Abstract
Background: The DurAVR transcatheter heart valve (THV) is a novel biomimetic balloon-expandable valve with promising early clinical results.
Aims: We aimed to assess the hydrodynamic performance of the DurAVR THV in native, valve-in-valve (ViV), and redo-transcatheter aortic valve implantation (TAVI) procedures against commercially available THVs on the bench.
Methods: The hydrodynamic function of the DurAVR THV was assessed by simulating native valve deployments at 0 mm, 3 mm, and 6 mm depths, compared to SAPIEN 3 (S3), Evolut PRO, Navitor, and ACURATE neo2 (ACn2) valves. For ViV simulations, THVs were implanted in 21 mm and 23 mm Magna Ease, Mosaic, and Hancock bioprostheses. For redo-TAVI simulations, the DurAVR THV was assessed within S3, Evolut PRO, Navitor, and ACn2 valves.
Results: For native TAVI simulations, the DurAVR THV demonstrated superior or comparable hydrodynamic performance, independent of implant depth, with an effective orifice area (EOA) ≥3 cm2 and a mean gradient (MG) <6 mmHg. The DurAVR THV had nil to mild pinwheeling (0-2%) at all depths, while the S3 and Evolut PRO showed moderate pinwheeling at 6 mm depth. For ViV simulations, the DurAVR THV exhibited larger EOAs and lower MGs than the comparator THVs and showed no more than mild pinwheeling in all ViV configurations. For redo-TAVI simulations, the DurAVR THV exhibited larger EOAs and lower MGs in each simulation compared to all other THVs tested, with no more than mild pinwheeling observed in all configurations except when implanted within the Evolut PRO.
Conclusions: In this bench study, the DurAVR THV demonstrated excellent hydrodynamic performance in native, ViV, and redo-TAVI simulations. Future large-scale studies are needed to confirm these findings in clinical application and further characterise the valve’s short- and long-term performance.
The use of transcatheter aortic valve implantation (TAVI) is now transitioning towards a younger patient population with greater anticipated longevity1. Thus, there is an increasing need for devices with optimal haemodynamic performance in both the short and long term. One of the main challenges for current and future transcatheter heart valve (THV) platforms is to engineer leaflets resistant to different modes of degeneration2. Post-TAVI thrombosis is poorly understood, and one mechanism may be related to prosthesis expansion and flow within the neosinuses34. Therefore, a THV that functions well across a wide range of deployment configurations and/or restores near-physiological flow may be advantageous and help improve long-term durability.
Presently, operators can choose between short-frame balloon-expandable (BE) and tall-frame self-expanding (SE) THVs. SE valves may have a better haemodynamic profile, particularly in the context of a small annulus, valve-in-valve (ViV) TAVI, or redo-TAVI, even if the impact on hard clinical endpoints and durability is debated567. Conversely, BE THVs are associated with lower pacemaker rates and improved coronary access in clinical practice8. On the bench, BE valves are generally associated with a higher degree of pinwheeling, a phenomenon that is potentially linked to reduced leaflet durability910. Thus, a short-frame BE THV with little to no pinwheeling and improved haemodynamics would be of great interest.
The recently developed DurAVR system (Anteris Technologies) is a TAVI platform built around a biomimetic BE THV with a single-piece, moulded bovine pericardial tissue leaflet intended to mimic the shape of the native aortic valve. The first-in-human implantations of the DurAVR valve have been performed successfully, with excellent early clinical outcomes and restoration of near-physiological aortic flow11. While clinical data on the DurAVR THV is growing, additional insight is needed. Therefore, to further evaluate the capabilities of this novel BE THV, we performed an in vitro bench study to assess the performance of the valve in three different important procedural settings: native valve TAVI, ViV TAVI, and redo-TAVI, in comparison to other commercially available THV devices.
Methods
Objectives
The present study had the following objectives:
1) To assess the hydrodynamic function of the DurAVR THV on the bench at three different depths (0 mm, 3 mm, and 6 mm) and compare this to four commercially available THV platforms: SAPIEN 3 (S3 [Edwards Lifesciences]), Evolut PRO (Medtronic), Navitor (Abbott), and ACURATE neo2 (ACn2 [Boston Scientific]).
2) To evaluate the hydrodynamic function of the DurAVR THV when used in a ViV context within different types of surgical bioprostheses (Magna Ease [Edwards Lifesciences], Mosaic [Medtronic], and Hancock [Medtronic]) and compare this to four commercially available THV platforms (S3, Evolut PRO, Navitor, ACn2).
3) To evaluate the hydrodynamic function of the DurAVR THV when used in a redo-TAVI context within different types of THVs and compare this to the use of other THVs (S3, Evolut PRO, Navitor, ACn2).
Study methods
In vitro testing was performed in collaboration with the Cardiovascular Translational Laboratory (Vancouver, Canada) and Anteris Technologies (Minneapolis, MN, USA). This study was performed under physiological test conditions with no human or animal participants, and thus ethical approval was not required.
Valves
The DurAVR THV is a BE THV, currently available in one size, with a short cobalt-chromium frame and large top cell intended for the treatment of native aortic annuli of 21 mm to 24 mm area-derived diameter, with a valve height of 23 mm. The THV has a trileaflet configuration built from a single piece of moulded bovine pericardium treated with an anticalcification tissue process (ADAPT [Anteris Technologies])12 (Figure 1A).
S3 is a BE THV with a cobalt-chromium frame, trileaflet bovine pericardial tissue valve, and polyethylene terephthalate fabric skirt.
Navitor is an SE THV incorporating a nitinol frame and an active sealing cuff. Unlike other SE THVs, the trileaflet bovine pericardial leaflets are positioned lower in the stent frame in an intra-annular position.
ACn2 incorporates an SE nitinol frame with three large open stabilisation arches, supra-annular trileaflet porcine pericardial leaflets, and a polyester fabric sealing skirt.
Evolut PRO is also designed around an SE nitinol frame with supra-annular trileaflet porcine pericardial leaflets and a porcine pericardial skirt.
The DurAVR valve was assessed in comparison to 23 mm S3, 25 mm Navitor, 23 mm and 26 mm Evolut PRO, and ACn2 THVs. Size selection for the comparators was matched to the size range currently covered by the DurAVR THV. The THVs used in the study are shown in Figure 1B and Figure 1C.
For the ViV component of the study, three types of surgical heart valves (SHVs) were used as the modelled “failed” index valves: 21 mm and 23 mm Magna Ease (true internal diameter [ID] 19 mm and 21 mm, respectively), 21 mm and 23 mm Mosaic (true ID 17 mm and 19 mm, respectively), and 21 mm and 23 mm Hancock (true ID 17 mm and 19 mm, respectively). Size selection for the SHV was based on recommendations from the Aortic ViV app (KRUTSCH). Finally, for the redo-TAVI section of the study, a total of four THVs were used as “failed” index valves: 23 mm S3, 26 mm Evolut PRO, small ACURATE neo2, and 25 mm Navitor.
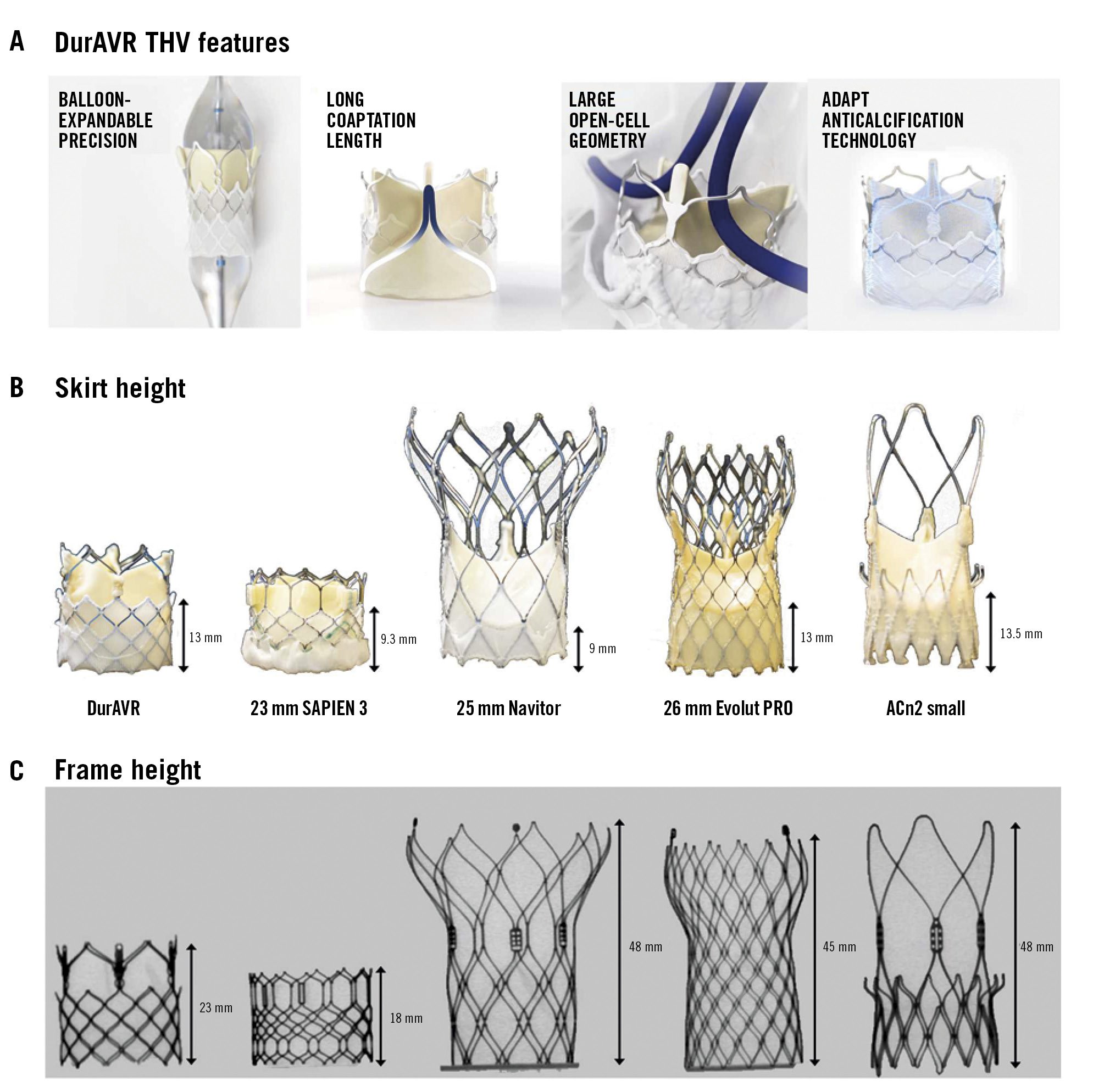
Figure 1. DurAVR THV design analysis in comparison to leading THVs. A) Novel design and components of the DurAVR THV. B) Photographic images of the DurAVR THV and commercially available THVs used comparing skirt heights. C) Fluoroscopy of the DurAVR THV and comparators showing overall valve heights. ACn2: ACURATE neo2; THV: transcatheter heart valve
THV configurations
Supplementary Figure 1 outlines the overall configurations used in testing for native TAVI, ViV TAVI, and redo-TAVI. For native TAVI testing, the DurAVR THV, 23 mm S3, 25 mm Navitor, 26 mm Evolut PRO, and small ACn2 were utilised. For each THV, three different implant depths were used: 0 mm, 3 mm, and 6 mm. For the ViV TAVI testing, the DurAVR THV, 23 mm S3, 23 mm or 26 mm Evolut PRO, 25 mm Navitor, and small ACn2 were used. These THVs were implanted in three types of SHV (Magna Ease, Mosaic, and Hancock) with two sizes for each SHV (21 mm and 23 mm) as described above. This represents a total of 27 ViV configurations, as the Navitor was not tested in the 21 mm SHVs because an appropriately sized Navitor was not available. Only one implant depth of 0 mm was tested for all ViV configurations. Images and fluoroscopy of ViV configurations are shown in Supplementary Figure 2 and Supplementary Figure 3.
Redo-TAVI performance was assessed using the 23 mm S3, 26 mm Evolut PRO, small ACn2 and 25 mm Navitor as the “failed” index THVs. The DurAVR THV and 23 mm S3 were implanted in all redo-TAVI configurations. However, as the implant of a tall-frame THV in a failed tall-frame valve is an unlikely clinical combination, ACn2, Navitor, and Evolut PRO were not assessed in other tall-frame valves and were only evaluated when implanted in S3 THVs. Overall, a total of 11 combinations were tested. For redo-TAVI, when Evolut PRO served as the “failed” index valve, the S3 and DurAVR THV were placed at node 5, as is commonly performed clinically. All deployments were performed in nominal conditions, and images and fluoroscopy of redo-TAVI configurations are shown in Supplementary Figure 4.
Hydrodynamic assessment
A pulse duplicator test system (BDC Laboratories) was used to assess hydrodynamic performance for all TAVI configurations. The THVs, index SHVs and index THVs were placed into a holder fabricated from silicone. The holder compliance was chosen in accordance with International Organization for Standardization (ISO) 5840-3:202113. For assessment, THVs were then directly deployed into holders for TAVI or into index SHVs and index THVs placed in holders for ViV TAVI and redo-TAVI testing, respectively. No post-dilatation was performed.
All testing was completed in a saline solution at 37°C, and results were taken for 20 consecutive cycles, repeated 3 times, and then averaged. High-speed video filming was performed to assess index valve kinematics from the outflow and inflow. Pulsatile forward flow performance was measured at a nominal beat rate of 70±1 beats/min, systolic duration of 35±5%, mean aortic pressure of 100±2 mmHg, and simulated cardiac output of 5±0.1 L/min. The mean gradient (MG; mmHg), regurgitant fraction (%), and effective orifice area (EOA; cm2) were assessed. The maximum allowable regurgitant fraction in accordance with ISO 5840-3:2021 is <20%13. The total regurgitant fraction, which included closing and intervalvular regurgitation, was assessed. The EOA was computed according to a simplified version of the Bernoulli equation, as previously described in ISO 584013. Of note, for ACn2, hydrodynamic data are only available for 0 mm and 3 mm depths due to interaction between the holder and the THV upper crown at 6 mm depth. The opening index was calculated from still imaging taken from the hydrodynamic video and represents the ratio of the maximal area for opening and the dimension of the free edge in systole.
Imaging protocol
Fluoroscopy of the THVs alone and in ViV combinations was performed using the OEC 9900 Elite Mobile C-arm X-ray system (GE HealthCare). High-resolution photography was performed at a prespecified magnification and fixed camera height. High-speed video from the hydrodynamic testing was also captured.
Pinwheeling
Pinwheeling, as defined by the ISO guideline for THV testing, refers to twisting of the free edges of the leaflets, resulting from excessive leaflet redundancy. A pinwheeling index (PWI) was calculated as previously reported14, using images from the high-speed videos. The PWI of each leaflet’s free edge was measured, and the mean PWI (%) of the valve was obtained from the average. Pinwheeling was classified as mild (<5%), moderate (5-9%) or severe (≥10%).
Statistics
Hydrodynamic variables, EOA, pressure gradients, and total regurgitation fraction are reported as mean values.
Results
Hydrodynamic performance for the native simulations
Photographic and fluoroscopic images of the DurAVR THV and comparator THVs are shown in Figure 1, along with skirt and frame heights. Overall, use of the DurAVR THV resulted in excellent hydrodynamic performance, independent of implant depth (Figure 2A, Figure 2B). The EOA was consistently ≥3 cm2 and MG <6 mmHg. The other THVs used as comparator valves also showed good performance, independent of implant depth, with an EOA >2 cm2 and an MG <10 mmHg. However, the EOA was greater and the MG was lower for the DurAVR THV at all three implant depths compared to the other THVs tested, with the increased opening index of DurAVR THV being visually appreciable (Figure 1C). Comparison of the DurAVR THV to the S3 at 3 mm depth exemplifies this, with an 82% opening index for the DurAVR THV compared to 72% for the S3. Representative cycles of hydrodynamic function at 3 mm implant depth are shown in Moving image 1.
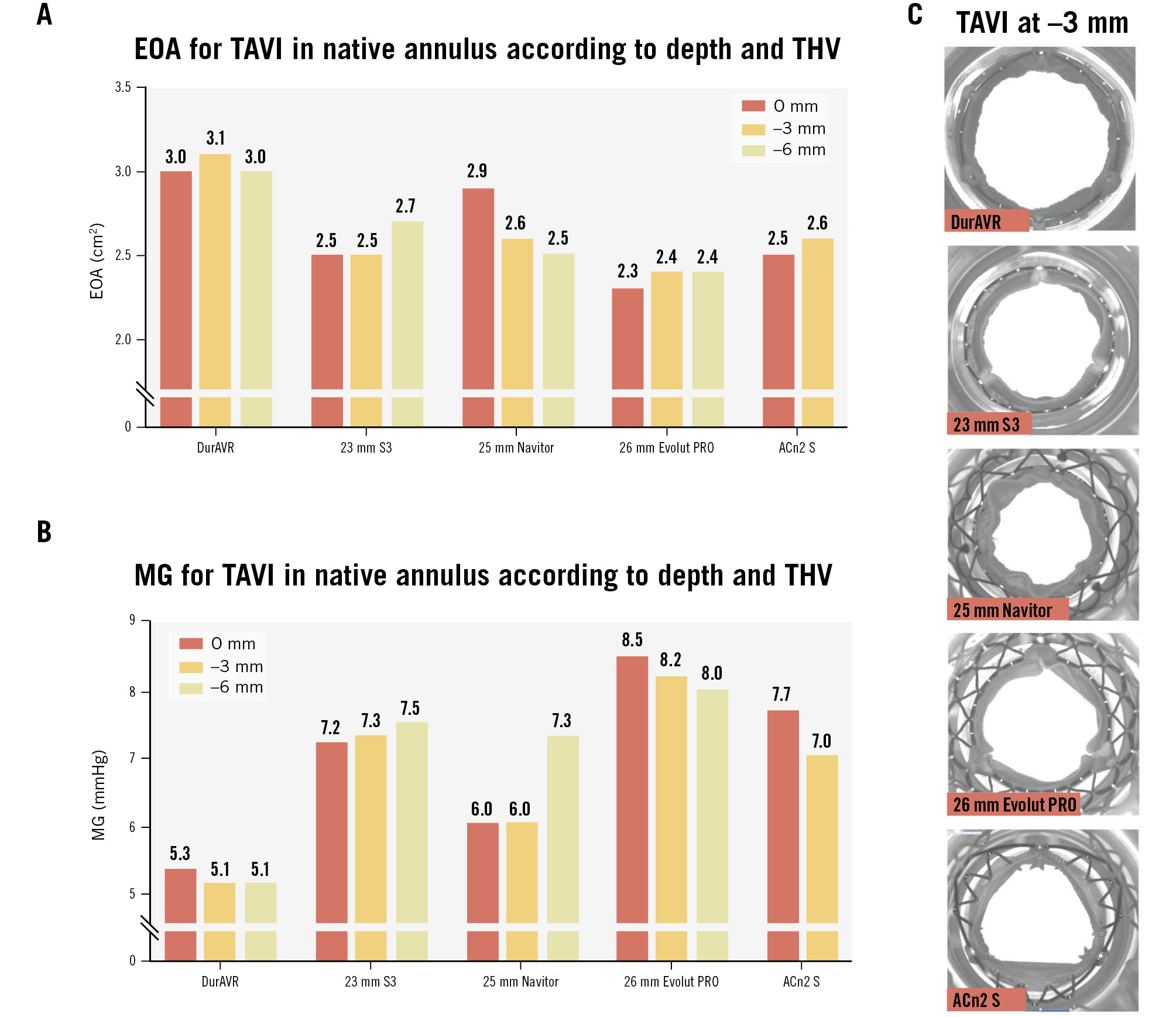
Figure 2. Effective orifice area and mean gradient of the THVs in the native TAVI simulations at varying depths. EOA (A) and mean gradient (B) of the THVs in the native simulations at 0 mm, 3 mm, and 6 mm depths with representative images of valve opening at 3 mm depth (C). ACn2 S: ACURATE neo2 small; EOA: effective orifice area; MG: mean gradient; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
Pinwheeling for the native TAVI simulations
Analysis of video from the hydrodynamic assessment of THVs simulating native TAVI implantion showed that the DurAVR THV resulted in very mild pinwheeling (between 0% and 2%) at all implant depths tested. This was also the case for ACn2 and Navitor. However, Evolut PRO displayed moderate pinwheeling at 6 mm depth, and S3 displayed moderate pinwheeling at 3 and 6 mm depths (Figure 3).
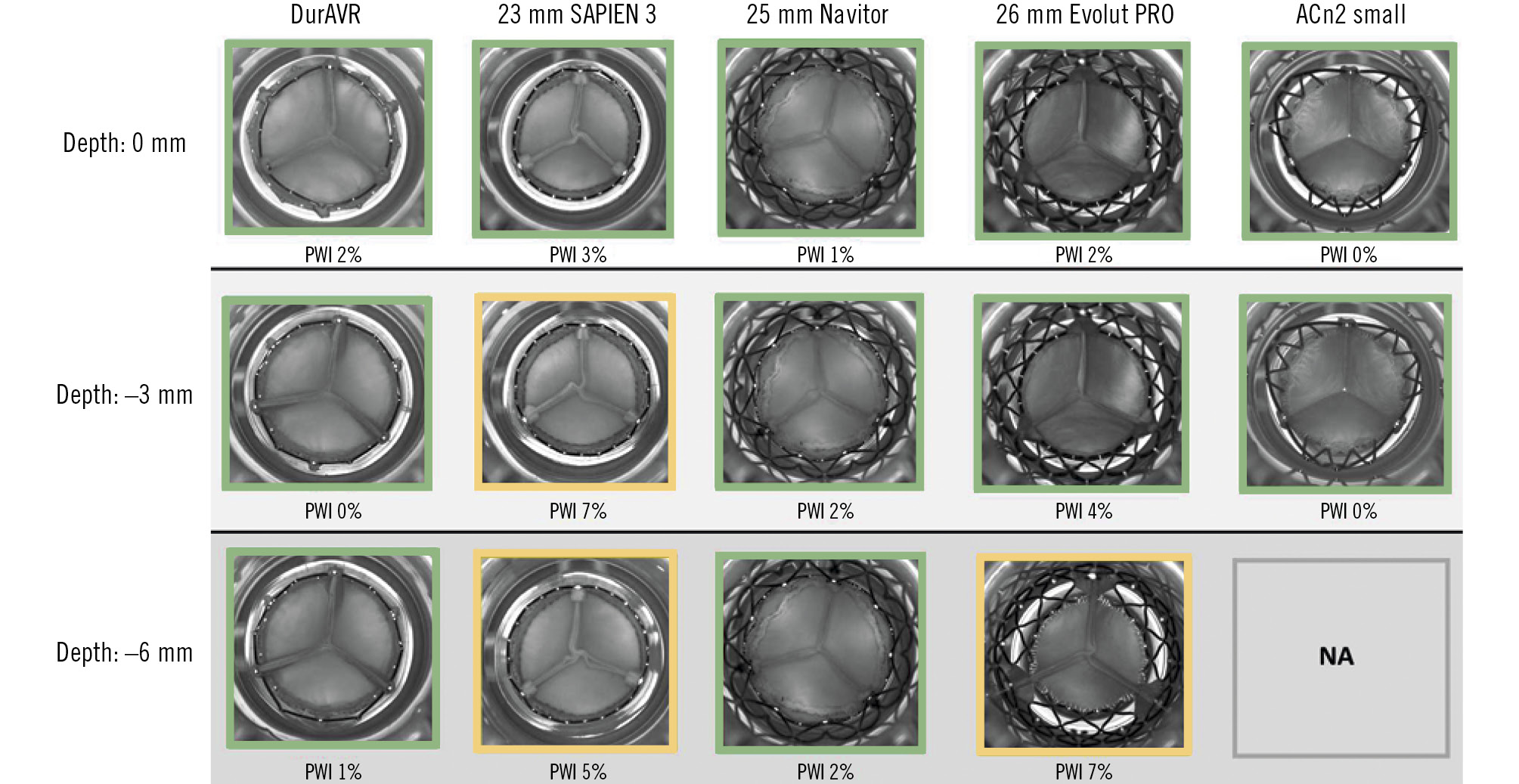
Figure 3. Pinwheeling index and representative hydrodynamic testing images in diastole of the THVs in the native simulations according to implant depth. ACn2 could not be assessed at –6 mm. Green: mild; yellow: moderate. ACn2: ACURATE neo2; NA: not applicable; PWI: pinwheeling index; THV: transcatheter heart valve
Hydrodynamic performance for the ViV TAVI configurations
Photographic and fluoroscopic images of the ViV combinations are displayed in Supplementary Figure 2 and Supplementary Figure 3. ViV configurations resulted in diminished hydrodynamic performance compared to native THVs. However, in the ViV context, the DurAVR THV was associated with larger EOAs and lower MGs than the comparators, with the greatest difference seen with implants in the 21 mm surgical valves (Figure 4A, Figure 4B). In particular, within the 21 mm Hancock, the DurAVR THV was the only THV with an MG below the acceptable threshold according to ISO guidelines (16.4 mmHg for DurAVR THV vs 19.7 mmHg, 21.1 mmHg, and 20.2 mmHg for S3, Evolut PRO, and ACn2, respectively). The smallest difference was noted for the 23 mm Magna Ease, where all THVs had an MG <10 mmHg. Of note, the advantage of the DurAVR valve was more marked in the porcine leaflet valves (Hancock and Mosaic) which had smaller true ID than in the Magna Ease (pericardial leaflets). Moving image 2 shows hydrodynamic function examples for ViV.
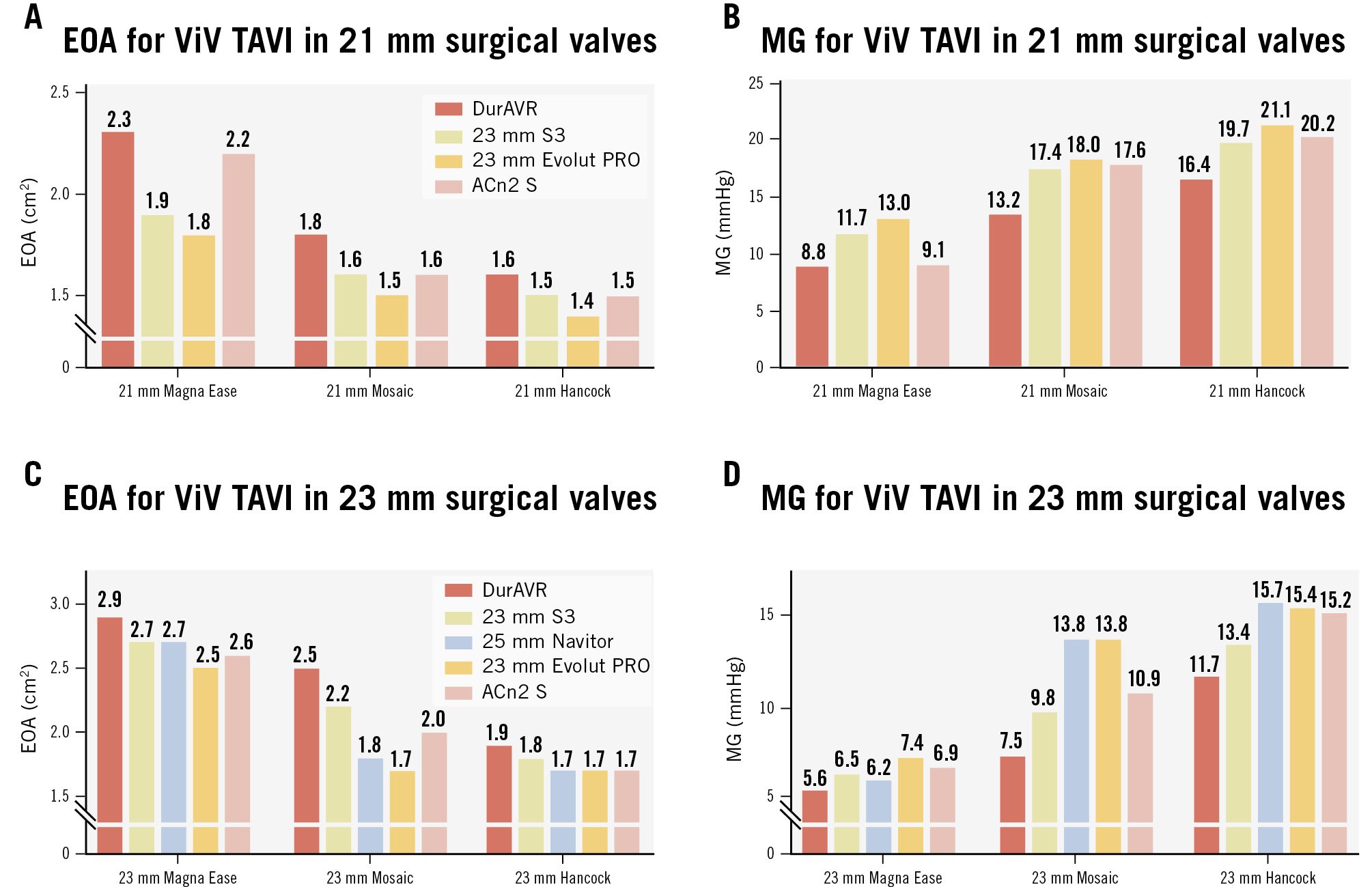
Figure 4. Effective orifice area and mean gradient in ViV TAVI simulations. Effective orifice area (EOA) and mean gradient (MG) of ViV configuration in 21 mm index surgical valves (A, B) and in 23 mm index surgical valves (C, D). ACn2 S: ACURATE neo2 small; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation; ViV: valve-in-valve
Pinwheeling for the ViV TAVI configurations
Pinwheeling was generally more marked in the ViV configurations than for the native THV implant alone, except for the DurAVR THV, which showed no more than mild pinwheeling (range 0-3%) for any of the surgical valve sizes and types tested (Figure 5). Similar to native TAVI configurations, a lower extent of pinwheeling was seen for ACn2, except for the 21 mm and 23 mm Hancock valves, where moderate pinwheeling was noted. Evolut PRO had severe pinwheeling in the 21 mm surgical valves and moderate pinwheeling in the 23 mm surgical valves. S3 had severe pinwheeling in the 21 mm surgical valves and moderate to severe pinwheeling in the 23 mm surgical valves. Finally, Navitor had a variable (from mild to severe) degree of pinwheeling depending on the surgical valve type.
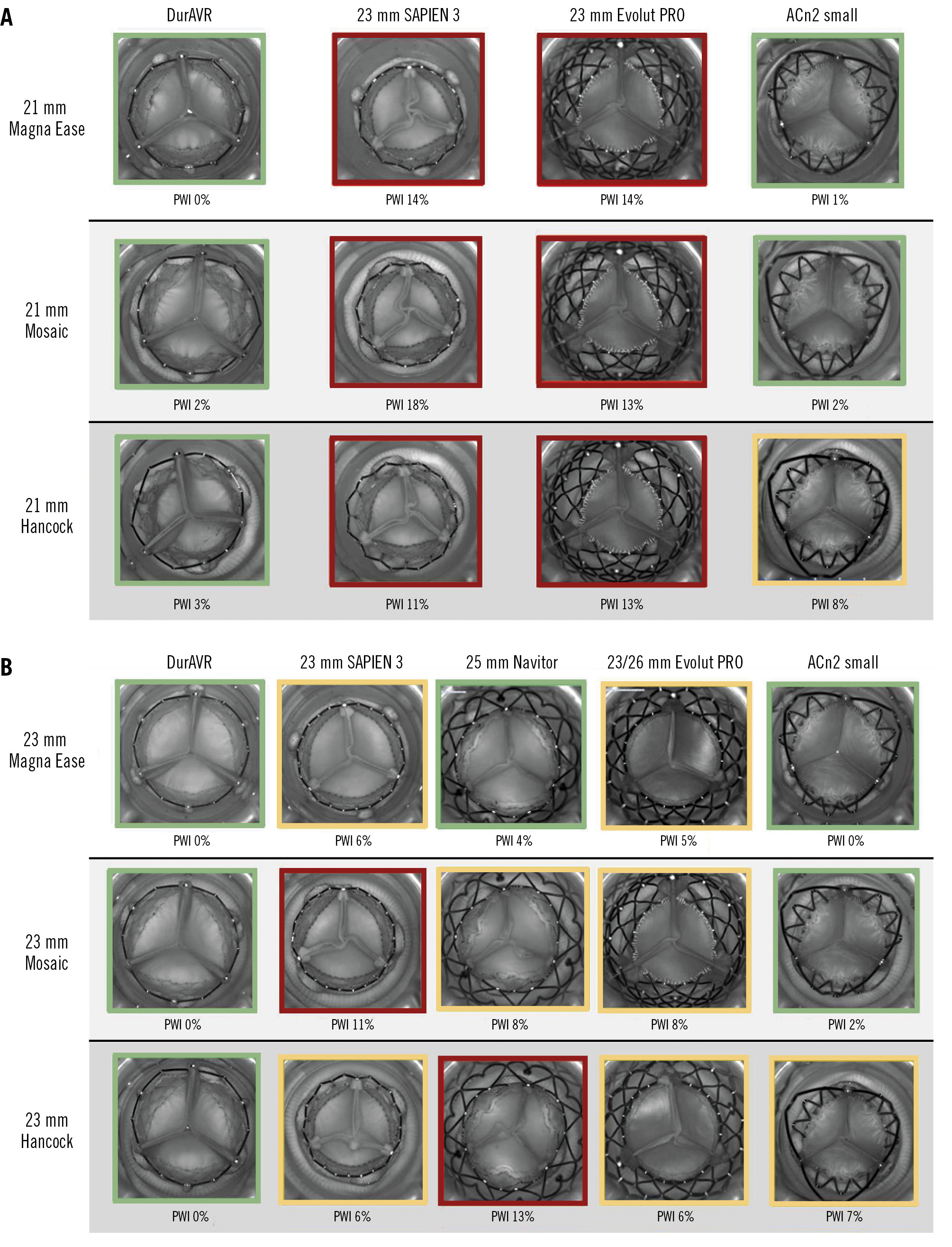
Figure 5. Pinwheeling index and representative hydrodynamic testing images in diastole of the THVs in ViV configurations. PWI of ViV configurations in 21 mm index surgical valves (A) and in 23 mm index surgical valves (B). Green: mild; yellow: moderate; red: severe. ACn2: ACURATE neo2; PWI: pinwheeling index; THV: transcatheter heart valve; ViV: valve-in-valve
Hydrodynamic performance for the redo-TAVI configurations
Supplementary Figure 4 displays photographic and fluoroscopic images of the redo-TAVI simulations. The DurAVR THV exhibited larger EOAs and lower MGs than its comparators in each index THV configuration (Figure 6A, Figure 6B). In all four configurations, the DurAVR THV demonstrated an MG ≤8 mmHg and an EOA ≥2.4 cm2.
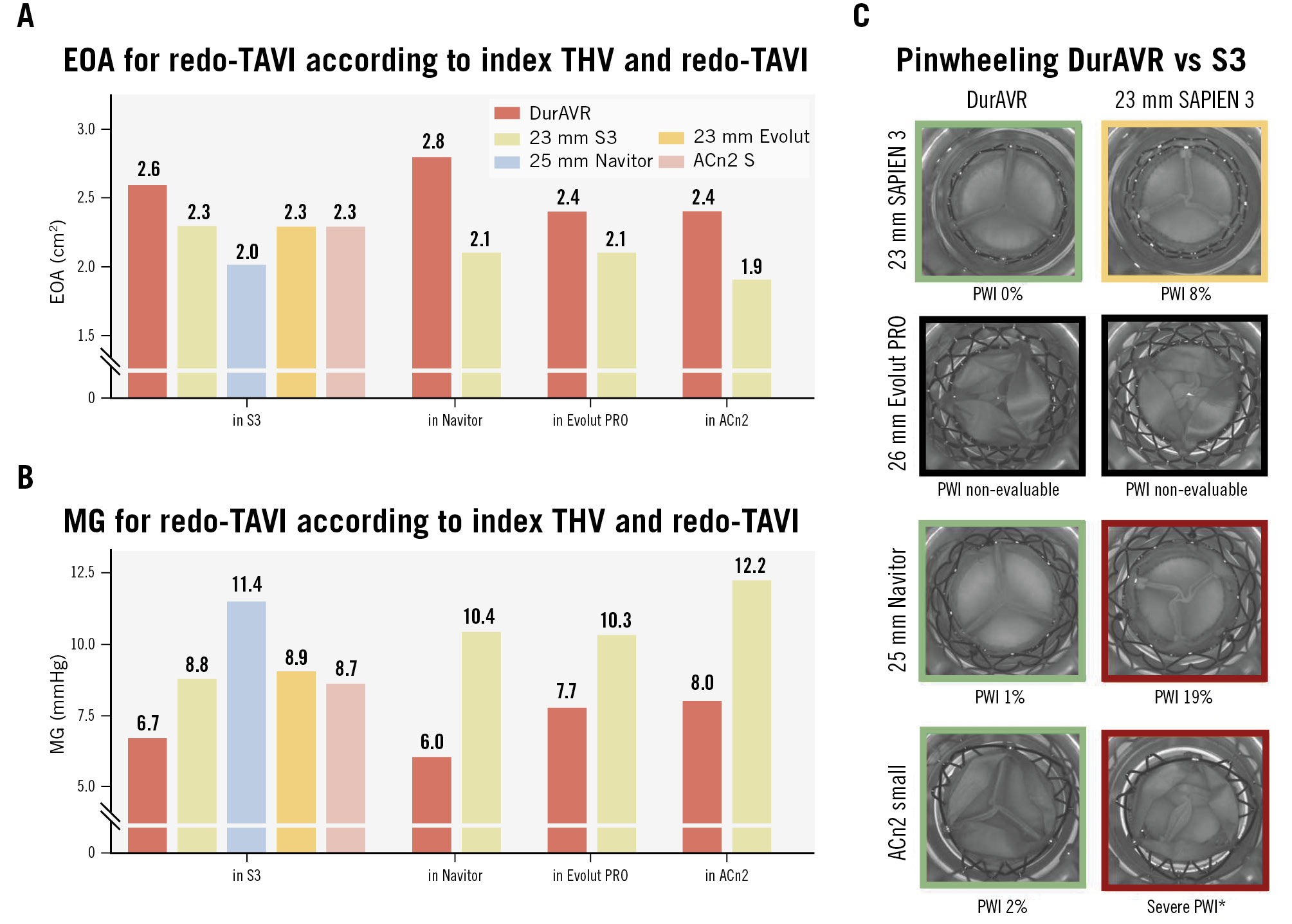
Figure 6. Effective orifice area, mean gradient and pinwheeling index in redo-TAVI simulations. Effective orifice area (EOA), mean gradient (MG) (A, B) and pinwheeling index (PWI) with representative hydrodynamic testing images in diastole (C). Green: mild, yellow: moderate, red: severe. *Visual estimation. ACn2 S: ACURATE neo2 small; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation
Pinwheeling for the redo-TAVI configurations
Figure 6C, Supplementary Figure 5 and Moving image 3 present the different degrees of pinwheeling observed in the various redo-TAVI configurations. When redo-TAVI was modelled in a failed S3, the DurAVR THV and ACn2 showed no pinwheeling (0%). In contrast, other THVs (Evolut PRO, Navitor, S3) demonstrated moderate to severe pinwheeling when implanted in an S3. In the case of redo-TAVI modelled in a failed Navitor, ACn2, and S3, DurAVR THV was compared to S3 only. In these configurations, the DurAVR THV showed notably less pinwheeling than the S3. However, due to the extent of leaflet overhang, pinwheeling measurements were not possible at all in the cases modelled in a failed Evolut PRO, and in one case modelled in a failed ACn2, only visual estimation was possible.
Discussion
In this bench evaluation of the DurAVR TAVI system, we demonstrate that this new platform has (1) excellent hydrodynamic performance in native simulations compared to other platforms; (2) superior hydrodynamic performance to other platforms when tested in ViV TAVI and redo-TAVI configurations; and (3) less susceptibility to pinwheeling than other platforms (Central illustration).
Early clinical experience has shown promising valve performance with the DurAVR TAVI system11. In lieu of clinical comparative data with other commercially available THVs, we show on the bench that the DurAVR THV is consistently associated with MGs <10 mmHg and large EOAs, independent of implant depth. These results are consistent with the observed data in the first-in-human experience11, which demonstrated stable haemodynamics up to 1 year. Although the DurAVR THV presented with numerically lower values than the comparators, MGs were similar across platforms.
In the ViV TAVI configurations, where THVs are generally constrained by the surgical valve, the DurAVR THV performed better than the comparator THV platforms. This benefit was more pronounced in the smaller SHVs and was different according to SHV type. This is a potentially important finding since ViV TAVI is often associated with an elevated residual gradient15, and some data have suggested that in small SHVs, BE short-frame THVs might have higher residual gradients6. Moreover, there is perception that tall-frame SE THVs are associated with a better haemodynamic profile for both index and ViV TAVI. Here, we show the importance of performing a detailed assessment of a new THV design without assuming a class effect. Indeed, the short-frame BE DurAVR THV had a comparative or better hydrodynamic profile than the SE comparators. This may be related to the moulded single-leaflet design.
In the redo-TAVI setting, the DurAVR THV displayed larger EOAs and lower, single-digit gradients compared to the other THV platforms, including SE tall-frame valves. This is a key finding to note, as redo-TAVI with tall-frame SE platforms has generally been associated with superior haemodynamics16.
Another important finding of this study is the minimal degree of pinwheeling observed in the DurAVR THV in native, ViV and redo-TAVI simulations. The difference compared to the other THV platforms was, however, much more marked in the ViV and redo-TAVI simulations. With the current THV platforms, pinwheeling is a phenomenon generally seen in the context of valve underexpansion17, which, in turn, has been associated with increased propensity for leaflet thrombus formation and may have negative clinical implications for the patient1819. On the bench, pinwheeling has been shown to cause more leaflet strain, impact turbulent flow and reduce durability. Studies assessing underexpanded S3 and ACn2 valves subjected to pinwheeling through 200 million cycles in ViV configurations showed a larger degree of leaflet histological ultrastructure damage compared to fully expanded valves10202122. However, despite its short frame, which is only slightly taller than the S3, and its visible underexpansion in the ViV configurations, the DurAVR THV displayed almost no pinwheeling. This suggests that the single-piece leaflet design with a long coaptation surface area could potentially offer a protective effect against pinwheeling, which, in turn, could reflect the near-physiological flow that has been observed during early clinical experience.
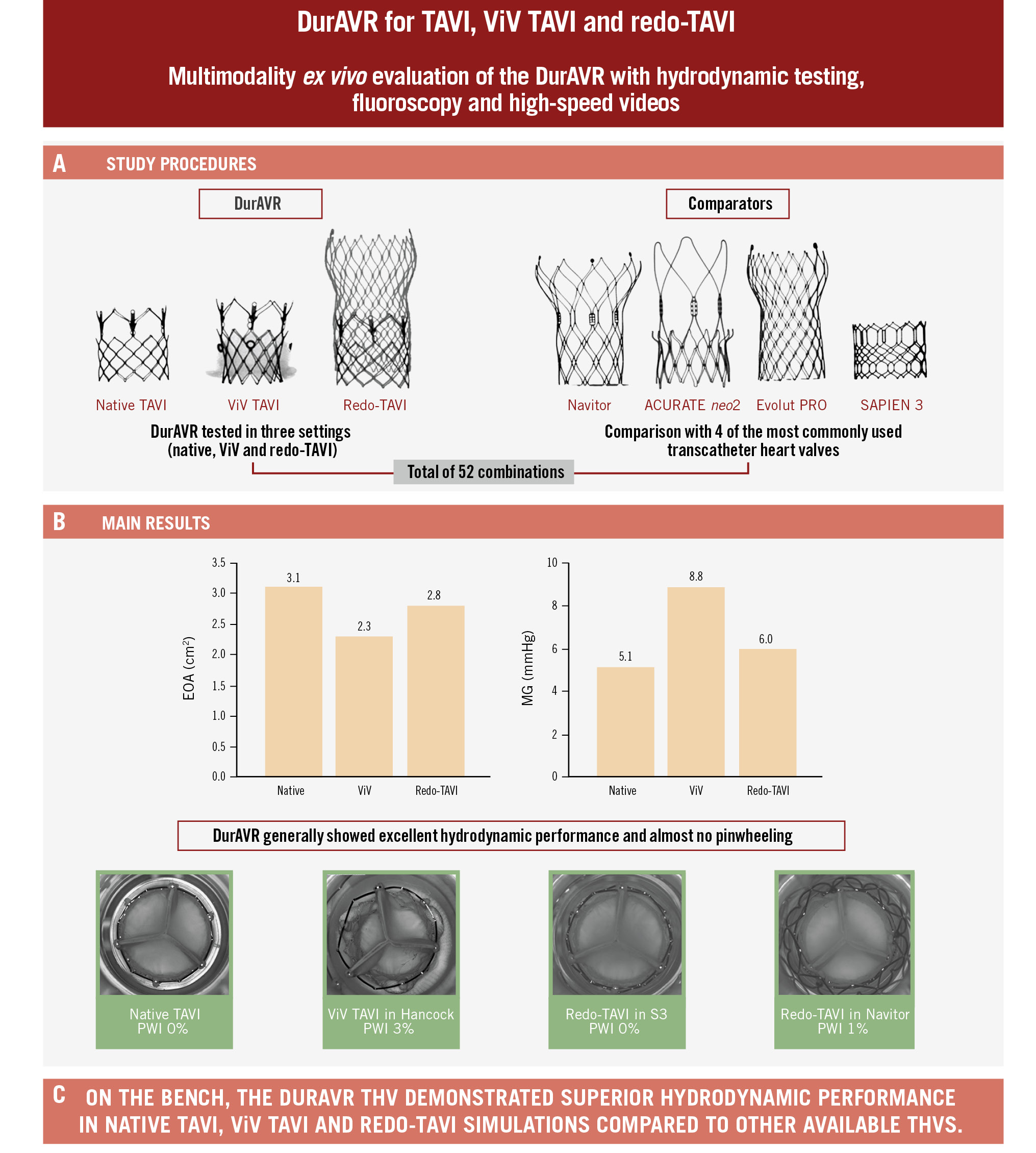
Central illustration. Functional assessment of the DurAVR THV in TAVI simulations. A) Functional assessment of the DurAVR THV and other THV platforms was performed (total of 52 combinations) with pulsatile testing allowing for measurement of MG/EOA. High-speed videos were recorded to assess leaflet motion and pinwheeling. B) Main results of bench testing of EOA and MG for DurAVR at 3 mm depth of native TAVI, in 21 mm Magna Ease ViV, and in Navitor redo-TAVI configurations. C) Study conclusions. EOA: effective orifice area; MG: mean gradient; PWI: pinwheeling index; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; ViV: valve-in-valve
Limitations
The study is presented with the known limitations inherent to bench testing. THV expansion might be somewhat different within a calcified annulus or a degenerated SHV or THV, as these factors were not represented in this study. Nevertheless, the present work suggests that in similar conditions, the DurAVR THV performs at least as well as its comparators. Additionally, only one size of THV was tested and one sample was used for each combination, and it remains unknown whether similar findings would have been obtained with larger index surgical valves and THVs. Additional bench studies are needed to investigate the impact of varying degrees of commissural alignment on hydrodynamic function, the outcomes of accelerated wear testing on function and leaflet integrity, and the implications of the DurAVR valve design on neoskirt height and any subsequent impact on the feasibility of coronary access. Future studies could also consider comparing systolic and diastolic flow between platforms and assessing susceptibility to calcification or thrombosis. While this bench study provides needed insight into the function of the DurAVR THV compared to other THV platforms, ultimately the evaluation of valve function and durability will need to be determined through ongoing and future clinical trials.
Conclusions
In this study, the novel DurAVR THV demonstrated excellent hydrodynamic performance in native TAVI, ViV TAVI, and redo-TAVI simulations compared to other commercially available balloon-expandable and self-expanding THVs.
Impact on daily practice
The DurAVR transcatheter heart valve (THV) is an intra-annular balloon-expandable THV that has shown promising results in early feasibility clinical experience. In this bench study, the DurAVR THV showed excellent hydrodynamic performance in native transcatheter aortic valve implantation (TAVI), valve-in-valve TAVI, and redo-TAVI simulations, compared to SAPIEN 3, Navitor, Evolut PRO, and ACURATE neo2 valves. The performance and characteristics of both the current and anticipated larger DurAVR THV sizes need to be assessed in future in vitro testing, and a larger clinical series is required to validate these findings in vivo.
Funding
This work was supported by Anteris Technologies.
Conflict of interest statement
D. Meier has received an institutional grant from Edwards Lifesciences. S.L. Sellers is a consultant for Edwards Lifesciences, Anteris Technologies, Excision Medical, and Medtronic; and has received research support from Edwards Lifesciences, Medtronic, and HeartFlow. J.L. Cavalcante has received consulting fees from 4C Medical, Abbott, Anteris Technologies, Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic; has received research grants from Abbott, Allina Health Foundation, JenaValve, and NIH/NHLBI; and he has received honoraria or consultation fees from 4C Medical, Abbott, Alleviant, Anteris Technologies, Boston Scientific, Edwards Lifesciences, JenaValve, JC Medical, Medtronic, Novo Nordisk, Siemens Healthineers, VDyne, and Zoll. M. Settergren is a consultant for Anteris Technologies, Edwards Lifesciences, and Boston Scientific. C.U. Meduri is the chief medical officer of Anteris Technologies; and serves as a consultant to Boston Scientific, Abbott, Alleviant, VDyne, Cardiovalve, xDot Medical, and V2V; he has received research funding from Edwards Lifesciences and Medtronic. A.W. Asgar has been a consultant for/on an advisory board for Medtronic and Abbott; and has been a consultant for Edwards Lifesciences. R.T. Hahn reports speaker fees from Abbott, Baylis Medical, Edwards Lifesciences, Medtronic, Philips, and Siemens Healthineers; institutional consulting contracts for which she receives no direct compensation with Abbott, Edwards Lifesciences, Medtronic, and Novartis; and she is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored tricuspid valve trials, for which she receives no direct industry compensation. J. Sathananthan is an employee of Boston Scientific; has been a consultant to Edwards Lifesciences, Medtronic, and Anteris Technologies; and has received research support from Medtronic, ViVitro Labs, and Edwards Lifesciences. The other authors have no relevant conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Hydrodynamic testing for native TAVI implants at 3 mm depth.
Moving image 2. Hydrodynamic testing for valve-in-valve implants in 21 mm Magna Ease.
Moving image 3. Hydrodynamic testing for redo-TAVI configurations with implantation in SAPIEN 3.

