Abstract
Aims: Current guidelines recommend the use of dual antiplatelet therapy (DAT) (aspirin+clopidogrel) for patients after acute myocardial infarction (MI). In relation to this, we sought to examine the adherence to this recommended treatment regimen in a population of patients admitted with MI and subsequent percutaneous coronary intervention (PCI).
Methods and results: A cohort study was conducted using data from the main health insurance reimbursement database of South West France. Patients hospitalised for MI in 2008 were identified, and then their reimbursement form of DAT for the subsequent 12 months was reviewed. Adherence was assessed by using the proportion of days covered by the treatment and persistence. Among the 634 patients included in the study, 34 had no reimbursement for DAT immediately after discharge. For the remaining patients, the probability of stopping DAT at least for one month was 18.6% (95% CI [15.4;21.8]) during the first three months and 49.1% (95% CI [44.9;53.2]) at 12 months, although the medication availability was 90%.
Conclusions: These results suggest that while this medication is available to patients, the treatment is often stopped before one year. This may account for what has been reported as “resistance” to antiplatelet therapy described in a subset of patients.
Introduction
Treatment of acute coronary syndrome (ACS) has improved markedly over the last decade, and dual antiplatelet therapy (DAT) with aspirin (acetylsalicylic acid [ASA]) and clopidogrel are now cornerstones of treatment1. In accordance with European recommendations on secondary prevention for patients with ACS2, French guidelines recommend clopidogrel treatment for 12 months after an ACS in addition to other evidence-based treatments (beta-blockers, statins, aspirin and angiotensin-converting enzymes inhibitors3,4). To prevent recurrent events, clopidogrel is also routinely added to aspirin in treatment of patients undergoing coronary stenting. Premature cessation of one of the drugs has been associated with increased risk of thrombotic events, including stent thrombosis5,6. Stent thrombosis remains an important complication after stent implantation, despite the use of DAT. Several studies have shown an increased risk of thrombotic events in patients with resistance to clopidogrel7,8. As highlighted in a recent meta-analysis, laboratory clopidogrel non-responsiveness can be found in approximately 20% of patients undergoing PCI9. The response variability is seen in patients treated with or without ASA added to clopidogrel treatment and has a dose-dependent component10-12. In addition to more complex mechanisms, including drug-drug interactions, genetic polymorphisms and clinical factors, it has been suggested that a component of the low response could be due to poor adherence or compliance with treatment12,13.
Few “real-world” results have been reported regarding the impact on morbidity of short- and long-term adherence to DAT. Moreover, there is a lack of published data on the adherence of patients to the recommended duration of treatment with a DAT regimen. In the current study, we investigated initiation and adherence with DAT in a population of patients admitted with myocardial infarction (MI) with subsequent PCI.
Methods
STUDY DESIGN AND SOURCE OF DATA
A retrospective cohort study was conducted using data from the main French health insurance reimbursement database of the Aquitaine region (South West France)14. In this region, which includes approximately 5% of the French population, 2.5 million patients are covered by this insurance.
INCLUSION AND EXCLUSION CRITERIA
Patients hospitalised in public hospitals for acute MI in between 1 January and 31 December 2008 in the Aquitaine region were first identified. Identification of MI was based on either “groupe homogène de séjour” (GHS), which allowed identification of patients hospitalised for MI and vascular endoprothesis, and/or “discipline médico-tarifaire” (DMT), which allowed identification of patients hospitalised in intensive care or for cardiac resuscitation. These codes are identical in all French hospitals and are disease-specific. The database contains no information about the type of stent that was implanted. We then looked for records of new reimbursement for dual antiplatelet therapy (DAT) treatment (aspirin indicated in cardiovascular diseases + clopidogrel) during the first month after discharge. Patients were included if they had dual antiplatelet therapy after discharge. A new treatment was defined as no reimbursement of DAT for two months previous to this.
Drugs of interest were identified in the database according to their “Club Inter Pharmaceutique” (CIP) code, which is a unique product identifier15. We considered the treatment as DAT if the antiplatelets had the same date of prescription. Exclusion criteria included patients with anticoagulant drugs. Records for all patients included in this retrospective study were reviewed for a 12-month period following the initial event/hospitalisation.
Prasugrel was not considered in the analysis, as this drug was not available in France during the study period.
We performed an observational study on anonymous data. Thus, based on the French legislation, it did not need to be approved by an ethics committee, although this study did conform to the principles of the Helsinki Declaration.
ASSESSMENT OF ADHERENCE
Adherence was assessed by using two parameters: medication availability and persistence.
1) The medication availability is defined as the proportion of days during the period in which patients could have been on therapy with that therapy in their possession. The medication availability (also termed proportion of days covered) was estimated using the “continuous multiple-interval measures of medication availability” (CMA) definition16-19. The CMA is defined as the sum of the all of the days’ supply of medication divided by the number of days between the first fill and the last refill. The theoretical day’s supply is calculated by dividing the number of units dispensed by the daily dose for the drug considered. The daily dose is the recommended dose per day for its main indication in adults. The CMA was assessed using both a cutoff at 80% (a CMA lower than 80% was considered as unsatisfactory in several previous studies for treatment of cardiovascular diseases) and as a continuous variable to characterise the distribution of the CMA in the study population. We further assessed adherence using the medical refill adherence (MRA19) to determine if there was consistency in the measures of the adherence between these two parameters.
2) Persistence was measured as the duration of uninterrupted therapy of the patient’s index product using the Kaplan-Meier survival curve analysis20 regardless of whether the treatment was actually taken. In France, these treatments are delivered for a one-month period (28 or 30 tablets per box), therefore discontinuation was defined as a minimum gap of 30 days between the theoretical end date of when the prescription was due for renewal/reimbursement (based on a days’ supply) and the actual starting date of the next renewal/reimbursement. In order to provide additional and more sensitive analysis and to avoid misclassification, we also used a second discontinuation definition, which was a gap of 60 days (i.e., two boxes of drugs).
The analysis of both persistence and medication availability allows the assessment of whether patients continue their treatment for a long period (persistence) and if, whatever the duration of their treatment, there is no gap long enough to induce recurrences or worsening (medication availability).
ASSESSMENT OF MEDICAL CONDITIONS
During the study period, the number of consultations to physicians, i.e., general practitioners, cardiologists or endocrinologists, were also assessed for all patients.
In addition, patients were assessed for re-hospitalisation for MI (same codes for identification) and/or death regardless of the cause, during the study period.
STATISTICAL ANALYSIS
Analysis was performed using SAS software version 9.1 for PC (SAS Institute Inc., Cary, NC, USA); the Student’s t and chi-squared tests were used. The Kaplan-Meier analysis was used to estimate persistence rates as it takes into account censored observations. The level of statistical significance was set at 0.05 and the statistical uncertainty of the estimates was assessed by 95% confidence intervals (95% CI).
Results
The study included 634 patients with MI and PCI in 2008 that had no reimbursement of DAT in the previous two months. The sex ratio was 2.6 and the mean age was 65.5 (CI 95%[64.5;66.6]). Among them, 34 patients (5.4%) were reimbursed for single antiplatelet therapy immediately after discharge and six additional patients died during the first month (0.9%). The mean age of these 34 patients was 69.5 (CI 95% [65.0;74.0]) and three of them were treated with oral anticoagulant drugs (Figure 1). Therefore, the final cohort for complete analysis consisted of 594 patients and then 94.6% initiated treatment with DAT. The mean age of the cohort was 62.1 (CI 95% [59.8;64.4]) and the ratio of male to female was 2.9.
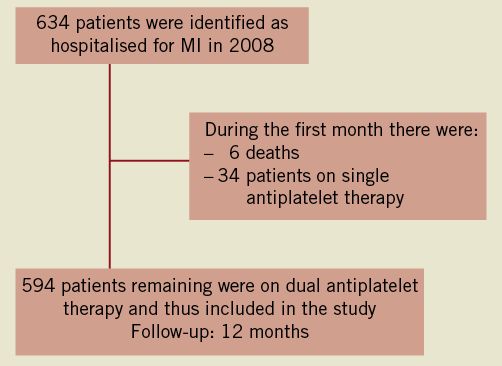
Figure 1. Flow diagram of the study.
ASSESSMENT OF ADHERENCE
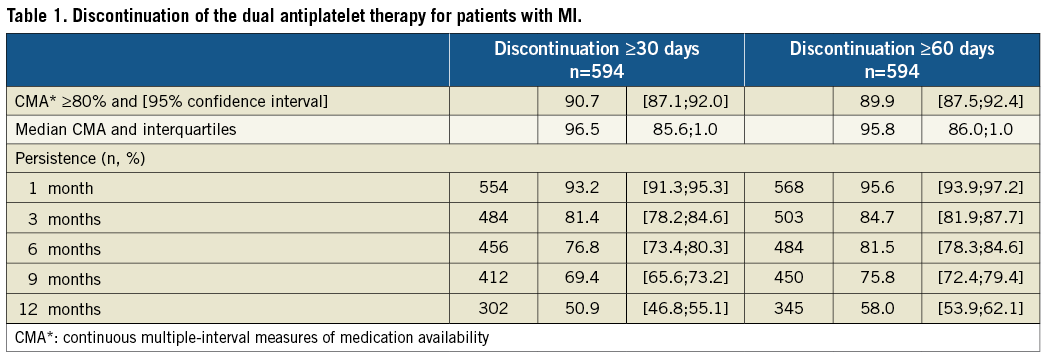
During the first three months, the probability of stopping DAT (meaning both aspirin and clopidogrel) for at least for one month was 18.6% for the remaining 594 patients (Figure 2 and Table 1). In other words, 18.6% of the patients that purchased at least one month’s supply of DAT in the first three months had a gap of at least 30 days or they died during this period. Taking into account the additional 34 patients who did not apply for any reimbursement, at three months after MI and stent placement, 22.9% of the total group of patients had either not received or discontinued their DAT for at least one month.
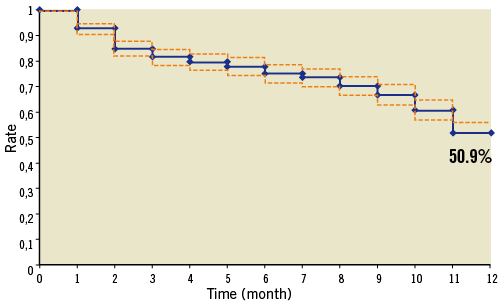
Figure 2. Persistence with dual antiplatelet therapy during 12 months after hospitalisation for MI (definition of discontinuation: 30 days after DAT stopped)
To strengthen these results, we considered a discontinuation of reimbursements for 60 days instead of 30 days in the remaining 594 patients, and found that 15.2% of the patients did not follow the recommended DAT regimen during the first three months.
During the 12-month follow-up, the probability of stopping the DAT regimen for at least one month was 49.1% and the probability of stopping it for two months was 42%. For 50.9% of patients without a gap of at least 30 days in their DAT, their median medication availability during the 12 months (i.e., having enough treatment in stock to be treated) was 90%. In other words, the patients had sufficient treatment to be treated for 90% of the study period, i.e., 329 days. When using MRA, the results remained unchanged throughout the study period.
At 12 months, 73% of the patients were still treated with aspirin alone and 47% were still treated with clopidogrel alone.
ASSESSMENT OF MEDICAL CONDITIONS
The mean number of medical consultations was identical in the group who did not stop the DAT for more than 30 consecutive days over 12 months after MI and the group of patients who stopped the treatment: n=15 consultations, (CI 95%[14;16]) compared to n=14 consultations, (CI 95%[12;16]).
Forty patients were hospitalised for MI in the 12 months following their first hospitalisation. Among them, three patients belong to the group that had stopped the treatment for at least one month (after 10, 30 and 266 days). Thus, 1% of patients who stopped the DAT regimen and 13% of patients who continued the regimen were hospitalised for MI in the 12-month follow-up period (p<10–3).
Finally, during the study period, 41 patients died (i.e., 6.9%), 27 in the group stopped the DAT regimen (i.e., 8.7%) and 14 in the group continued the regimen (i.e., 5.8%). In both groups, one patient died after their hospitalisation for MI.
Discussion
This study investigated the initiation and adherence (medication availability and persistence) with both clopidogrel and aspirin treatment (recommended DAT regimen) in a selected cohort of patients surviving an MI + PCI during 2008 in the Aquitaine region of France.
There are three major findings: 1) At three months after MI + PCI, the probability of stopping the recommended DAT regimen, for at least one month, was approximately 20%; 2) At twelve months, the probability of stopping the recommended DAT regimen, for at least one month, was approximately 50%; and 3) The rate of recurrent MI was higher in the population that was adherent to the regimen, as compared to non-adherent patients.
The results were strengthened by the demonstration that the probability of stopping the recommended DAT regimen for 60 days was in the same range as stopping for 30 days.
Initiation of the recommended DAT regimen was high, 94.6% filling the prescription for the two drugs within 30 days after hospital discharge.
Guidelines for duration of aspirin and clopidogrel treatment were clear in 2008 and it was recommended to continue the DAT regimen for 12 months after MI with PCI.
Persistence rates among patients in the MI + PCI cohorts were remarkably low, with only 81.4% of patients completing 90 continuous days of treatment and 50.9% continuing the regimen throughout the full 12 months. Our results further indicated that patients who discontinued the recommended regimen had the same number of medical consultations as those who remained on it. This indicates that either the physicians had decided to stop the treatment prematurely or patients stopped treatment and their physicians were either in agreement with this decision or were not made aware of it.
Few adherence studies have been reported for the recommended DAT regimen, in part because aspirin is available over the counter (OTC) and/or is not reimbursed in most countries. Despite the fact that aspirin is available OTC in France, the pharmaceutical form used most frequently for cardiovascular diseases (75 or 160 mg) is found on the same prescription as other cardiovascular drugs, thus there is no reason to think that OTC may have greatly modified the results. Moreover, when we modified the definition of the discontinuation, i.e., 60 days versus 30 days, the results remained unchanged, which supports this hypothesis.
In one study devoted to post-MI treatment adherence, where adherence was estimated by interviews, more than 15% of the patients discontinued aspirin during the month following MI. The largest decline in rates of medication use occurred between hospital discharge and one month. Thereafter, medication use rates remained relatively stable21. In a recent report in France, the authors reported that 18.3% of patients had medication availability lower than 80% for aspirin/clopidogrel, in a 30-month follow-up period after MI22, which can be considered to be in accordance with our results of 9.3% at 12 months.
There have been few studies devoted to long-term clopidogrel treatment and assessment and adherence differs according to studies. One study reported that among PCI patients approximately 75% were treated for 12 months. The study used patient reports every six months as a source of determining adherence with clopidogrel treatment among PCI patients and did not describe breaks in therapy within the treatment period23 as we did, which may have overestimated the adherence. Another study from Denmark found that persistence rates of clopidogrel among MI patients with PCI were remarkably high: 89.4% completed 90 days of treatment, 82.2% 180 days and 77.5% were treated throughout nine months in 2004–2005. However 26.3% of patients had a break of >30 days24.
To our knowledge, no studies have been conducted to specifically investigate DAT adherence during the early phase post-MI+PCI.
Evidence has arisen that stopping aspirin in patients with stable coronary artery disease (CAD) treated with aspirin alone leads to an increased risk of ACS and death25,26. The risks due to DAT therapy interruption in patients treated with bare metal stents and drug-eluting stents is even higher23,27-30. Each of the few available studies addressing the issue of aspirin withdrawal clearly demonstrate the relevant hazards inherent in interrupting aspirin treatment in patients in primary or secondary prevention; meta-analysis further demonstrates the significant increase in the risk of adverse events after an average of 10 days of discontinuing aspirin, across a broad spectrum of patients at risk of or with CAD25.
Surprisingly, in our study, significantly fewer patients in the non-adherent group presented a reinfarction. There was no correlation between the discontinuation of the antiplatelet used and events as previously reported. However, first, current data focusing on aspirin discontinuation are still limited and observational in nature. Most of the other studies for clopidogrel or aspirin use have been self-reported. Patients were asked if they discontinued antiplatelet treatment or if they were withdrawn from their medication. In our study we used a reimbursement database. However, if patients who do not buy medication do not take it, it is also possible that patients who do buy medication might not take it. Secondly, patients may have had other types of events. Nevertheless, as this result is surprising, it may be due to confounding factors and thus further studies should be conducted to further clarify this outcome.
Anyway, this suboptimal adherence and poor persistence could in part explain the inter-patient variation in the effect of clopidogrel treatment also referred to as “resistance” to antiplatelet drugs, which has also been previously described31-33. Even if resistance to treatment due to genetic polymorphisms exists, resistance to taking the drug should also be assessed.
Reasons for discontinuation commonly reported in treatment indicated in cardiovascular disease were adverse drug reactions34-36, multiple medications, depressive symptoms36,37 or cost34,38. Moreover, several studies have tried to describe independent factors affecting persistence or medication availability with therapy, but with conflicting results. Factors such as gender, age >80 years, diagnosis of MI without PCI or heart failure, multiple drug therapy and consultation with a cardiologist have been associated with both good and bad persistence with ASA39. To date there is no study that has assessed these factors with clopidogrel and there are few studies that have assessed the persistence with or medication availability of this drug.
STRENGTHS AND LIMITATIONS
Due to the use of a reimbursement database, this retrospective study has the advantage of not altering the behaviour of either prescribers or patients. As adherence is a multifaceted question, two standardised parameters were used. These have been extensively discussed in the literature40. One factor that may be debated is the use of 80% treatment coverage as the cutoff for assessing adherence. This is commonly used in the literature, yet it may not be appropriate for all types of drugs41. Nevertheless, for antiplatelet drugs, there has been clear, strong evidence that stopping the treatment has led to the rapid occurrence of cardiovascular events33,42 thus, considering a cutoff of 80% may be too low, which is why we also used the median CMA to describe the medication availability.
In the current study, discontinuation was defined as the absence of any filed antiplatelet reimbursement during at least 30 consecutive days and we can see that results remained unchanged when we considered a period of 60 consecutive days. We performed the sensitivity analysis to limit DAT disruption due to non-cardiac surgery, in cases where the DAT would be stopped for eight to 10 days.
The type of implanted stent initially may explain the low duration of DAT observed in our study. However, the guidelines recommend a DAT for at least three months regardless of the type of stent placed and our results suggested that at three months the treatment is still suboptimal.
However, a study conducted using a reimbursement database suffers from some limitations. The results presented are likely to be an overestimation of adherence since some patients who buy their treatment may not take it. Patients may have been classified as having discontinued if they moved outside the administrative region (Aquitaine) but this can be considered as only marginal as the population over 50 years has been geographically quite stable. Discontinuation for financial reasons is also improbable owing to the extent of the national health insurance system, which offers full coverage for underprivileged and unemployed people. Conversely, the cost of clopidogrel is high enough to practically preclude claims for reimbursement not being made and the electronic system used in France for reimbursement (SESAM-Vitale14) guarantees the exhaustive capture of deliveries in the database in real time. Moreover, there are no OTC forms of clopidogrel in France.
Conclusions
These results suggest that while the patients have good medication availability, the treatment is often stopped before one year. At three months after MI + PCI, the probability of having a gap in the dual antiplatelet therapy (DAT) for at least one month, was almost 20%. The results presented are likely to be an underestimation of adherence since some patients who buy their treatment may not take it. The problem of adherence to antiplatelet therapy should also be taken into account when assessing the effectiveness of the drug regimen.
Conflict of interest statement
The authors have no conflicts of interest to declare.