Abstract
Background: There is no established technique for managing large thrombus burden (LTB) in patients with acute coronary syndrome (ACS).
Aims: The aim of this study was to assess the safety and efficacy of the NeVa (Vesalio) mechanical thrombectomy device (MTD) in ACS patients with LTB.
Methods: Consecutive patients with ACS and LTB were treated with the NeVa MTD as the primary vessel recanalisation and thrombus removal modality, followed by conventional intervention. We further developed a bench model and applied to a subset of patients, a vacuum-assisted aspiration technique, exploiting 6 Fr-compatible conventional guiding catheter extensions, as an adjudicative manoeuvre to the use of stent-based MTD. A core laboratory reviewed the angiographic images for procedural complications, Thrombolysis In Myocardial Infarction (TIMI) flow, myocardial blush grade (MBG) and TIMI thrombus grade (TTG).
Results: Between November 2019 and March 2021, 61 patients underwent thrombectomy with the NeVa device. Non-flow limiting and reversible coronary spasm occurred in 14 (23%) patients. One patient (#10) suffered from side branch embolisation, which was successfully treated with the NeVa, triggering the development of a vacuum-assisted aspiration technique in a bench model, which was then applied to the subsequent 51 patients. No other device-related complications occurred. After NeVa use, TIMI flow <3 decreased from 68.3% at baseline to 10.3% (p<0.001), MBG <2 from 65% to 27.6% (p<0.001), TTG ≥3 from 96.7% to 43.2% (p<0.001), respectively.
Conclusions: In patients with LTB, the NeVa MTD was safe and associated with high rates of vessel recanalisation and thrombus removal. The concomitant use of vacuum-assisted aspiration has potential to improve the effectiveness and safety of the technique.
Introduction
Large thrombus burden (LTB) can be found in up to 60% of patients with acute coronary syndrome (ACS)1 and in up to 75% of patients presenting with ST-elevation myocardial infarction (STEMI). It confers a twofold greater risk of mortality and from two to fourfold greater risk of major adverse cardiovascular events2.
Several thrombectomy devices and techniques have been investigated though none have been shown to improve outcomes3. The initial benefit of routine manual thrombus aspiration was not confirmed in subsequent multicentre trials45 and one study raised concerns about a possible increase in stroke rates56. Therefore, routine thrombus aspiration is not recommended7.
Stent-based mechanical thrombectomy devices (MTD), also called stent retrievers, are recoverable self-expanding nitinol stent-based devices developed to achieve immediate flow restoration upon device deployment, allowing thereafter the capture and clearance of the target thrombus for stroke intervention. The use of these devices as an alternative to, or preferably in association with, continuous aspiration techniques to avoid distal or side branch thrombotic debris embolisation and maximise thrombus extraction has become standard for stroke intervention8.
A few case reports have described the bail-out use of the Solitaire Platinum (Medtronic), without concomitant aspiration, in selected patients with large coronary thrombus after failed conventional treatment910.
The NeVa stent (Vesalio) is an MTD which has been designed to improve the incorporation and removal of organised thrombi. It showed a higher percentage of recanalisation after a single stent passage compared with other commercially available MTDs for stroke intervention1112. This device has been CE marked for coronary interventions since December 2019.
We conducted the first-in-human coronary use of the NeVa MTD in a consecutive series of ACS patients with LTB and developed a technique for continuous aspiration in a bench model using 6 Fr-compatible conventional guiding catheter extensions.
Methods
Bench model
A bench model was developed to investigate commercially available guiding catheter extensions as intermediate catheters located at the site of thrombotic occlusion to generate negative pressure for aspiration during NeVa MTD removal.
Two contiguous tanks (88 x 88 x 65 mm) were connected by waterproof Tuohy-Borst valves and filled with deionized water. The tip of a guiding catheter (Launcher 6 Fr; Medtronic) was kept in Tank I or advanced into Tank II according to the tested configuration (Figure 1A). The guiding catheter extension’s tip (Guidezilla II, inner diameter 0.057 inches; Boston Scientific) protruded into a 2.5 mm internal diameter polyvinyl chloride (PVC) tube, which was placed in Tank II and mimicked a coronary vessel (Figure 1B). A collateral pressure transducer (TruWave; Edwards LifeSciences) was placed in the proximity of the first dropping zone of the NeVa MTD. VacLok 60 ml vacuum pressure syringes (Merit Medical) were connected to the proximal extremity of the guiding catheter through the side port of an Honor Hemostasis Valve (also Merit Medical). Four different system configurations were analysed (Figure 1C). Every configuration was serially tested, applying one, two or three 60 ml VacLok vacuum pressure syringes, to assess suction as a function of the number of aspirating syringes. A pressure/time curve was recorded during each aspiration test, and the following parameters were measured:
– VolH2O (ml): water volume in VacLok syringe(s) at the end of aspiration;
– duration (sec): time lapse from the beginning to the end of aspiration;
– peak pressure (mmHg): maximal vacuum at the tip of the catheter;
– flow rate (ml/sec): VolH2O (ml)/duration (sec) ratio, to describe the average flow rate inside the aspiration system.
Each measure was repeated three times to analyse the measurements’ reproducibility.
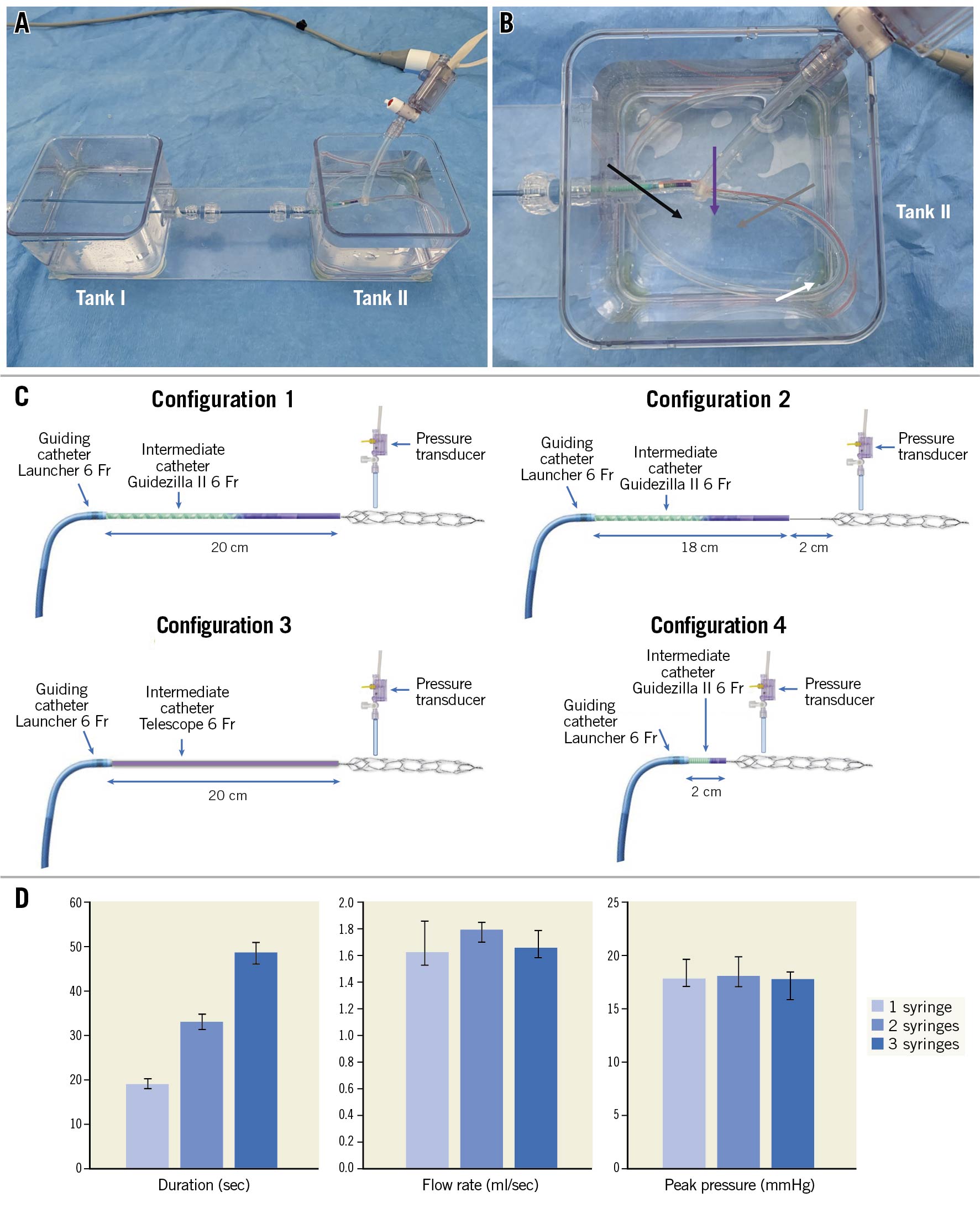
Figure 1. Bench model. A) Overall view: two tanks (I and II) are crossed by the tested catheters through a series of haemostatic valves. B) Detail of tank I: the tip of the intermediate catheter (black arrow) lies at the beginning of the coronary PVC tube (white arrow). The NeVa mechanical thrombectomy device (grey arrow) protrudes from the intermediate catheter into the same coronary tube. A collateral transducer measures pressures in the proximity of the NeVa first dropping zone (purple arrow). This figure mimics a proximal occlusion (configuration 4) in which also the tip of the guiding catheter is advanced up to tank II. In configurations 1-3 the guiding catheter lies in tank I. C) Schematic reproduction of the four configurations of the bench model. Configuration 1 modelled a maximised intermediate catheter protrusion (20 cm) simulating extremely distal occlusion sites, with the distal end adjacent to the NeVa stent. In configuration 2 the intermediate catheter was partially withdrawn 2 cm proximally (18 vs 20 cm) but leaving the NeVa stent in place, thus simulating the effect of a 2 cm gap between the intermediate/aspiration catheter and the NeVa stent. Configuration 3 reproduces configuration 1, except that the intermediate catheter was the Telescope; inner diameter 0.056 inches, (Medtronic). Finally, configuration 4 simulates a proximal occlusion site: the intermediate catheter protrudes only 2 cm from the guiding catheter and it is close to NeVa proximal edge. In every configuration, the pressure sensor overlaid the NeVa stent’s first dropping zone, where the clot is supposed to be engaged. D) Median values (and IQR) of the pressure/time parameters obtained from all four configurations according to the number of aspirating syringes. Increasing the number of syringes from 1 to 3 resulted in progressively more prolonged aspiration time (duration), whereas no relevant differences in maximal vacuum and flow rate were observed.
Population, event adjudication and core laboratory assessment
We included patients presenting with ACS and LTB, defined as Thrombolyis In Myocardial Infarction (TIMI) thrombus grade (TTG) ≥3 at coronary angiography according to operator judgement, who underwent treatment with the NeVa MTD as the primary approach. No anatomical or clinical exclusion criteria were applied. Except for primary use of the NeVa MTD, patients thereafter underwent standard PCI and received pharmacologic treatment according to international guidelines. The study was conducted at two centres in Switzerland (Cardiocentro Ticino Institute and Bern University Hospital). All patients gave informed consent, and the protocol was approved by both institutional ethics committees.
Angiographic images were analysed by an independent core laboratory (MedStar Washington Hospital Center, Washington, DC, USA), which assessed the occurrence of procedural angiographic complications and TIMI flow, myocardial blush grade (MBG) and TTG at baseline, after wire insertion, after treatment with the NeVa and at the end of the intervention. All patients were followed up to 30 days for the assessment of cardiac or cerebrovascular ischaemic events or bleeding. All clinical events were adjudicated against original source documents by one trained physician and verification was carried out by a second; disagreements were resolved through third-party adjudication.
Use of the NeVa mechanical thrombectomy device in the coronary arteries
The NeVa MTD is a self-expanding nitinol stent attached to a push wire (Supplementary Figure 1), which is temporarily inserted into the vasculature to restore blood flow upon stent deployment. It is available in different designs and sizes (Supplementary Table 1) and engineered with one or more dropping zones which are large, open pockets, offset at 90° to one another, serving as entry points for capturing thrombi inside the NeVa’s basket (Supplementary Figure 1).
Firstly we placed a standard 0.014” coronary guidewire distal to the occlusion/thrombotic site. This wire was left in place during the thrombectomy, allowing immediate access to the infarcted vessel after MTD removal, whereas over a second 0.014” coronary guidewire, a Rebar-18, 160 mm, microcatheter (Medtronic), was inserted (parallel wire technique) (Figure 2A). This is at variance with the standard technique used for stroke intervention in which a single wire is used to cross the occlusion site and the same wire used to deliver the microcatheter so that no additional wire is left in place after MTD removal. This technique modification was deemed necessary, anticipating the need of stent deployment after thrombus removal in coronary intervention, where plaque rupture or erosion is the leading cause of vessel occlusion rather than thrombotic emboli, as in stroke. The parallel wire technique concomitantly helps to avoid deep intubation of the guiding catheter during MTD removal. After positioning the microcatheter distal to the occlusion/thrombotic site, the guidewire was withdrawn and the NeVa MTD, selected based on the vessel diameter (typically oversized by at least 50%) and estimated occlusion/thrombotic length, was advanced into the microcatheter until its distal markers lined up with the end of the catheter (Figure 2B). The NeVa MTD was deployed by fixing the push wire in place and withdrawing the microcatheter in the proximal direction until complete removal (bare wire technique) (Figure 2C). For the first set of cases, the NeVa MTD was then gently withdrawn into the guiding catheter, making sure the guiding catheter’s tip was well engaged in the coronary ostium to avoid peripheral embolisation. After a case of embolisation occurred, from the first marginal branch to mid-circumflex artery during MTD removal, this manoeuvre was performed under continuous aspiration, achieved with one and up to 3 VacLok 60 ml vacuum pressure syringes connected at the side port of the guiding catheter haemostatic valve. In cases in which the occlusion/thrombotic site was proximal and the anatomy amenable to deep intubation (e.g., proximal right coronary or proximal left anterior descending arteries), the guiding catheter was advanced as close as possible to the thrombotic occlusion and no guiding catheter extension was used, so that aspiration could occur directly at the tip of the guiding catheter, leveraging a bigger lumen diameter and higher aspiration forces. In cases with more unfavourable anatomy (occlusion at distal sites or in the circumflex artery), a 6 Fr compatible guiding catheter extension was used as an intermediate catheter and inserted after MTD deployment by introducing the MTD push wire and the first 0.014” coronary guidewire into its lumen and advancing it in close proximity to the MTD (Figure 2C). During the simultaneous removal of the guiding catheter extension and the NeVa stent, the initial 0.014” coronary guidewire was kept in place for subsequent treatment (Figure 2D).
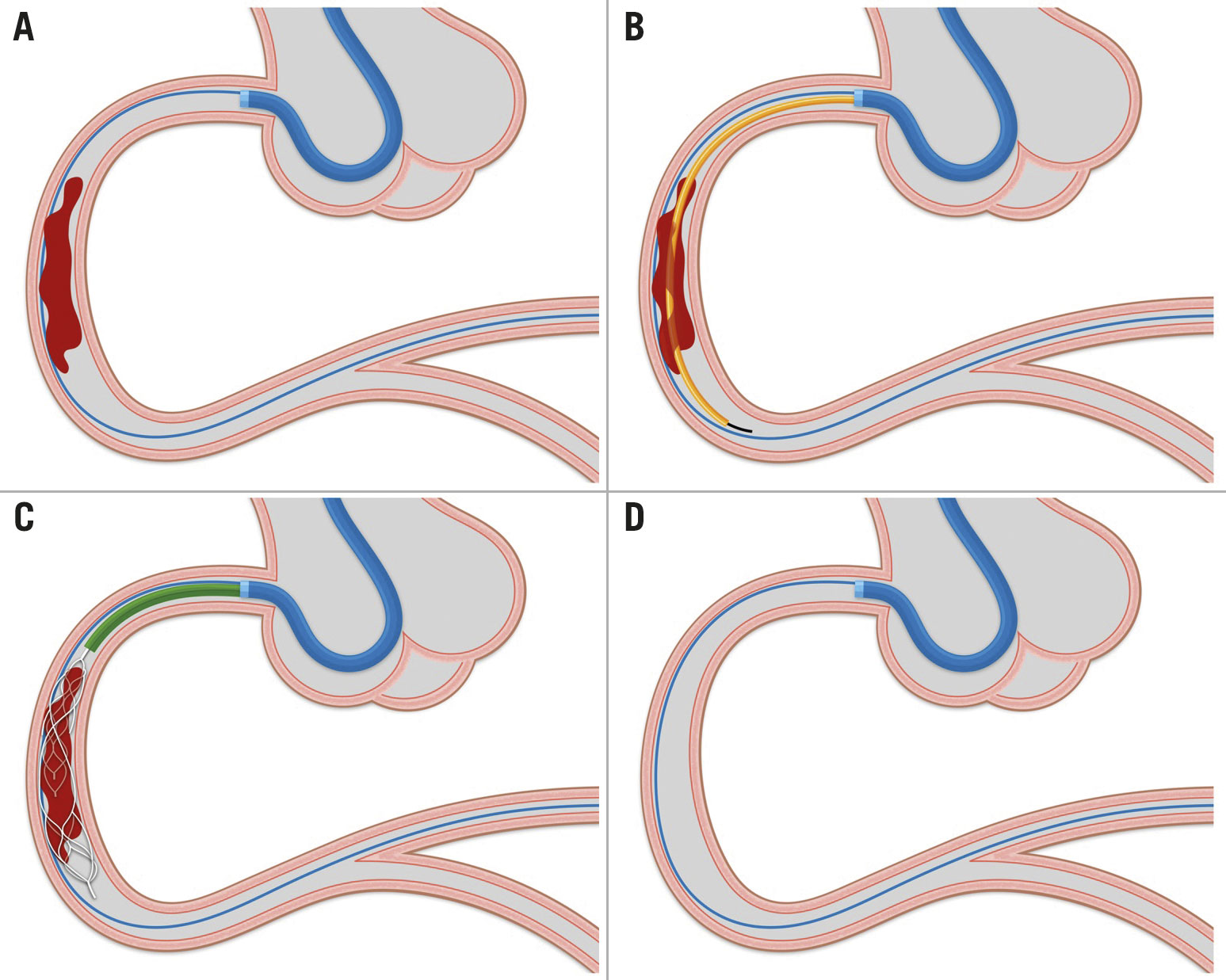
Figure 2. Procedural steps of thrombectomy with the NeVa MTD. A) Insertion of a coronary guidewire distal to the occlusion/thrombotic site. B) Positioning of the microcatheter distal to the occlusion/thrombotic site using a second coronary guidewire. C) Deployment of NeVa MTD at site of thrombus and insertion of a guiding catheter extension (intermediate catheter). D) Removal of the guiding catheter extension and NeVa MTD. See Methods section for a detailed explanation.
Endpoints
Safety outcomes were: 1) complications occurring during the PCI, either angiographic (coronary dissection, perforation, occlusion, spasm, embolisation) or clinical (cardiac tamponade, arrhythmias needing treatment), and 2) cardiovascular adverse events at 30 days (death, myocardial infarction, unplanned coronary revascularisation, definite stent thrombosis, stroke, bleeding according to the Bleeding Academic Research Consortium [BARC] classification, cardiac arrest, hospitalisation for cardiac causes).
Efficacy outcomes were TIMI flow, MBG and TTG after NeVa MTD use. Resolution of ST-elevation on 12-lead electrocardiogram (ECG) was assessed at the end of PCI in patients presenting with STEMI.
Procedural complications and cardiovascular events were defined in accordance with the Academic Research Consortium (ARC) standardised definitions13. Definition of TIMI flow, MBG and TTG are available in Supplementary Table 2.
Statistical analysis
Continuous variables were expressed as median and interquartile ranges and categorical variables as counts and percentages. TIMI flow grade, MBG and TTG at different time points were compared using a Wilcoxon signed-rank test. Logistic regression was used to find predictors of TTG ≤2 after NeVa use and of TTG reduction of at least 2 degrees after NeVa use. The following variables were retained in the final model: age, sex, time from symptom onset to PCI, TTG at baseline, ST-elevation on ECG, culprit vessel right coronary artery (RCA).
Results
Bench test
When the guiding catheter’s tip was kept in Tank I and the guiding catheter extension in Tank II, aspiration produced a fluid level only in the latter while Tank I remained dry.
For every configuration, increasing the number of aspirating syringes from one to three resulted in a greater aspiration duration (19 seconds [IQR 19-20] vs 33 seconds [IQR 30-35] vs 49 seconds [IQR 46-53] with one, two or three syringes, respectively), whereas maximal vacuum and flow rate remained stable (Figure 1D, Supplementary Table 3). At Configuration 2 with a single aspirating syringe, a slightly lower vacuum was measured at the first dropping zone in comparison to Configuration 1 (16.6 mmHg [IQR 15.8-17.4] vs 18.9 mmHg [IQR 17.3-19.0]: absolute difference 2.3 mmHg, relative difference 12.2%). However, the vacuum drop in Configuration 2 was minimised by increasing the number of syringes (relative reduction -12.2% vs 6.1% vs. 1.1% with one, two or three VacLoks, respectively) (Supplementary Table 3).
No significant differences were noted when the Telescope (Medtronic) was used instead of the Guidezilla II (Boston Scientific). Finally, in Configuration 4, a proximal occlusion site resulted in slightly higher vacuum (20.1 mmHg [IQR 20.0-20.9] vs 17.6 mmHg [IQR 16.8-18.8] with three syringes) and flow rates (1.80 ml/sec [IQR 1.78-2.0] vs 1.66 ml/sec [IQR 1.45-1.77]), whereas aspiration duration did not differ (Supplementary Table 3).
Patient and procedural characteristics
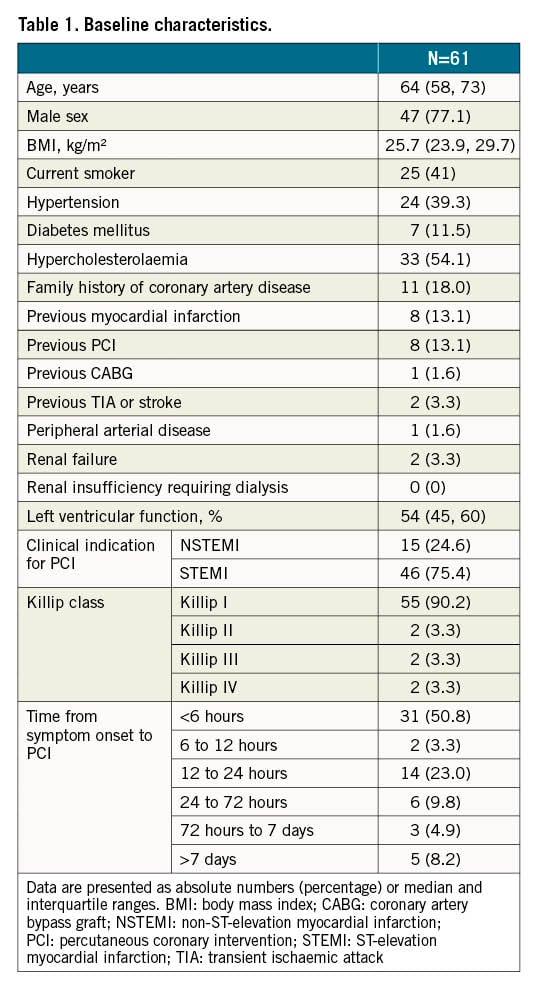
Between November 2019 and March 2021, 61 patients presenting with an ACS and LTB at coronary angiography were treated with the NeVa MTD. Median age was 64 years, 47 (77.1%) patients were male, eight (13.1%) had a history of myocardial infarction, eight (13.1%) had a previous PCI and one (1.6%) had a previous coronary artery bypass graft (CABG). At admission, 46 (75.4%) patients had ST-elevation on ECG and two (3.3%) were in Killip Class IV (Table 1). Culprit lesion location is shown in Table 2.
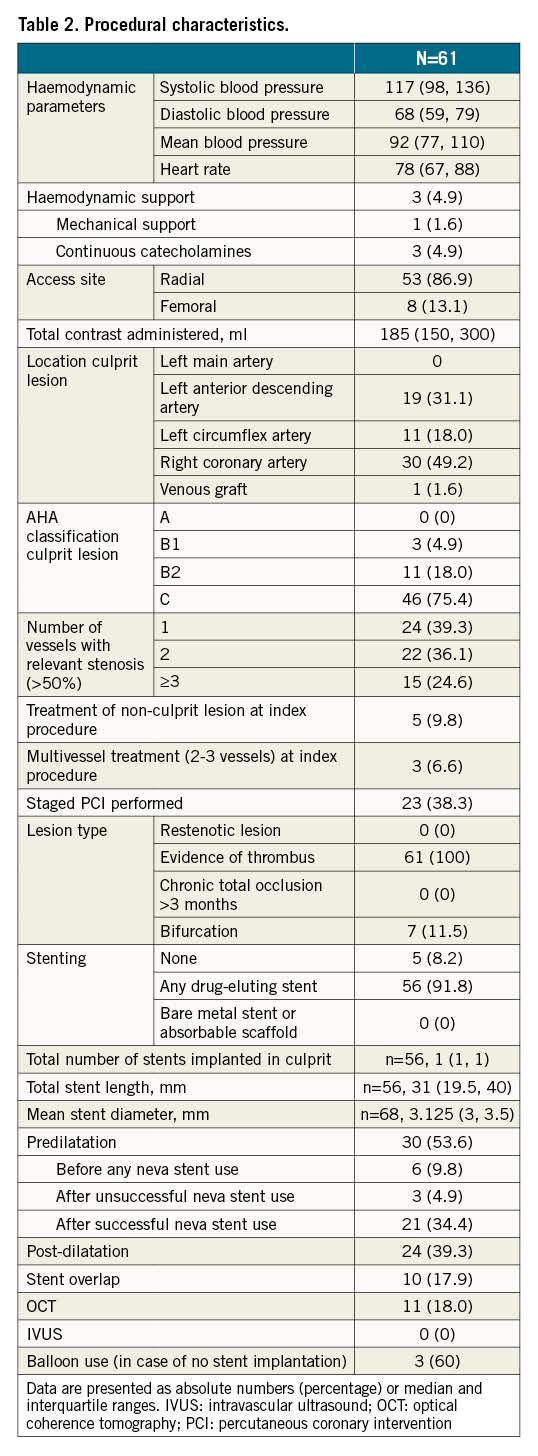
Forty-three patients (70.5%) received a single NeVa passage whereas in the remaining patients, two or more passages were deemed necessary with the same device in all but one patient, in whom a 4.5×37 mm NeVa was used for the mid-right coronary artery and a 4x30 mm was used to remove a previously embolised thrombus in the posterolateral branch. The most frequently used stent size was 4.5×37 mm (n=41, 67.2%), followed by 4x30 mm (n=9, 14.8%) and 4.5×44 mm (n=6, 9.8%) (Central illustration). The NeVa stent diameter, as compared to the target reference vessel at baseline quantitative coronary angiography (QCA), was on average 60.9% greater (Supplementary Table 4). Concomitant continuous aspiration was not performed for the first 10 patients but was systematically performed thereafter, following a case of side branch embolisation which occurred during NeVa retrieval (see text below and Figure 3). Aspiration was performed in the consecutive 51 (84%) patients, with a single VacLok syringe in 10 (20%), two syringes in 18 (35%) and three syringes in 23 patients (45%). A guiding catheter extension for continuous aspiration at the site of occlusion/thrombosis was used in 28 (55%) patients (Central illustration). In 32 patients (53%) a macroscopic thrombus was found in the NeVa MTD. Predilatation before NeVa use was performed in nine (14.7%) patients (in six [9.8%] patients in whom TIMI 0 persisted after wire insertion in order to restore flow immediately, per operator choice, before attempting to use the NeVa; in three [4.9%] patients after failed passage of the microcatheter in whom the NeVa was subsequently successfully deployed). Fifty-six patients (91.8%) underwent stent implantation (Table 2), of whom 29 (51.8%) received direct stenting. Glycoprotein IIb/IIIa inhibitors were used in nine patients (14.8%). Medications at discharge are shown in Supplementary Table 5.
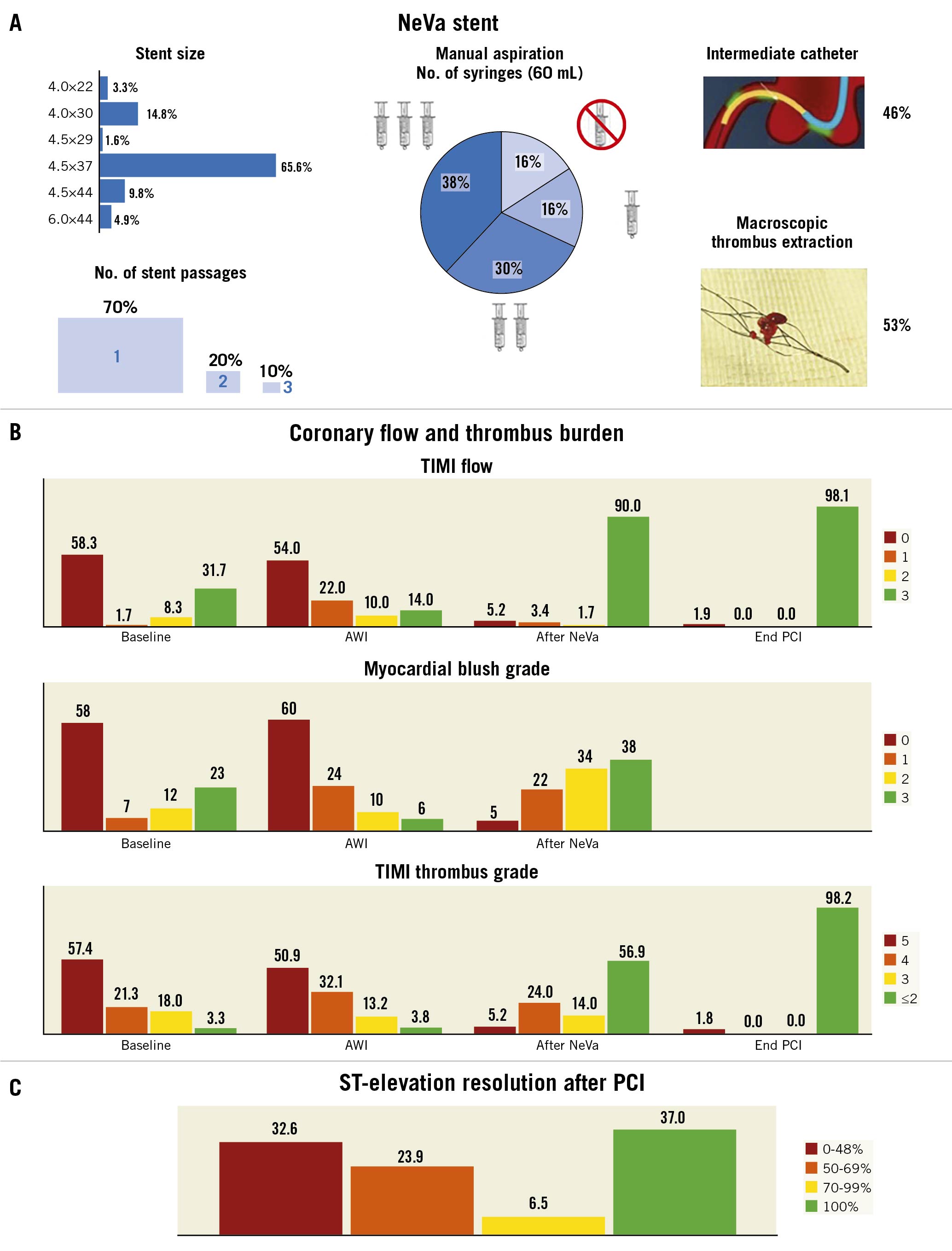
Central illustration. NeVa stent use and procedural efficacy outcomes. A) Procedural details on thrombectomy with the NeVa stent. B) TIMI flow, myocardial blush grade, TIMI thrombus grade at baseline, after wire insertion (AWI), after NeVa use, and at the end of PCI. C) ST-elevation resolution in 46 STEMI patients after PCI. Data are represented as percentages. †Wilcoxon signed-rank test. PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; TIMI: Thrombolysis In Myocardial Infarction
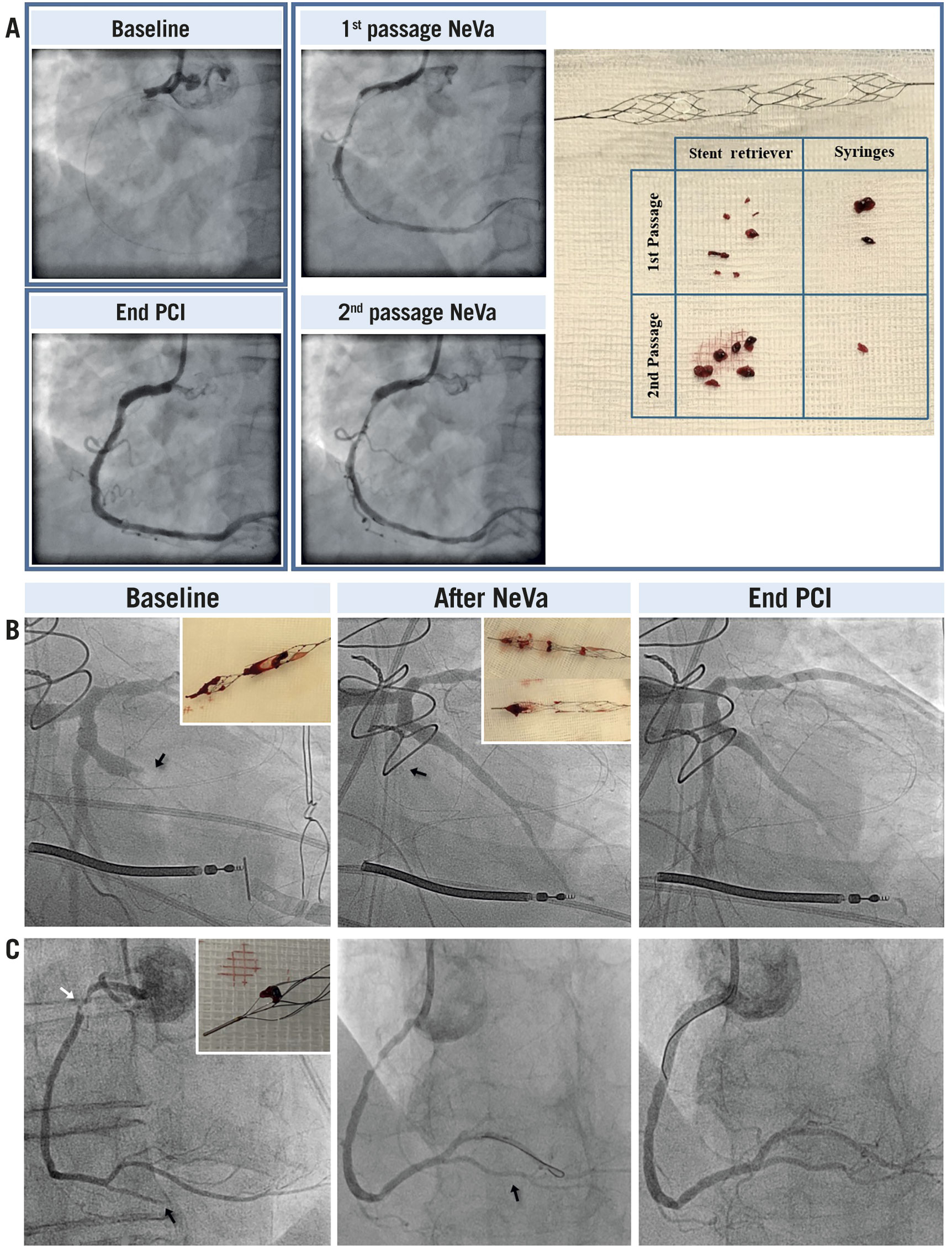
Figure 3. Angiographic results and macroscopic thrombus extracted in patients treated with the NeVa mechanical thrombectomy device. A) A 62-year-old man with STEMI and thrombotic occlusion of the proximal RCA. The patient was treated with two passages of the NeVa stent and then conventional PCI with stent implantation. A massive amount of thrombus was found in the NeVa stent and in the syringes used for manual aspiration. B) A 60-year-old man with NSTEMI three days after surgical replacement of the mitral valve and PFO closure. The three pictures from left to right show: the occluded first obtuse marginal branch of the LCX; the occlusion of the LCX through some thrombus fragments migrated during the withdrawal of the NeVa stent; the successful removal of thrombus from the LCX through a second employment of the NeVa stent. No stent implantation was needed. C) An 87-year-old woman with STEMI. The three pictures from left to right show: a thrombotic lesion in the proximal RCA (white arrow) and a distal embolisation, resulting in complete occlusion of the posterior descending artery (PDA) (black arrow); result after thrombectomy with the NeVa stent, showing complete restoration of flow and no residual thrombus (black arrow); final results at the end of PCI after stent implantation in the proximal RCA and in the PDA. LCX: left circumflex artery; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; PFO: patent foramen ovale; RCA: right coronary artery; STEMI: ST-elevation myocardial infarction
Safety outcomes up to 30 days
Non-flow-limiting coronary spasm occurred in 14 (23%) patients after NeVa retrieval and disappeared in all after intracoronary nitrate or spontaneously. In a single case, in which no continuous aspiration was performed, a side branch embolisation and subsequent occlusion of the mid-circumflex artery occurred during NeVa retrieval from the first obtuse marginal; recanalisation of the circumflex artery was achieved with two additional passages of the NeVa MTD (Figure 3). No cases of coronary dissection, perforation, cardiac tamponade, or life-threatening arrhythmias were observed (Table 3).
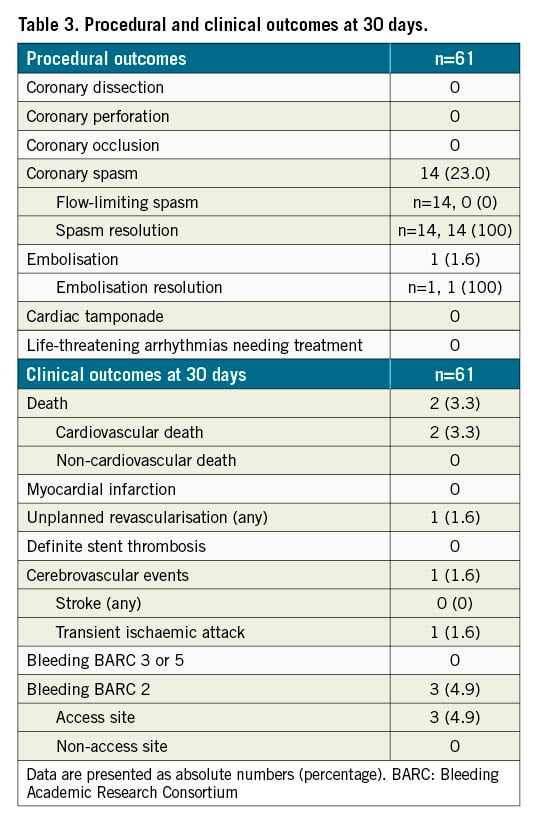
Two female patients (3.3%), with subacute STEMI (symptom onset >24 hrs before admission) in cardiogenic shock at admission died shortly after the procedure (which was unsuccessful in restoring coronary flow). One patient (1.6%) underwent unplanned revascularisation; at the time of a staged PCI, a stent implanted during the index procedure was deemed suboptimally expanded and with some traces of non-occlusive thrombus, and underwent post-dilatation.
One patient (1.6%) had a transient ischaemic attack at day 29, immediately after a staged PCI, in which the NeVa MTD was not used. No patient was hospitalised because of cardiac causes or suffered from cardiac arrest. Three patients (4.9%) had access-site related BARC 2 bleeding (Table 3).
Efficacy outcomes
At baseline, a TIMI flow <3 in the culprit lesion was present in 68.3% of the cases and decreased to 10.3% after NeVa use (p-value <0.001) and to 1.9% at the end of the procedure (p-value <0.001). MBG 0 or 1 was detected in 65% of patients at baseline and in 27% after NeVa use (p<0.001). TIMI thrombus grade ≥3 was confirmed in 96.7% of the patients at baseline and decreased to 43.2% after NeVa use (p<0.001) and to 1.8% after PCI (p<0.001) (Central illustration). The strongest predictors of TTG <2 after NeVa use or a TTG reduction ≥2 (difference between TTG at baseline and after NeVa use) were lower pre-PCI TTG and a longer interval between symptom onset and PCI (Supplementary Table 6).
ST-elevation resolution (≥50%) occurred in 33 out of 46 STEMI patients (71.7%) and it was complete (≥70%) in 20 patients (43.5%) (Central illustration). TIMI flow, MBG, and TTG are provided in the Supplementary Figure 2. Visual angiographic results of a few representative cases are available in Figure 3 and Supplementary Figure 3.
Discussion
The results of our bench study are as follows:
– The bench model proved the suitability of guiding catheter extensions as aspiration tools. No fluid aspiration occurred from the guiding catheter itself when the extension catheter was inserted. The vacuum pressure applied via VacLok syringes was directly measurable at the tip of guiding catheter extensions.
– The vacuum for extremely distal lesions was only minimally reduced compared with proximal locations and this minor drop was minimised by three VacLok syringes, which, in turn, permitted a remarkable prolongation of aspiration duration.
– The small differences in inner diameters among commercially available guiding catheter extensions are unlikely to impact on aspiration performances.
The main findings of the clinical study are as follows:
1) The use of the NeVa MTD for LTB was safe and was not associated with relevant procedural complications, including vessel dissection or perforation. The most common device-related adverse event was coronary spasm, observed in almost a quarter of the patients. This was never flow limiting and resolved spontaneously or after nitrate injection.
2) A single case of side branch embolisation occurred. This patient suffered from an occlusion of an ectatic marginal branch after surgical mitral intervention and PFO closure and was most likely due to thrombus embolisation considering that no flow limiting lesion was observed after thrombus removal. After the first MTD retrieval, a large amount of thrombotic material was observed in the NeVa MTD and a TIMI 3 flow with no residual thrombus at angiography was noted in the target vessel. However, during a MTD retrieval unprotected by concomitant aspiration, embolisation and occlusion of the mid-circumflex artery occurred, which was successfully treated with two additional NeVa passages. No further episode of thrombus embolisation occurred in the subsequent 51 cases performed with concomitant aspiration through the guiding catheter or the guiding catheter extension.
3) NeVa stent deployment was associated with immediate reperfusion in 85% and TIMI 3 flow in 74% of the patients with TIMI 0 after wire insertion. The use of the NeVa MTD retrieved macroscopic thrombotic material in slightly more than 50% of the cases and decreased the angiographic thrombus burden to ≤2 in 60% of the patients.
4) Among STEMI patients, ST-elevation resolution after PCI occurred in 71.7% and was complete in 43.5% of the patients.
The use of MTDs has become routine practice for stroke intervention. In this setting, the use of a large bore balloon guide catheter or continuous aspiration by means of an intermediate 5 or 6 Fr catheter in combination with MTD, also called stent retriever technologies, has proved to be the most effective way for vessel recanalisation while avoiding distal embolisation14.
We have developed a coronary adaptation and simplification of the Stent retriever Assisted Vacuum-locked Extraction (SAVE) technique15, which results in high rates of first-pass reperfusion for stroke intervention. The proposed approach for clot retrieval combines a distally placed stent-based MTD and a proximally placed aspiration catheter which act as a unit, offering a distal (stent) and proximal capture of the clot (aspiration catheter) while being withdrawn simultaneously under continuous proximal aspiration into the guiding catheter. The SAVE technique, as well as many other aspiration techniques for stroke intervention, uses long intermediate 5 or 6 Fr catheters to be inserted inside an 8 Fr guiding catheter, which typically has a pre-assembled child-mother configuration.
Guiding catheter extensions have been developed for complex coronary intervention, allowing stent delivery in difficult coronary anatomies. In this study, we showed that they can also be used for direct aspiration during MTD removal and that simultaneous aspiration with three VacLok syringes optimises aspiration duration and, to a lesser extent, maximum vacuum, especially for the treatment of distal lesions. Moreover, the use of guiding catheter extensions, unlike the pre-assembled child-mother configuration, allows leaving a coronary wire in place during the removal of the MTD, with direct access to the infarcted vessel after thrombus removal for stent implantation, if needed.
The NeVa MTD mimics other conventional stent-retriever technologies but carries unique features which have been engineered for maximising first pass thrombus extraction, such as the presence of the dropping zones. Few case reports exist on the use of the Solitaire revascularisation device in coronary cases910. This is the first series of patients with coronary LTB in whom the current conventional stroke intervention technique is adapted and embedded into standard coronary care in patients with LTB. Our first-in-human study on the use of the NeVa MTD for patients with LTB provides reassuring safety data on this device for use in coronary intervention. Our findings support the potential efficacy of the NeVa as a primary tool for vessel recanalisation in combination with proximal aspiration in LTB patients. This new approach was associated with a high rate of flow restoration, with relatively little need of lesion predilatation after use of the NeVa MTD, including in patients with persistent vessel occlusion after wire insertion, who are at high risk of suboptimal results and poor outcomes16. A macroscopic thrombus was found in the NeVa MTD in 53% of patients. The blood aspirated during the concomitant manual aspiration was not examined for thrombotic material. Therefore, the overall rate of macroscopic thrombus extracted may have been underestimated.
These favourable feasibility and safety data have prompted the conduct of the NeVa Thrombectomy device as Adjunctive Reperfusion Modality in the ST-segment Elevation Myocardial Infarction (NATURE) randomised trial (ClinicalTrials.gov: NCT04969471) to assess further this new reperfusion treatment modality against conventional treatment in patients with LTB.
Limitations
This study is a single-arm study, with inherent limitations. The low number of patients and the absence of a control group limit any conclusion about the safety and efficacy of this device as compared to standard PCI. Therefore, our results remain exploratory. Nevertheless, the independent angiographic assessment provided by an external core laboratory minimises the risk of bias caused by operator/investigator self-assessment.
Conclusion
In this first-in-human study, the NeVa MTD in combination with aspiration through the guiding catheter or a guiding catheter extension, proved safe and effective in removing coronary thrombus and allowed immediate prompt restoration of flow in a high proportion of patients with ACS and LTB. A randomised study to assess the comparative safety and effectiveness of this new treatment modality in addition to standard intervention among patients with STEMI and LTB is underway.
Impact on daily practice
Large thrombus burden is a frequent finding in patients with acute coronary syndrome and is associated with worse prognosis. To date, no thrombectomy device or technique has been shown to improve outcomes in patients with large thrombus burden. In this observational first-in-human study, the use of the NeVa mechanical thrombectomy device with concomitant manual aspiration through the guiding catheter or guiding catheter extension proved safe and effective in removing coronary thrombus. A randomised study is planned to assess further the comparative safety and effectiveness of this new treatment modality followed by standard percutaneous coronary intervention as compared with standard percutaneous coronary intervention alone.
Funding
This work was entirely supported by Cardiocentro Ticino Institute, Lugano and Inselspital, Bern, Switzerland and received no extramural funding. Vesalio supplied the study material for free to participating sites but had no role in the data collection, interpretation, analysis, in the preparation of the manuscript, or in the decision to submit the manuscript for publication.
Acknowledgements
We wish to thank Augusto Spirito for his support in the preparation of figures.
Conflict of interest statement
M. Valgimigli reports grants and personal fees from Terumo, personal fees from AstraZeneca, Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Bayer, CoreFlow, Idorsia Pharmaceuticals Ltd, Universität Basel/Dept. Klinische Forschung, Vifor, Bristol Myers Squibb SA, Biotronik, Boston Scientific, Medtronic, Vesalio, Novartis, Chiesi, and PhaseBio outside the submitted work. H.M. Garcia-Garcia reports the following institutional grant support: Biotronik, Boston Scientific, Medtronic, Abbott, Neovasc, Shockwave, Philips and CoreFlow outside the submitted work. A. Spirito declares grants/contracts from the Swiss National Science Foundation. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

