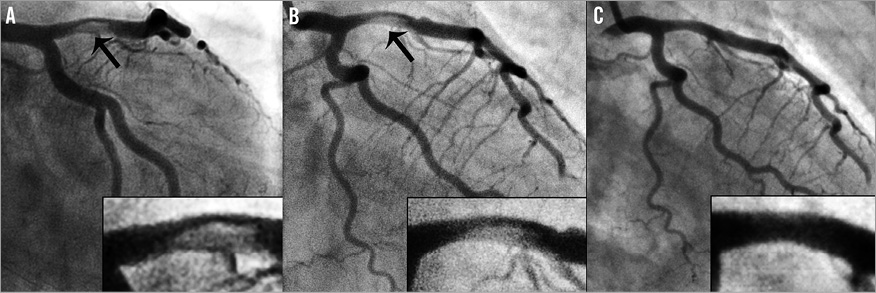
Figure 1. Patient 1: coronary angiography and Trevo procedure. A) Left coronary artery angiography at the end of the index procedure. A large intracoronary thrombus (arrow), comprising 80% of the coronary lumen, is present in the proximal left anterior descending artery (LAD). B) Initial left coronary artery angiography during the second catheterisation. The large thrombus still comprises 60% of the lumen in the LAD (arrow), with a flow-limiting distal LAD occlusion. C) Left coronary artery angiography after first Trevo device deployment and retraction. Although the distal flow-limiting occlusion persisted, the proximal thrombus was retrieved in its totality.
CASE 2
The second patient was a 66-year-old male with a non-ST-segment elevation acute coronary syndrome (NSTE-ACS), in whom diagnostic angiography at the referral hospital had revealed a proximal right coronary artery (RCA) occlusion (Moving image 14), likely recent, with collateral filling from the left circumflex coronary artery and LAD (Moving image 15). Due to ongoing symptoms, urgent PCI of the RCA was subsequently attempted in our institution (Moving image 16). Extensive manual thrombus aspiration (Export; Medtronic, Minneapolis, MN, USA) was performed, during which a large amount of macroscopic thrombus material and debris was retrieved without restoration of antegrade flow (Moving image 17). During subsequent multiple balloon dilatations under proximal embolic protection (Moving image 18-Moving image 21) (Proxis; St. Jude Medical, Maple Grove, MN, USA), a large amount of debris was retrieved and TIMI 3 flow was restored. However, final angiography showed an enormous residual thrombus at the transition of RCA segment 2 and 3, comprising 80% of the vessel lumen (Figure 2A, Moving image 22-Moving image 24). In addition to routine dual antiplatelet therapy with aspirin and prasugrel, the patient was treated with heparin intravenously for five days until planned re-catheterisation.
At re-catheterisation, the residual RCA thrombus still comprised 50% of the lumen diameter (Figure 2B, Moving image 25-Moving image 27). Mechanical thrombectomy with the Trevo Pro 4 device was attempted. The Trevo device could easily be positioned at the thrombus site (Moving image 28 and Moving image 29) and, after two successful stent-retriever passes (Moving image 30), complete removal of the intracoronary thrombus was achieved. During the retrieval, careful engagement of the guiding catheter in the ostium of the coronary artery was ensured to prevent systemic embolisation of the thrombus fragments. The final angiogram showed TIMI 3 flow and myocardial blush grade 3, with no residual thrombus in the RCA (Figure 2C, Moving image 31 and Moving image 32).
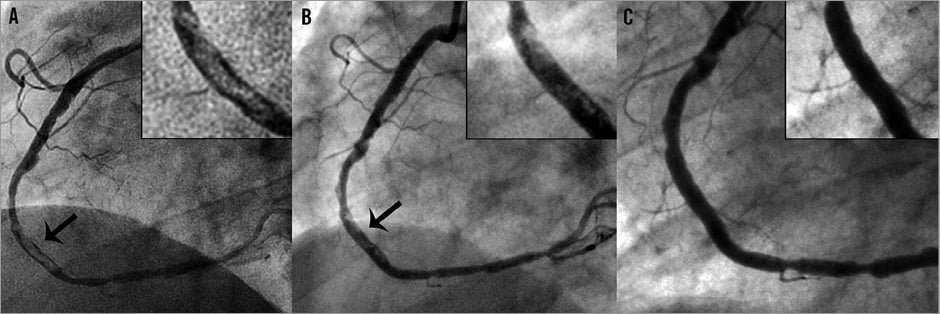
Figure 2. Patient 2: coronary angiography and Trevo procedure. A) Right coronary artery (RCA) angiography during the index procedure. A large thrombus is present (arrow) at the transition of RCA segment 2 and 3, comprising 80% of the coronary lumen. B) Initial RCA angiography during the second catheterisation. The large thrombus, although decreased in size, still comprises 50% of the coronary lumen (arrow). C) RCA angiography after two Trevo device deployments and retractions. The thrombus was retrieved in its totality.
Discussion
TREVO PRO 4 MECHANICAL THROMBECTOMY TECHNOLOGY
The Trevo Pro 4 (Figure 3) is a novel self-expandable mechanical thrombus retrieval device, designed for removal of occlusive thrombi in the setting of acute ischaemic stroke (AIS). The device is currently not certified for use in the coronary arteries, but recently received FDA approval for its use in AIS patients.
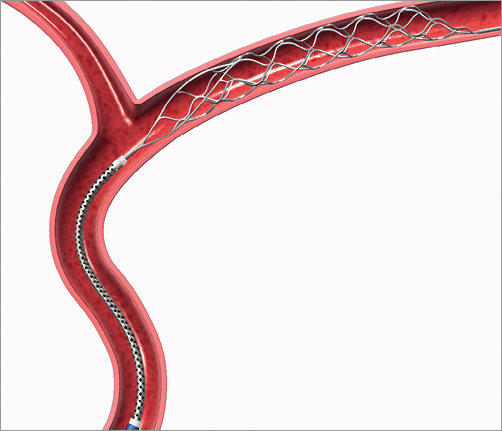
Figure 3. Trevo Pro 4 mechanical thrombectomy system. The Trevo Pro 4 mechanical thrombectomy device is designed for the removal of occlusive thrombi in vessels ranging from 1.5 to 3.5 mm in diameter. The device consists of a 0.018 inch core wire of 180 cm length, with a 75 cm long tapered transition, a subsequent stent-like shaped section of 44 mm length, and a guidewire-like tip at the distal end.
The use and handling of the device are relatively easy and straightforward. After wiring of an occlusive thrombus with a standard 0.014 inch coronary guidewire (Figure 4A), a 2.5 Fr microcatheter is introduced over-the-wire, and is positioned across the thrombus (Figure 4B). The guidewire is then retracted, and the Trevo device is advanced within the microcatheter until it is positioned to cover the full length of the thrombus (Figure 4C). By retracting the microcatheter, the Trevo stent retriever is subsequently deployed (Figure 4D). The outward radial force of the device displaces fragments of the thrombus against the vessel wall and entangles the thrombus within the closed-cell stent-like nitinol mesh. While restoration of antegrade flow is usually achieved immediately after device deployment4, retrieval of the thrombus is performed after four to five minutes by retracting the Trevo device and the microcatheter jointly while applying aspiration to the guide catheter until it is nearly withdrawn. Care should be taken to ensure optimal engagement of the guide catheter in the coronary ostium in order to prevent embolisation of thrombus fragments during retrieval.
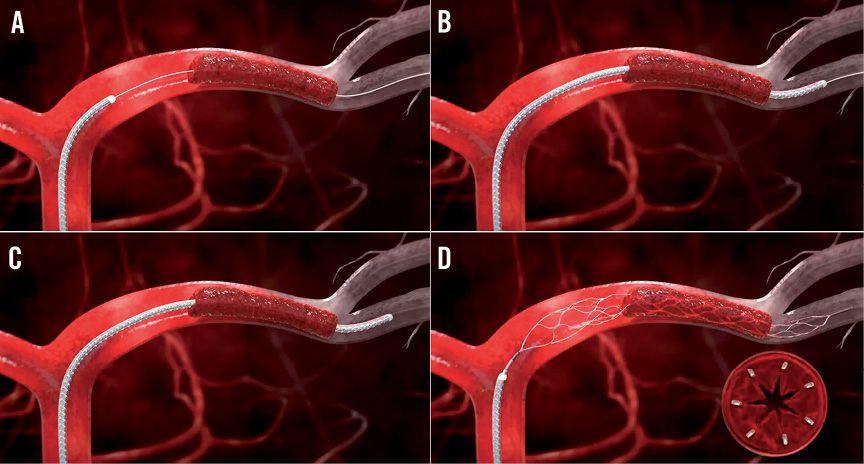
Figure 4. Trevo Pro 4 deployment procedure. A) The occlusive thrombus is wired with a standard 0.014” coronary guidewire. B) A 2.5 Fr microcatheter is advanced across the thrombus. C) The guidewire is retracted, and the Trevo device is advanced through the microcatheter until it is positioned to cover the full length of the thrombus. D) The Trevo device is deployed by retracting the microcatheter, resulting in self-deployment of the stent retriever.
PRECLINICAL AND CLINICAL EXPERIENCE IN ACUTE ISCHAEMIC STROKE
Preclinical evaluation showed that the Trevo Pro 4 device is highly effective for immediate reperfusion of occluded arteries4. Consistent with these findings, early non-randomised clinical evaluation in AIS patients has repeatedly yielded favourable clinical outcomes, with successful recanalisation rates of up to 92% without concerns regarding its safety3,5-8. Recently, a large randomised clinical trial showed the Trevo Pro 4 device to be the mechanical thrombectomy device of choice in patients with AIS, yielding the highest successful reperfusion rates2. The present report is the first to describe efficacy and safety of its use in coronary arteries.
Technique limitations
SYSTEMIC AND DISTAL EMBOLISATION
In the setting of AIS, the Trevo Pro device is used in conjunction with a balloon-tipped guiding catheter, allowing balloon occlusion of the proximal internal carotid artery during retraction of the thrombus to circumvent the risk of systemic embolisation. Concomitantly, manual aspiration is applied to the balloon guide catheter to minimise the risk of distal embolisation of thrombus fragments. In the absence of balloon-tipped guide catheters for coronary use, the risk of systemic embolisation of thrombus fragments may exist. Therefore, we performed Trevo retraction under careful engagement of the guide catheter into the coronary ostium, while applying manual aspiration to the guide catheter, minimising the risk of systemic and distal embolisation. Availability of balloon-tipped guide catheters for coronary use may further limit the risk of systemic embolisation of thrombus fragments.
Disengagement of the guide catheter from the coronary ostium may lead to an increased risk of systemic embolisation. However, the Trevo concept is based on thrombus integration within the stent retriever’s nitinol mesh, securing the thrombus to the device. In comparison with manual thrombus aspiration of large intracoronary thrombi, in which the manual aspiration force applied to the thrombus secures the thrombus to the catheter, the risk of systemic embolisation in case of disengagement of the guide catheter may be considered relatively limited.
VASCULAR DISRUPTION
Intuitively, vascular wall injury could be an important concern with a stent-like retrieval device, a concept based on outward radial force to the vessel wall in order to incorporate and retrieve the intra-arterial thrombus. Although in general there is a paucity of data on the risk of vascular injury after the use of mechanical thrombectomy devices, the adverse effects of the Trevo Pro 4 device have been evaluated in a preclinical study by Nogueira et al, which assessed the adverse effects of the Trevo device on vascular wall integrity by means of histology4. Although superficial intimal disruption occurred after passing the vessels with the Trevo device, no haemorrhage of the media/adventitia or evidence of transmural medial dissection was present. The authors concluded that the Trevo device does not cause any clinically significant disruption of vascular integrity. These findings, as well as the favourable safety profile of the device in the early clinical evaluation in AIS patients, substantiate a safe use of the Trevo Pro 4 device in human arteries.
Conclusions
The current report describes the first successful use of the novel Trevo Pro 4 stent retriever in the setting of coronary interventions after failed manual thrombus aspiration. It is simple to use, does not require complex preparations and the handling is straightforward, making it a practical, feasible alternative after failed manual thrombus aspiration. A larger study to assess the safety and efficacy of this device in the setting of acute coronary syndromes, as well as its potential as a first-in-line thrombectomy device, is warranted.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
CASE 1.
INDEX PROCEDURE
Moving image 1-3. Left coronary artery angiography at immediate angiography.
Moving image 4. Left coronary artery angiography after manual thrombus aspiration in the proximal left anterior descending coronary artery.
Moving image 5. Left coronary artery angiography after manual thrombus aspiration in the distal left anterior descending coronary artery.
Moving image 6-7. Left coronary artery angiography at the end of the index procedure.
TREVO PROCEDURE
Moving image 8. Left coronary artery angiography at the start of the Trevo procedure.
Moving image 9. The 2.5 Fr microcatheter is advanced over-the-wire, across the distal left anterior descending coronary artery occlusion.
Moving image 10. Trevo stent retriever is deployed at the site of the distal left anterior descending coronary artery occlusion.
Moving image 11. Retraction of the Trevo stent retriever under manual aspiration on the guide catheter.
Moving image 12-13. Final left coronary artery angiography.
CASE 2.
REFERRAL HOSPITAL DIAGNOSTIC PROCEDURE
Moving image 14. Right coronary artery angiography during the diagnostic catheterisation at the referral hospital.
Moving image 15. Left coronary artery angiography during the diagnostic catheterisation at the referral hospital.
URGENT PERCUTANEOUS CORONARY INTERVENTION PROCEDURE
Moving image 16. Right coronary artery angiography after successful wiring, prior to manual thrombus aspiration.
Moving image 17. Right coronary artery angiography after manual thrombus aspiration.
Moving image 18-21. Multiple balloon dilatations of the right coronary artery under proximal embolic protection (Proxis; St. Jude Medical, Maple Grove, MN, USA).
Moving image 22-24. Final right coronary artery angiography.TREVO PROCEDURE
Moving image 25-27. Right coronary artery angiography at the start of the Trevo procedure.
Moving image 28. The 2.5 Fr microcatheter is positioned across the right coronary artery thrombus, and the Trevo stent retriever is advanced through the microcatheter.
Moving image 29. The Trevo stent retriever is positioned across the right coronary artery thrombus.
Moving image 30. Retraction of the Trevo stent retriever under manual aspiration on the guide catheter.
Moving image 31-32. Final right coronary artery angiography.

