Abstract
Aims: Rates of stent thrombosis (ST) after ST-elevation myocardial infarction (STEMI) may vary over time and the relationship of this complication with non-adherence to dual antiplatelet therapy (DAPT) during long-term follow-up remains unclear.
Methods and results: We analysed 2,997 patients who were treated with at least one stent and in whom a non-target vessel ST did not occur during follow-up from the large-scale Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial of patients with STEMI undergoing primary percutaneous coronary intervention (PCI). Aspirin was prescribed indefinitely, and a thienopyridine for at least six months. DAPT usage was evaluated according to the development of ST in four time periods (<1 month, 1-6 months, 6-12 months and >1 year from index stent implantation). DAPT non-usage was lowest within the first month, but was strongly associated with ST. During the 1-6 month period the relationship remained strong, but was absent in the 6-12 month period. Beyond one year, ST was associated with non-usage of aspirin but was paradoxically more common in patients taking a thienopyridine.
Conclusions: The relationship between stent thrombosis and non-adherence to DAPT varies over time. It is strong within the first month, remains important until six-month follow-up but fades afterwards. Very late ST is associated with both DAPT and aspirin alone but not with thienopyridine non-adherence. Clinical trial registration: HORIZONS-AMI - registered at clinicaltrials.gov #NCT00433966.
Introduction
Stent thrombosis is a serious complication of percutaneous coronary intervention (PCI) with stent implantation. Dual antiplatelet therapy (DAPT) has been shown to reduce subacute stent thrombosis1 and prolonged use of DAPT has been shown to reduce major adverse cardiac events after treatment of acute coronary syndromes with or without PCI2. Following concerns regarding drug-eluting stent (DES) thrombosis at late and very late time frames, a set of standardised definitions has been applied3, and current guidelines suggest the use of DAPT for at least one year post-stenting even for non-urgent PCI with stent implantation. The utility of DAPT beyond one year is the subject of a large trial, but smaller scale studies have questioned its efficacy in low-risk groups4. Among other risk factors5,6, non-adherence to DAPT is considered a risk factor of stent thrombosis7. However, the relationship between timing of DAPT non-adherence and the risk of stent thrombosis has not been investigated. We sought to analyse this association in the HORIZONS-AMI trial database, which enrolled patients with ST-segment elevation myocardial infarction (STEMI) who fulfil the criteria for prolonged DAPT after PCI with stent implantation.
Methods
The HORIZONS-AMI trial methodology has been published previously8. In brief, STEMI patients treated with immediate cardiac catheterisation and primary angioplasty were included. A total of 3,602 patients were primarily randomised 1:1 to treatment with bivalirudin plus a provisional glycoprotein IIb/IIIa inhibitor (GPI) versus unfractionated heparin plus a routinely administered GPI. Subsequently, 3,006 patients eligible for stent implantation underwent a secondary randomisation 3:1 to a paclitaxel-eluting stent (Taxus®; Boston Scientific, Natick, MA, USA) versus an identical bare metal stent (Express®; Boston Scientific, Natick, MA, USA). For the purpose of the present analysis (n=2,997), we only considered patients who were treated with at least one stent (n=3,006) and did not sustain a non-target vessel stent thrombosis (n=9). Stent thrombosis at follow-up was defined as occurring in the original target vessel treated during the STEMI admission and trial enrolment, and was defined as definite or probable according to the Academic Research Consortium criteria3.
Patients without stent thrombosis were questioned about DAPT adherence at the 1, 6, 12, 24 and 36-month follow-up time points. Patients were then assigned one of three possible classifications: 1) those who were on DAPT during the complete follow-up interval; 2) those who were off DAPT during the complete follow-up interval; or 3) those who were not faithfully compliant to the daily dosage of DAPT or were unclear about what regimen they followed were classified as following “irregular use” of DAPT. In our primary analysis we compared together patients on DAPT with those off DAPT in combination with those reporting irregular use of DAPT. A secondary analysis was performed only on confirmed cases, i.e., after exclusion of the “irregular use” cases. The overall patient population was analysed first, and then the analysis was repeated among patients who received a DES during the index PCI.
Stent underexpansion was defined as a minimum stent area <5.0 mm by intravascular ultrasound imaging (IVUS). Stent malapposition was defined as the presence of blood speckles behind stent struts by IVUS. The presence of residual dissection or residual thrombus adjacent to a stent was assessed by IVUS.
The relationship between DAPT adherence status and the occurrence of definite/probable stent thrombosis was examined in a total of four time frames: up to 30 days, 1-6 months, 6-12 months and beyond one year from the index PCI with stent implantation for STEMI. Within each time frame, patients were grouped according to the occurrence of stent thrombosis. The rate of DAPT adherence was derived and compared between the groups using the Fisher’s exact test. The significance level was set at p<0.05. Descriptive statistics were used to report the rate of stent thrombosis related events that might have contributed to this event according to the relevant case report form.
Results
The summary of results in the overall population is given in Table 1 and Figure 1 and Figure 2. With respect to subacute stent thrombosis after index STEMI and primary angioplasty with stent implantation, the rate of discontinuation or irregular aspirin, thienopyridine or combined DAPT therapy was low, but also associated with stent thrombosis. Stent underexpansion, dissection and adjacent thrombus were reported. Although the confirmed DAPT discontinuation cases were rare, a statistical trend was documented towards discontinuation of either aspirin or thienopyridine and subacute thrombosis.
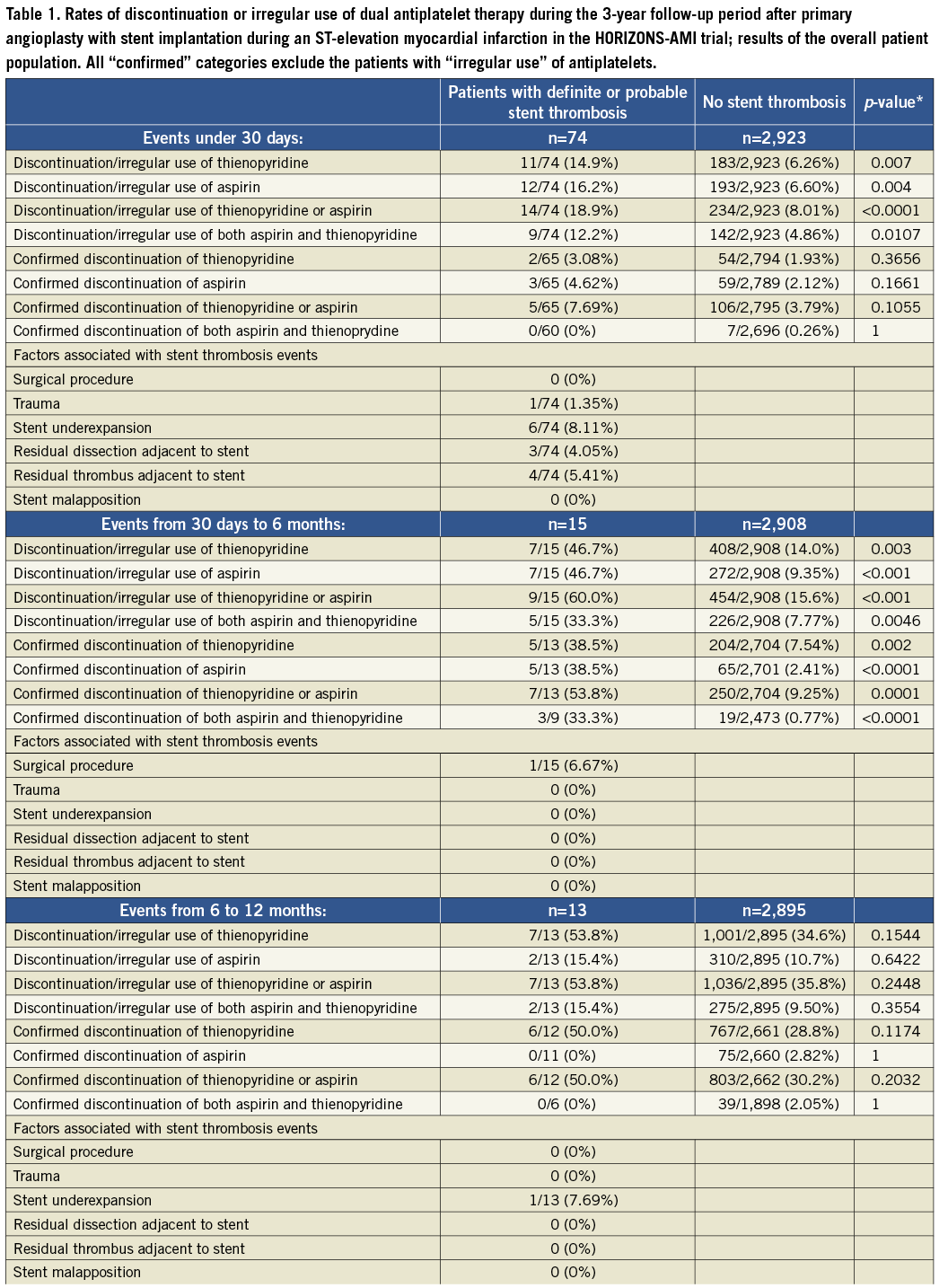
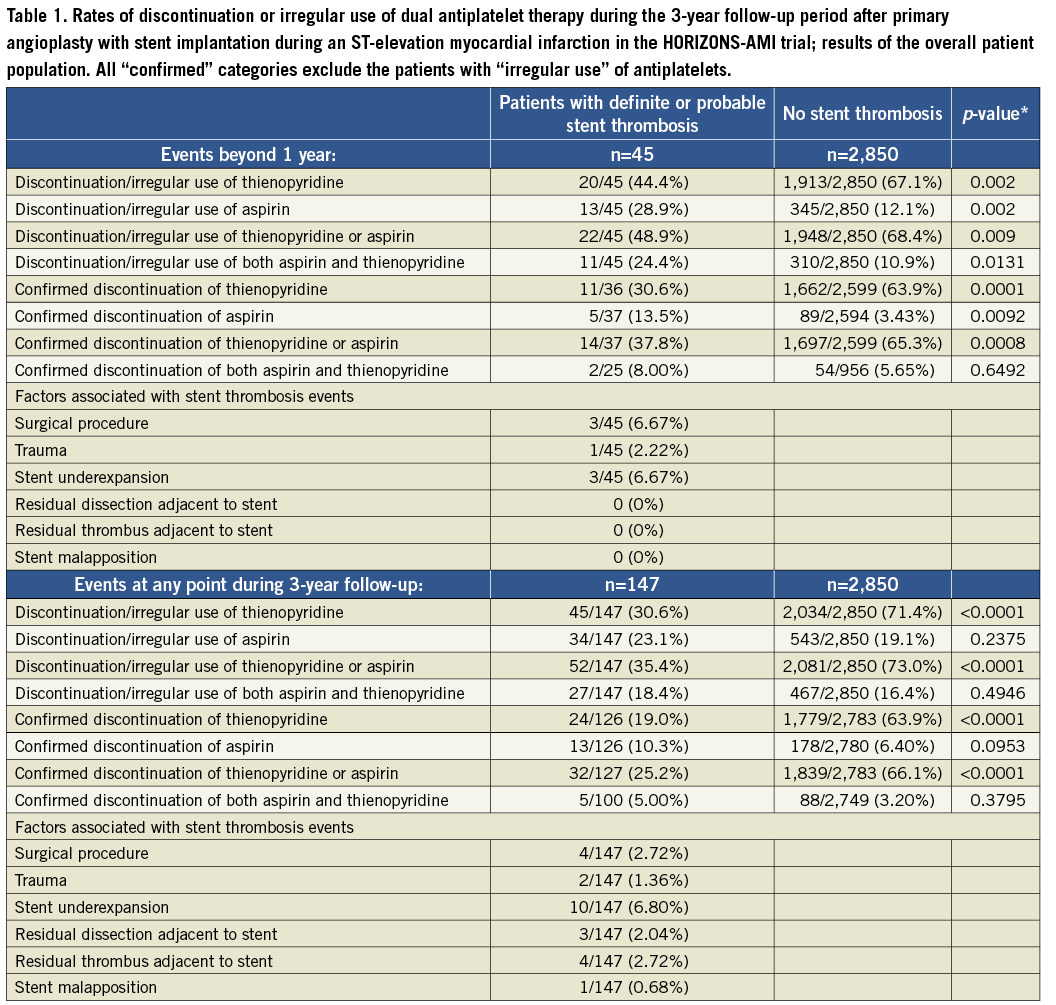
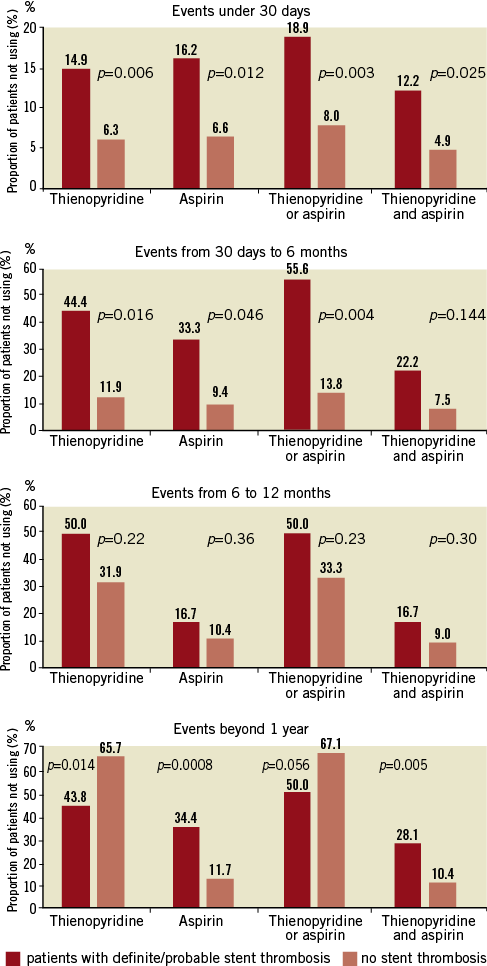
Figure 1. Rates of discontinuation or irregular use of dual antiplatelet therapy during the 3-year follow-up period in patients suffering a definite/probable stent thrombosis and those without stent thrombosis.
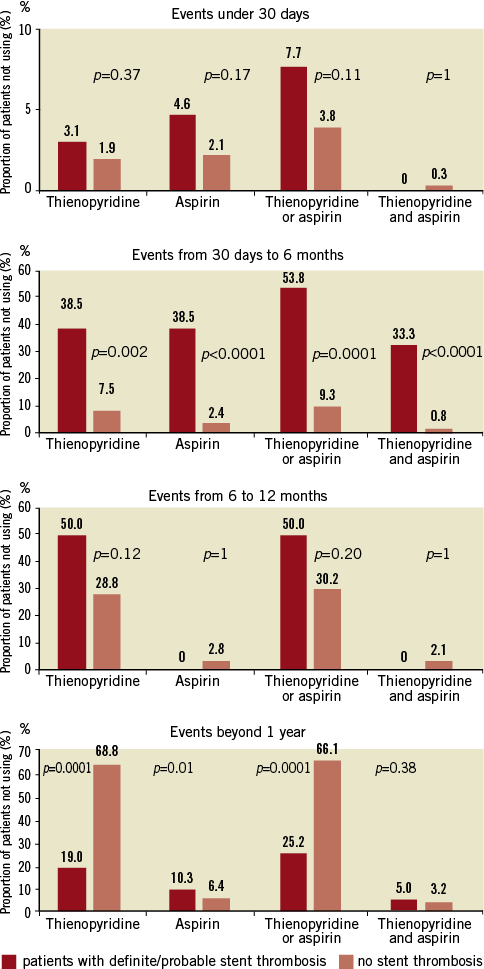
Figure 2. Rates of confirmed discontinuation of dual antiplatelet therapy during the 3-year follow-up period in patients suffering a definite/probable stent thrombosis and those without stent thrombosis.
With respect to late stent thrombosis between one and six months post implantation, we found a strong association between this complication and discontinuation of one or both antiplatelet drugs. These results were unaffected when only the confirmed DAPT discontinuation cases were considered. The only event reported as potentially relating to stent thrombosis was a surgical procedure.
Discontinuation of either aspirin, thienopyridine or both among patients with late stent thrombosis between six and 12 months was considerable (up to 54%), but not significantly different than in patients without stent thrombosis (35.8%, p=0.24). Results were similar when only confirmed DAPT discontinuation cases were considered. The only event reported as potentially relating to stent thrombosis was stent underexpansion.
Very late stent thrombosis was associated with higher discontinuation or irregular use of aspirin and of both DAPT drugs, but less discontinuation of thienopyridine in comparison to patients without very late stent thrombosis. Analysis of only the confirmed cases revealed largely concordant results. Few surgical procedures, a case of trauma and few cases of stent underexpansion were reported in relation to very late stent thrombosis.
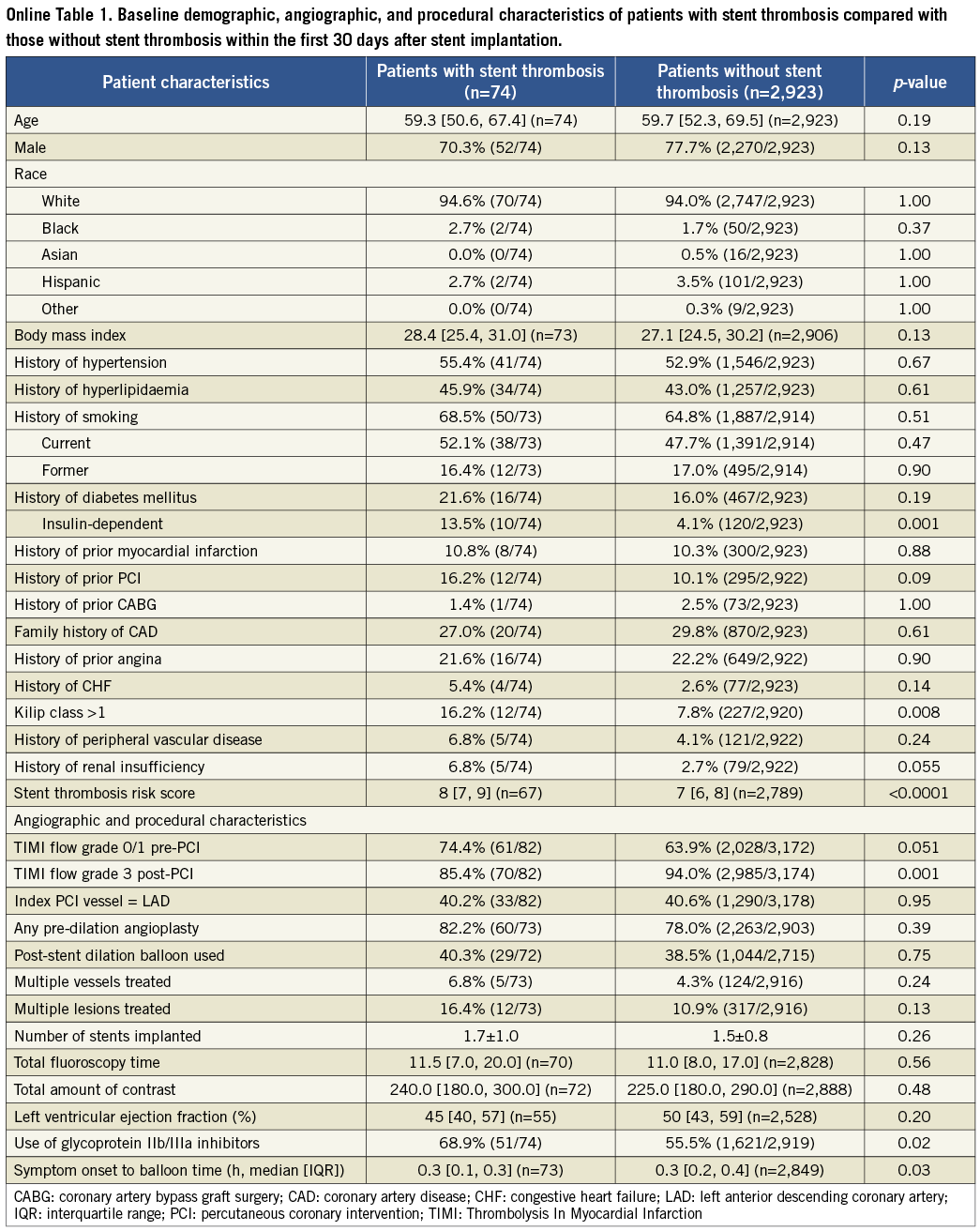
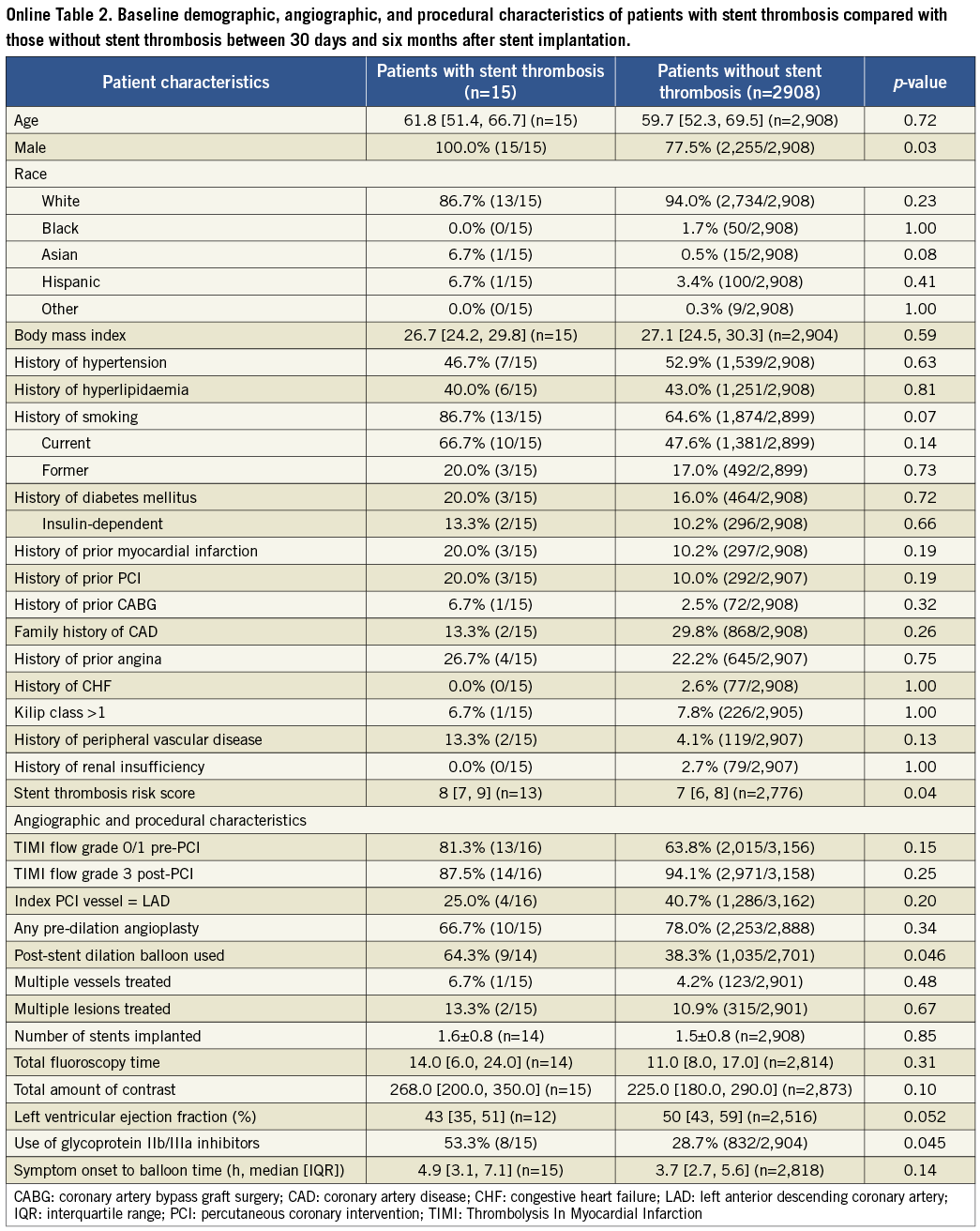
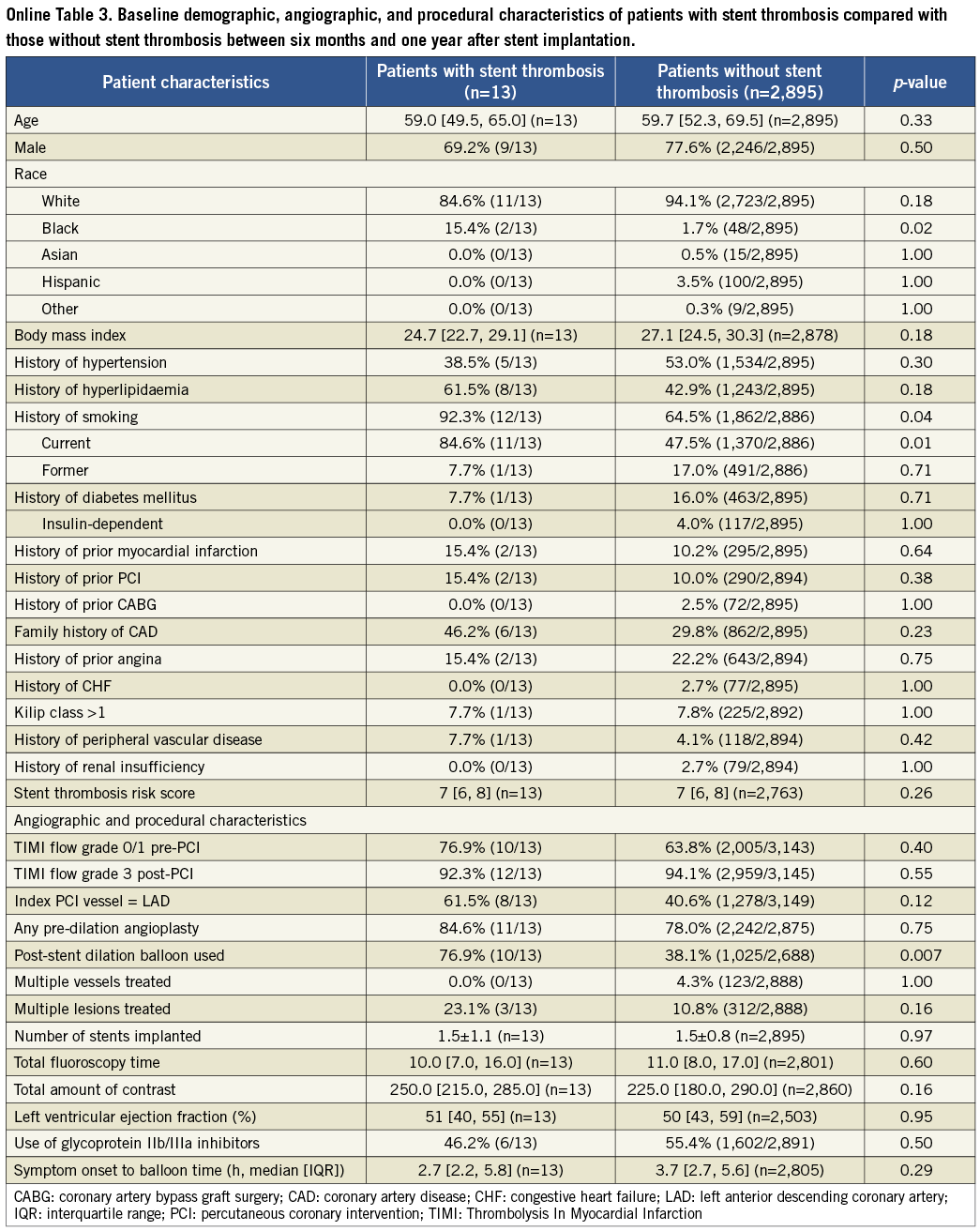
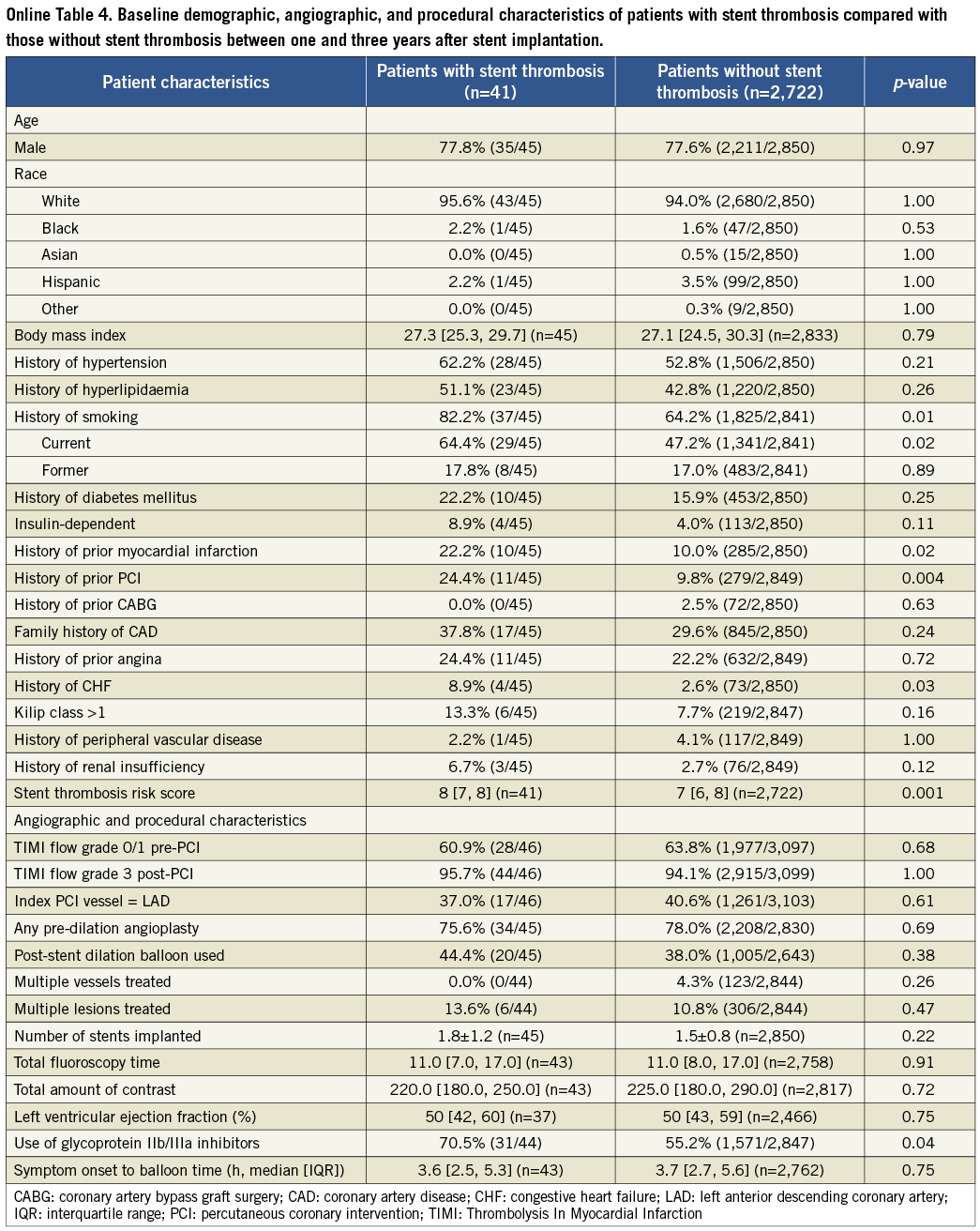
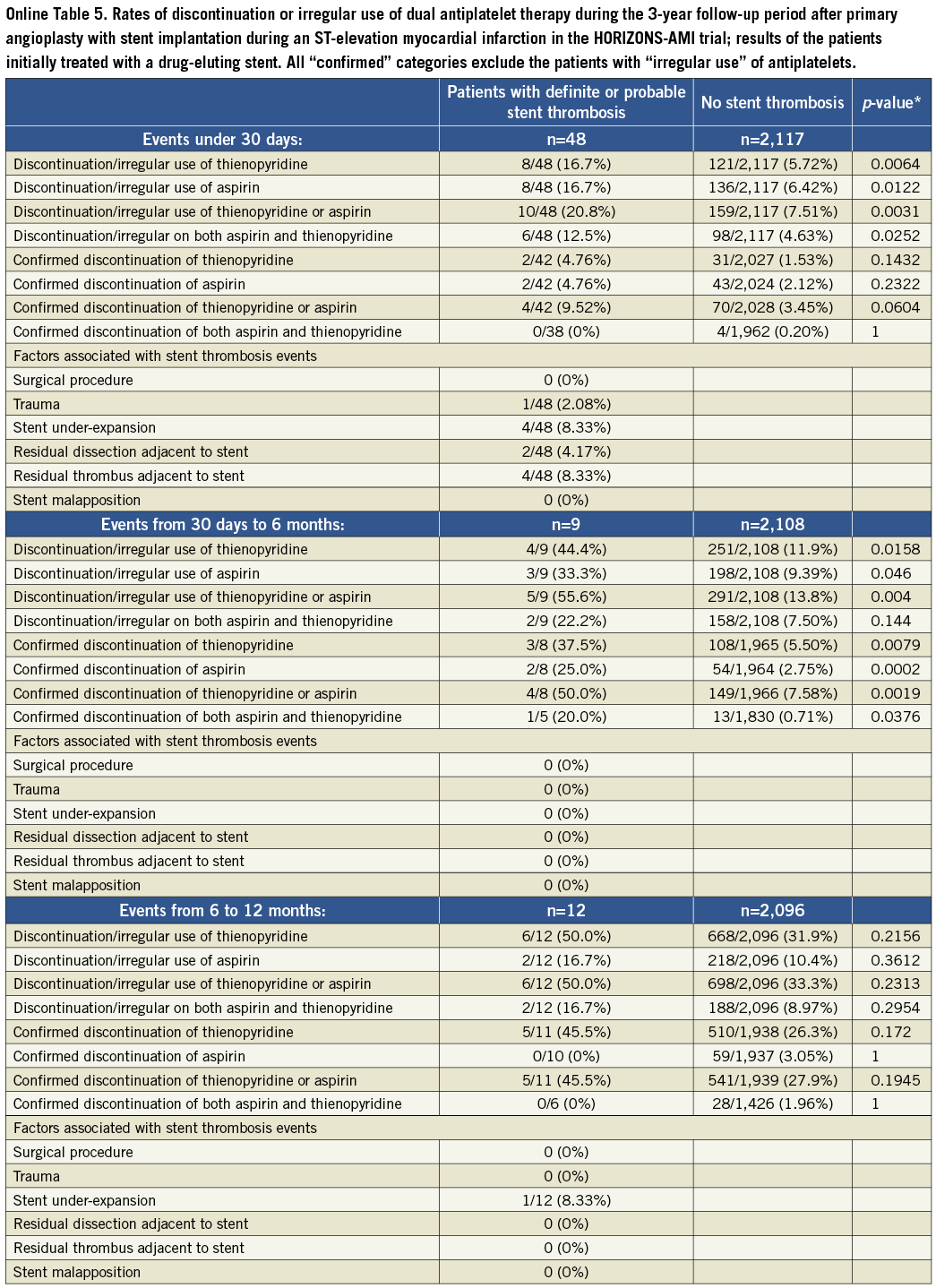
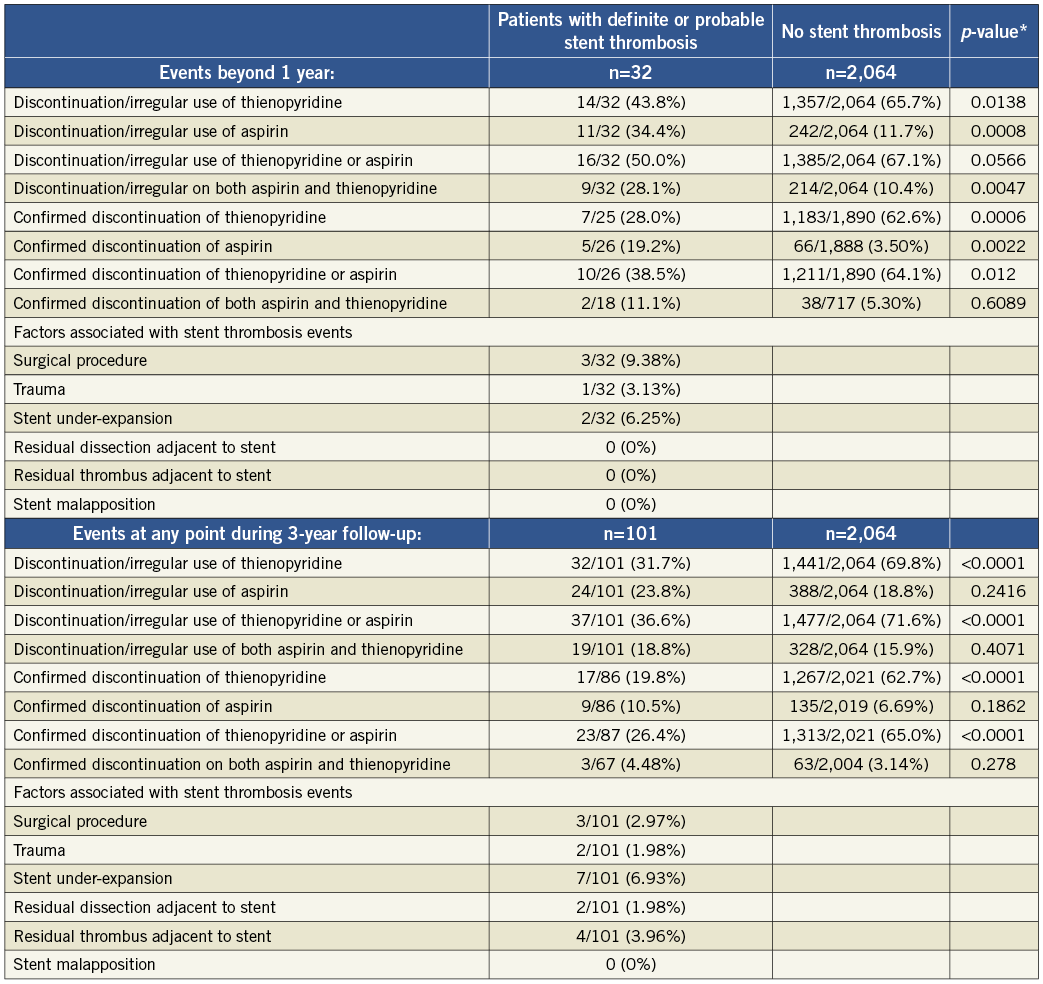
The results of the cohort of patients who received a drug-eluting stent during the original index procedure were qualitatively concordant with the above findings in the overall population, and consistent among the four time frames of stent thrombosis occurrence. These findings are summarised in Online Table 2. The Online appendix also includes baseline, procedure and angiographic data comparisons between the two groups that were compared at each time point.
Discussion
The rate of discontinuation or irregular use of one or both components of DAPT therapy after stent implantation occurs in variable rates during different post-procedure time frames but, as could be expected, increased beyond 12 months post-procedure. The recommended duration of DAPT after STEMI is 12 months and bears a Class I indication in both USA and European practice guidelines9, 10. However, there are subtle differences in the recommended duration of DAPT after elective PCI in both guidelines. Therefore, DAPT non-usage should be even more variable after stent implantation for non-urgent indications or outside the selection process for inclusion in a prospective randomised trial.
We found the relationship between DAPT usage and stent thrombosis to be quite variable over time. Subacute thrombosis has a strong relationship with discontinuation or irregular use of either one or both DAPT components. At the same time, DAPT non-adherence is rather low during the first month post-procedure. The “fresh” not yet endothelialised stent surface and the reported underexpanded struts and local dissections may explain why even not clearly confirmed DAPT non-adherence may be implicated in such events.
Late stent thrombosis appears to have a relationship to DAPT non-adherence during the 1-6 month time frame, and this relation appeared to fade during the 6-12 month time frame. These events remained largely unexplained, as only isolated events were reported preceding these adverse events. These findings support the results of the recently published PRODIGY (Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study) trial11. In PRODIGY 2,013 patients receiving a mixture of bare metal stents (BMS) and DES were randomised to a regimen of six or 24 months of clopidogrel therapy in addition to aspirin and showed that a 24-month regimen was not more effective than a six-month regimen.
Our findings on very late stent thrombosis were intriguing. First, the rate of DAPT non-usage was generally higher beyond one-year follow-up; thienopyridine discontinuation was over 50%. Second, several events were reported in association with stent thrombosis, including surgery, trauma and stent underexpansion. On the one hand, practice guidelines recommend postponing a surgical procedure, whenever possible, beyond one year after STEMI treated with PCI and stent implantation. Therefore, the above findings are understandable. On the other hand, surgery as well as all other reported events prior to stent thrombosis might indeed have created a prothrombotic milieu in certain cases. Discontinuation of aspirin or both DAPT components appeared to be strongly associated with stent thrombosis, whereas the opposite was evident for non-usage of thienopyridine alone.
Since our study utilised an imbalanced randomisation to drug-eluting versus bare metal stents (3:1), it is not surprising that the drug-eluting stent cohort yielded concordant results with the overall study population.
IMPLICATIONS
Previous studies have shown a considerable increase in the risk of developing ST in patients. Iakovou et al reported that premature antiplatelet therapy discontinuation was associated with an increased rate of ST. However, follow-up in this study was limited to nine months7. Airoldi et al also reported that discontinuation of thienopyridine was an important determinant of ST within the first six months of follow-up. With patients followed up to 18 months, the follow-up duration was again significantly shorter than in the current study12. The current study, which exclusively enrolled STEMI patients, therefore adds further insight into the relationship between DAPT non-usage and the occurrence of ST within a three-year follow-up period in this subgroup of patients at an increased risk of developing ST.
Based on our findings, DAPT therapy appears to be most important with respect to stent thrombosis avoidance during the first six months after stent implantation. Beyond one year, aspirin usage is very important and discontinuation of both agents should be avoided in relation to surgical procedures whenever feasible. These results may differ with non-paclitaxel-eluting DES, since lower stent thrombosis rates may be achieved with everolimus-eluting DES13,14. Randomised trials should further evaluate the utility of DAPT beyond six and 12 months after stenting.
Limitations
In this analysis, we did not have active surveillance for all non-cardiac surgical procedures or trauma that might have occurred in the group of patients who did not sustain a stent thrombosis event. In addition, clinical events that might have preceded a stent thrombosis event were collected by investigator initiative and were not adjudicated. The non-adherence to DAPT was assessed by a set questionnaire and did not include a pill count or other dedicated procedure. Both DAPT agents were not considered “investigational drugs” and were therefore administered through the regular prescription system of the treating cardiologists. However, we believe that the inclusion of STEMI patients who received a stent provided for a very strong clinical initiative to recommend and prescribe prolonged DAPT. Finally there was no periodic assessment of platelet inhibition in any of the study groups.
Funding
The HORIZONS-AMI trial was supported by the Cardiovascular Research Foundation, with grant support from Boston Scientific and The Medicines Company.
Conflict of interest statement
G.D. Dangas has received speaker honoraria from Astra Zeneca, Bristol-Meiers Squibb, The Medicines Company, Sanofi Aventis, and Abbott Vascular. R. Mehran has received a research grant from Sanofi Aventis, and honoraria from The Medicines Company, Abbott Vascular, Sanofi Aventis, Bristol Meiers Squibb, Cordis, and Astra Zeneca. G.W. Stone is on the scientific advisory boards for and has received honoraria from Abbott Vascular and Boston Scientific, and has served as a consultant to The Medicines Company, Eli Lilly BMS/Sanofi and AstraZeneca. The other authors have no conflicts of interest to declare.
Online data supplement
Table 1. Patient characteristics for time period 0-30 days after stent implantation
Table 2. Patient characteristics for time period 30 days to six months after stent implantation
Table 3. Patient characteristics for time period six months to one year after stent implantation
Table 4. Patient characteristics for time period 1-3 years after stent implantation
Table 5. Rates of discontinuation of antiplatelet therapy during 3 year follow-up in patients receiving drug-eluting stents