Abstract
Background: The role of direct oral anticoagulants (DOACs) in the treatment of left ventricular thrombus (LVT) after ST-elevation myocardial infarction (STEMI) remains uncertain.
Aims: We aimed to compare the effect of rivaroxaban versus warfarin in patients with STEMI complicated by LVT.
Methods: Adult patients with STEMI and two-dimensional transthoracic echocardiography showing LVT were assigned to rivaroxaban (15 mg once daily) or warfarin (international normalised ratio goal of 2.0-2.5) in an open-label, randomised clinical trial (RCT). A prospective pooled analysis was planned comparing DOAC- versus warfarin-based anticoagulation for the same indication. The main outcome of the RCT was complete LVT resolution at 3 months, determined by a blinded imaging core laboratory. Complete LVT resolution and bleeding were investigated in the pooled analysis.
Results: A total of 50 patients (median age: 55 years, 18% females) were enrolled from June 2020 to November 2022. Three-month complete LVT resolution occurred in 19/25 (76.0%) patients assigned to rivaroxaban and 13/24 (54.2%) assigned to warfarin (relative risk [RR] 1.40, 95% confidence interval [CI]: 0.91-2.15; p=0.12) with no thrombotic or major bleeding events. Pooled analysis showed numerically better complete LVT resolution with DOACs (rivaroxaban and apixaban; 93/115 [80.8%] vs 79/112 [70.5%], RR 1.14, 95% CI: 0.98-1.32; p=0.08) and less major bleeding (2/116 [1.7%] and 9/112 [8.0%], risk difference −0.06, 95% CI: −0.12 to 0.00; p=0.05) than with warfarin.
Conclusions: Although the findings are limited by a small sample size, the results suggest that DOACs are safe with at least similar outcomes concerning LVT resolution and major bleeding compared with warfarin. (ClinicalTrials.gov: NCT05705089)
Early revascularisation with primary percutaneous coronary intervention (pPCI) has reduced the incidence of left ventricular thrombus (LVT) formation1. However, according to a pooled analysis of 2,072 patients with recent ST-elevation myocardial infarction (STEMI), LVT is still observed in 6% of patients; this increases up to 19% in patients with anterior STEMI and reduced left ventricular (LV) function12. If left untreated, LVT is associated with a 4-fold increase in stroke/systemic embolisation and a 2-fold increase in long-term mortality3.
Warfarin has historically been used for treating LVT, and direct oral anticoagulants (DOACs) have recently gained attention, with studies in routine practice indicating their frequent use. However, there is scant evidence to support (or refute) the effectiveness of DOACs in leading to LVT resolution and their safety with respect to bleeding events45. Recently, a few, small randomised clinical trials (RCTs) showed promising early results for DOACs compared with warfarin in patients with LVT, but there is a need for more evidence6.
We compared 3-month core laboratory-confirmed imaging findings and clinical outcomes in patients with STEMI randomised to rivaroxaban or warfarin in a pilot clinical trial. Recognising that the RCT was planned with a relatively small number of enrollees, a priori, a meta-analysis was planned and registered (PROSPERO: CRD42023455855) to evaluate existing RCTs that compared DOACs versus warfarin in patients with STEMI complicated by LVT.
Methods
Trial oversight and design
Rivaroxaban vErsus Warfarin for Antithrombotic TheRapy in Patients with LeFt Ventricular Thrombus After Acute ST-Elevation Myocardial Infarction (REWARF-STEMI) was an open-label, parallel-group, blinded-outcome pilot RCT conducted at two large tertiary cardiovascular centres in Tehran, Iran: Tehran Heart Center and Rajaie Cardiovascular Institute. The study protocol (Supplementary Appendix) was approved by the ethics committees of both centres (Ethics code: IR.TUMS.THC.REC.1399.004). All patients provided written informed consent. A clinical events committee (CEC) blinded to assigned treatments adjudicated all the clinical outcomes (Supplementary Appendix). An independent data and safety monitoring committee monitored the trial results (Supplementary Appendix).
Study population
Adult patients aged between 18 and 80 years old presenting with LVT, confirmed by non-contrast two-dimensional transthoracic echocardiography (2D TTE), within 2 weeks of confirmed STEMI7 were eligible for the study. Patients with contraindications to DOACs (such as a mechanical prosthetic heart valve implantation, rheumatic heart disease, or antiphospholipid syndrome [APS]48), active bleeding, cardiogenic shock, estimated glomerular filtration rate (eGFR) <30 ml/min or those already receiving anticoagulation for other indications were excluded from the study. A full description of eligibility criteria is listed in the study protocol (Supplementary Appendix).
Randomisation and treatment strategy
Randomisation was performed via a permuted block method with a block size of 4, using a web-based application with a 1:1 allocation ratio. Patients were randomised to receive either rivaroxaban- or warfarin-based antithrombotic regimens. Those assigned to rivaroxaban received rivaroxaban (15 mg once daily, orally) plus clopidogrel (75 mg daily, orally) and aspirin (80 mg once daily, orally). In the warfarin-based antithrombotic therapy group, patients received warfarin (overlapping with enoxaparin until reaching an international normalised ratio [INR] goal of 2.0-2.5) plus clopidogrel (75 mg once daily, orally) and aspirin (80 mg once daily, orally). In both groups, aspirin was discontinued within the first 7 days of the STEMI diagnosis. Time in the therapeutic range (TTR) was calculated based on the Rosendaal method9.
Clinical follow-up
Following randomisation, patients were visited weekly during the first month and monthly thereafter, until the end of the 3-month follow-up. At each visit, patients’ new complaints and anticoagulation status were recorded. INR monitoring was planned during each visit for patients allocated to warfarin. For patients with non-therapeutic INR levels, shorter monitoring intervals were scheduled until reaching a therapeutic INR.
Echocardiographic assessment
The diagnosis and follow-up of LVT were based on non-contrast 2D TTE, mainly due to the unavailability of echocardiographic contrast agents in Iran. Although the sensitivity of contrast echocardiography is higher than non-contrast echocardiography (61% vs 33%) in diagnosing LVT, the specificity of non-contrast echocardiography is high (94%)10. Cardiac magnetic resonance was not selected because of limited resources.
Patients with STEMI routinely underwent non-contrast 2D TTE, performed by the on-call cardiologist during the first 24 hours of hospitalisation, at both enrolling centres. All patients with new LVT according to the on-call cardiologist were subsequently assessed by an expert cardiologist with a subspeciality in echocardiology, blinded to the assigned treatment, to confirm the diagnosis before the enrolment in the trial.
The same assigned expert cardiologist obtained the follow-up images for each patient who completed 3-month follow-up. All follow-up images were stored, deidentified, and sent for evaluation by the trial imaging core laboratory, consisting of two independent echocardiologists who remained blinded to the assigned treatments. All conventional measurements were carried out following the latest recommendations by the American Society of Echocardiography and the European Association of Cardiovascular Imaging11. Intra- and interobserver variability were tested by assessment of a series of deidentified cases for a second evaluation by the same operator and evaluation by a second operator of the core laboratory, respectively. A third operator resolved any discrepancies. Echocardiograms were acquired in the standard parasternal short- and long-axis views and apical 2-, 3- and 4-chamber view imaging planes (Supplementary Appendix).
Study outcomes
The primary outcome was complete LVT resolution at the 3-month follow-up based on non-contrast 2D TTE, determined by the imaging core laboratory. Other outcomes were the proportion of patients with adjudicated stroke and systemic embolism (SSE), major adverse cardiac events (MACE; a composite of death from cardiovascular causes, myocardial infarction [MI], or SSE), and all-cause death at 3 months from enrolment. The main prespecified safety outcome was the proportion of patients with adjudicated major bleeding events based on the International Society on Thrombosis and Haemostasis (ISTH) definition at 3 months from enrolment. Clinically relevant non-major bleeding (CRNMB) events based on the ISTH definition were also ascertained (Supplementary Appendix). All outcomes were adjudicated by a CEC blinded to assigned treatments.
Systematic literature review and meta-analysis
A systematic review and meta-analysis were planned, and the protocol was registered in PROSPERO (CRD42023455855) before the results of the current RCT were known. It was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline12. A systematic syntax search was devised using the relevant keywords to search the literature in MEDLINE (via PubMed) and the Cochrane Library up to 9 November 2023 (Supplementary Appendix, Supplementary Table 1). The records were deemed eligible if they had an RCT design including adult patients (aged ≥18 years) with MI being treated in two distinct arms of antithrombotic regimens with DOACs versus warfarin and if they reported LVT resolution during follow-up at 3 months or a time interval close to that. The main outcomes for the prospective meta-analysis mirrored those of the currently reported trial. The risk of bias was assessed using the Cochrane Risk of Bias tool version 2 (RoB 2) (Supplementary Appendix)13.
Statistical analysis
Categorical variables are expressed as frequency counts with percentages. Continuous variables are described as the mean and standard error of the mean if normal distribution was confirmed, and if the variables were not normally distributed, data are described as median (interquartile range [IQR]). Due to the exploratory nature of the pilot RCT, no formal sample size calculation was carried out. A sample size of 25 in each arm was planned. The primary outcome, complete LVT resolution at the 3-month follow-up, was analysed in patients with valid values, i.e., those who were alive and agreed to participate in the 3-month follow-up visit. Other outcomes were analysed in all randomly assigned patients.
The effect of the intervention on the outcomes was reported with relative risk (RR) and risk difference as the measures of effect, with their respective 95% confidence intervals (CIs).
For the meta-analysis, complete LVT resolution was the main efficacy outcome, and major bleeding was the safety outcome, comparing the pooled effectiveness of DOACs versus warfarin. The meta-analysis used the common-effect Mantel-Haenszel method, and the overall effect was reported with RR as the effect measure, except for outcomes with zero events in some trials, in which case risk difference was used as the effect measure. Further information on the methodology and statistical considerations for the meta-analysis are summarised in the Supplementary Appendix. Statistical analyses were performed using R version 4.2.1 (R Foundation for Statistical Computing).
Results
From June 2020 to November 2022, 55 patients with STEMI and LVT were screened for eligibility. Four patients did not consent, and one was excluded because of an eGFR below 30 ml/min; thus, 50 patients (median age [IQR]: 55505152535455565758596061 years; 9 females [18%]) were included in the study, of whom 26 and 24 patients were randomly assigned to the rivaroxaban- and warfarin-based antithrombotic regimens, respectively (Figure 1, Supplementary Appendix).
The two study groups were balanced regarding baseline clinical, procedural, and imaging characteristics (Table 1). The majority of patients (45/50 [90%]) had anterior STEMI (Supplementary Table 2). Forty-nine (98%) patients underwent pPCI, and one patient in the rivaroxaban group proceeded to emergent balloon angioplasty, followed by urgent coronary bypass graft surgery due to significant thrombotic involvement of the left main coronary artery in the diagnostic coronary angiography. All patients in both groups were compliant with and adherent to the assigned treatments. One patient in the rivaroxaban group had sudden death while sleeping 31 days post-randomisation. Consequently, 49 out of 50 (98%) patients completed the 3-month follow-up required for the primary outcome assessment. In patients treated with warfarin, the median TTR was 63% (IQR 61-66%) according to the Rosendaal method.
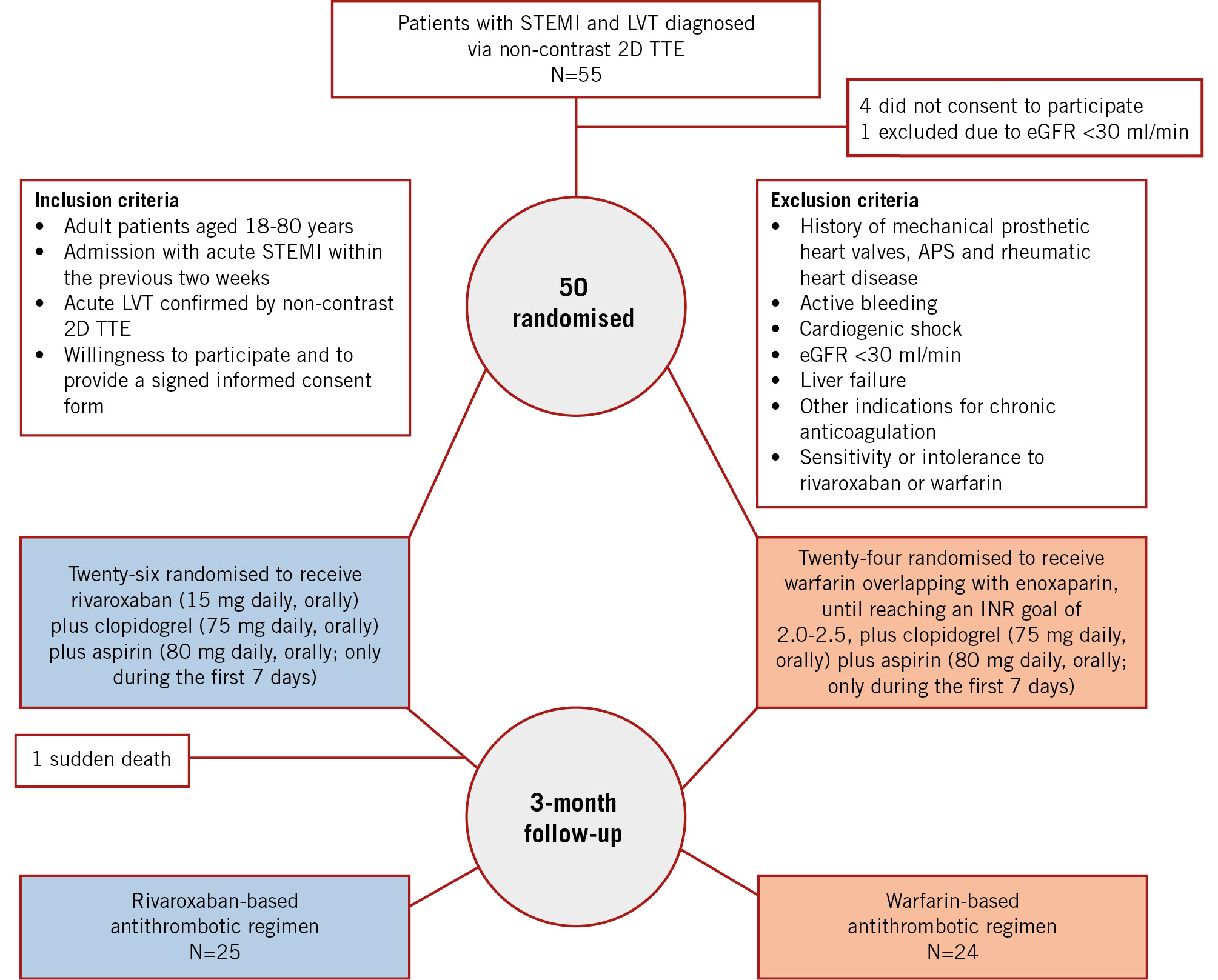
Figure 1. Trial flow diagram. 2D: two-dimensional; APS: antiphospholipid syndrome; eGFR: estimated glomerular filtration rate; INR: international normalised ratio; LVT: left ventricular thrombus; STEMI: ST-elevation myocardial infarction; TTE: transthoracic echocardiography
Table 1. Baseline clinical, imaging, and procedural characteristics in REWARF-STEMI.
| Rivaroxaban (N=26) | Warfarin (N=24) | |
|---|---|---|
| Age, years | 55 (50-60) | 55 (50.00-62.75) |
| Female sex | 4 (15.3) | 5 (20.8) |
| Body mass index, kg/m2 | 26.3 (24.5-27.7) | 25 (23-28) |
| Previous medical condition | ||
| Diabetes mellitus | 7 (26.9) | 5 (20.8) |
| Hypertension | 9 (34.6) | 14 (58.3) |
| Current smoker | 11 (42.3) | 10 (41.7) |
| Coronary artery disease | 9 (34.6) | 6 (25.0) |
| Ischaemic stroke | 3 (11.5) | 1 (4.1) |
| Previous coronary revascularisation | ||
| Percutaneous coronary intervention | 5 (19.2) | 3 (12.5) |
| Coronary artery bypass graft | 1 (3.8) | 0 (0) |
| Laboratory values at baseline | ||
| Creatinine, mg/dl | 1.1 (1.02-1.23) | 1.1 (0.9-1.3) |
| Haemoglobin, mg/dl | 14.8 (14.1-16.1) | 14.7 (13.2-15.7) |
| Platelets x 103/µl | 211.5 (193-247) | 210 (187-297) |
| Imaging characteristics | ||
| Left ventricular ejection fraction, % | 32 (25-40) | 30 (25-35) |
| Thrombus long-axis diameter, mm | 15 (9.75-18) | 18 (14-22.7) |
| Thrombus short-axis diameter, mm | 8 (5-10) | 9 (5-17) |
| Revascularisation strategy for acute myocardial infarction | ||
| Primary percutaneous coronary intervention | 25 (96.1) | 24 (100) |
| Coronary artery bypass graft | 1 (3.9) | 0 (0) |
| Data are presented as median (25th-75th percentile) or n (%). | ||
Primary outcome
Three-month complete LVT resolution occurred in 19/25 (76.0%) patients assigned to rivaroxaban versus 13/24 (54.2%) patients assigned to warfarin (RR 1.40, 95% CI: 0.91-2.15; p=0.12) (Table 2).
Table 2. Three-month study outcomes in the REWARF-STEMI trial population.
| Outcome | Rivaroxaban N=26 | Warfarin N=24 | Relative risk (95% CI) | Risk difference (95% CI) | p-value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Complete LVT resolution | 19/25 (76.0)* | 13/24 (54.2) | 1.40 (0.91-2.15) | 0.22 (−0.04 to 0.48) | 0.12 |
| Other outcomes | |||||
| All-cause death | 1/26 (3.8) | 0 | NA | 0.04 (−0.03 to 0.11)† | 0.30 |
| MACE | 1/26 (3.8) | 0 | NA | 0.04 (−0.03 to 0.11)† | 0.30 |
| SSE | 0 | 0 | NA | NA | NA |
| Major bleeding | 0 | 0 | NA | NA | NA |
| CRNMB | 2/26 (7.7) | 0 | NA | 0.07 (−0.02 to 0.18)† | 0.14 |
| Data are presented as n/N (%). *Calculated based on the population who completed the 3-month follow-up (i.e., all participants except the one who died before the 3-month follow-up). †For events with zero incidence in one group, only risk difference was reported. CI: confidence interval; CRNMB: clinically relevant non-major bleeding; LVT: left ventricular thrombus; MACE: major adverse cardiac events; NA: not applicable; SSE: stroke and systemic emboli | |||||
Other outcomes
There were no SSE events. Two CRNMB events occurred in the rivaroxaban group: one patient had haematuria and one had rectorrhagia, which were both treated conservatively in the outpatient setting. No major bleeding occurred in any of the patients during the study follow-up time (Table 2).
Meta-analysis
The screening process, data extraction, synthesis, and systematic review of the included RCTs are described in detail in the Supplementary Appendix and in Supplementary Figure 1. Based on the eligibility criteria, four prior RCTs, along with the current study, were included in the meta-analysis14151617, including a total of 228 patients with post-MI LVT. Of these, 116 patients were assigned to DOACs (51 patients to apixaban and 65 to rivaroxaban, respectively), and 112 patients were assigned to warfarin14151617 (Figure 2).
Complete LVT resolution occurred in 93/115 (80.8%) patients in the DOAC-based regimen and 79/112 (70.5%) in the warfarin-based regimen (RR 1.14, 95% CI: 0.98-1.32; p=0.08) (Figure 3A, Supplementary Figure 2A).
Major bleeding occurred in 2/116 (1.7%) and 9/112 (8%) patients in the DOAC- and warfarin-based regimens, respectively (risk difference −0.06, 95% CI: −0.12 to 0.00; p=0.05) (Central illustration, Figure 3B, Supplementary Figure 2B).
One study (Isa et al)17 had an overall “low risk”, and the other studies141516 (including REWARF-STEMI) had “some concerns” in terms of risk of bias (Supplementary Appendix, Supplementary Figure 3). No evidence of publication bias was identified (Supplementary Figure 4A-Supplementary Figure4B).
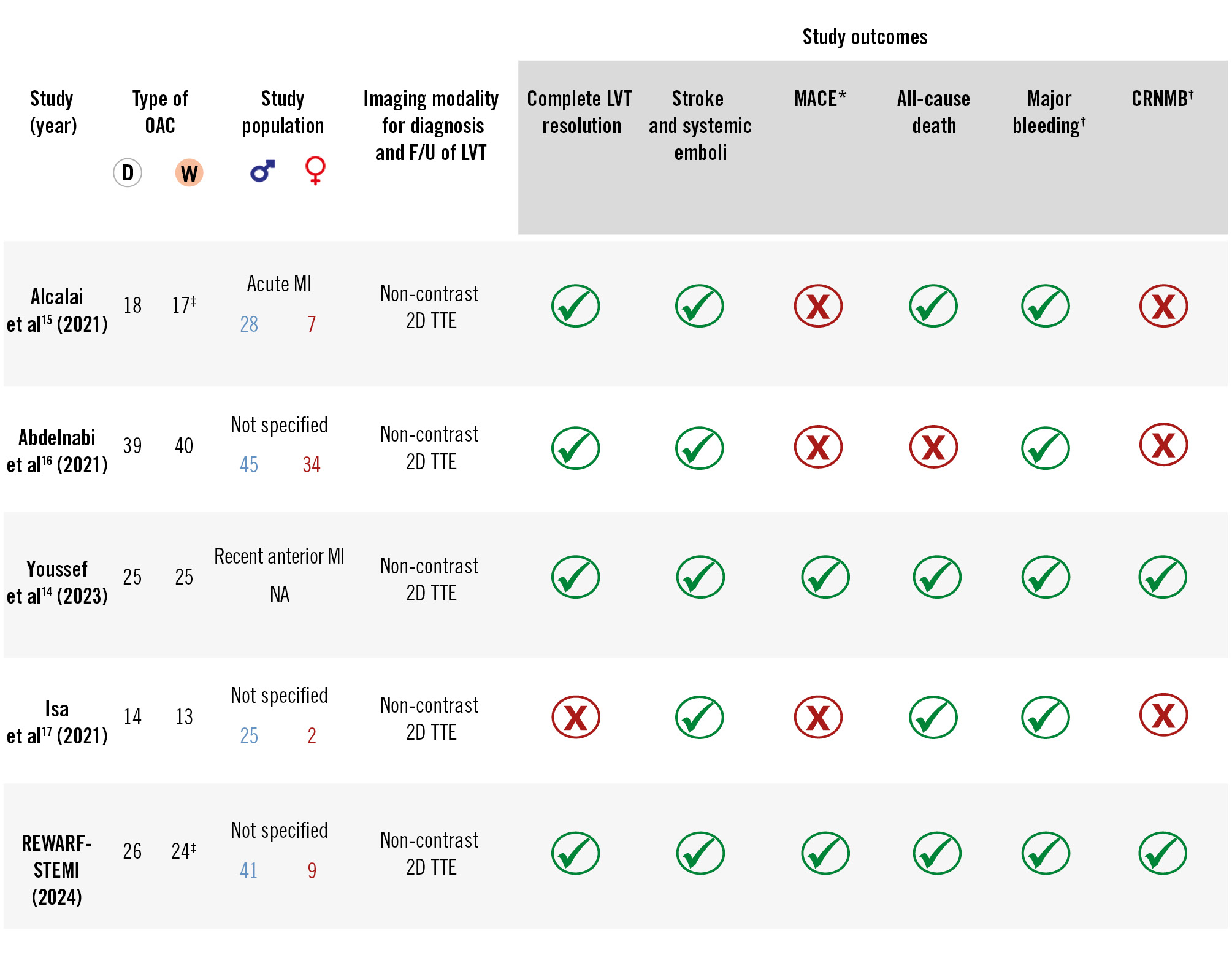
Figure 2. Summary of the baseline characteristics and reported outcomes in randomised clinical trials included in the pooled analysis. Note: In all studies, apixaban 5 mg was administered (except for Abdelnabi et al16 and REWARF-STEMI administering rivaroxaban 20 mg and 15 mg, respectively), and warfarin was adjusted to achieve an INR of 2-3. *MACE: a composite of death from cardiovascular causes, MI, or stroke. †Three trials (Alcalai et al15, Abdelnabi et al16, and REWARF-STEMI) and one trial (Youssef et al14) defined bleeding based on ISTH and BARC definitions, respectively, and one trial (Isa et al17) did not use a specific definition. ‡In the RCT by Alcalai et al15, at 3 months, 17 and 15 patients were followed up in the DOAC and warfarin groups, respectively; in REWARF-STEMI, at 3 months, 25 and 24 patients were followed up in the DOAC and warfarin groups, respectively. 2D: two-dimensional; BARC: Bleeding Academic Research Consortium; CRNMB: clinically relevant non-major bleeding; D: direct; F/U: follow-up; INR: international normalised ratio; ISTH: International Society on Thrombosis and Haemostasis; LV: left ventricular; LVT: left ventricular thrombus; MACE: major adverse cardiac events; MI: myocardial infarction; NA: not available; OAC: oral anticoagulant; TTE: transthoracic echocardiography; W: warfarin
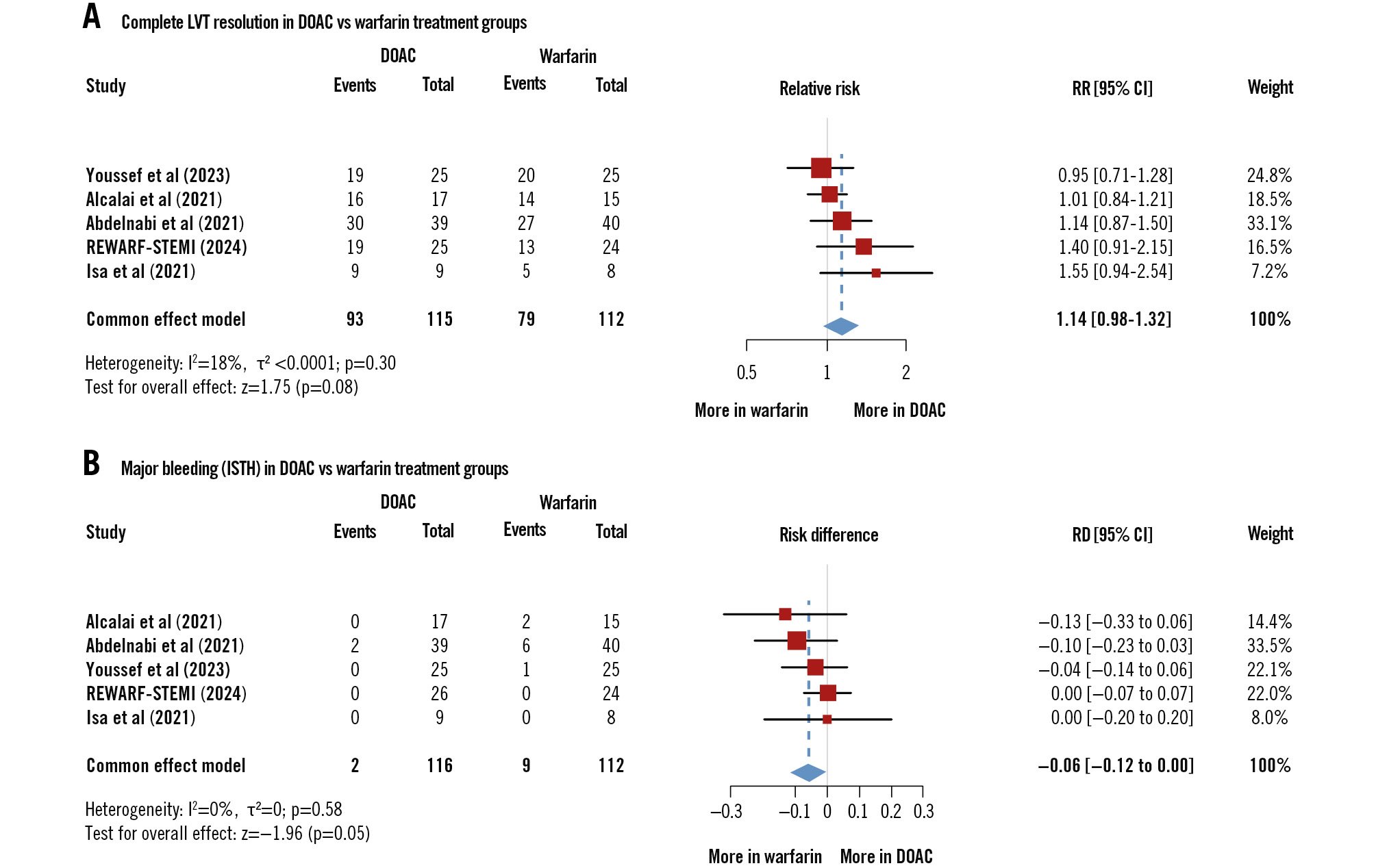
Figure 3. Pooled analysis of complete left ventricular thrombus resolution and major bleeding. A) Complete LVT resolution in DOAC versus warfarin treatment groups. B) Major bleeding in DOAC and warfarin treatment groups. Note: The study by Isa et al17 was conducted on patients with LVT, out of whom only patients with post-AMI LVT were included in the meta-analysis, as confirmed by the corresponding author. Similarly, the study by Abdelnabi et al16 did not differentiate between post-AMI LVT and LVT due to other causes. However, they did not respond to our multiple inquiries. AMI: acute myocardial infarction; CI: confidence interval; DOAC: direct oral anticoagulant; ISTH: International Society on Thrombosis and Haemostasis; LVT: left ventricular thrombus; RD: risk difference; RR: relative risk
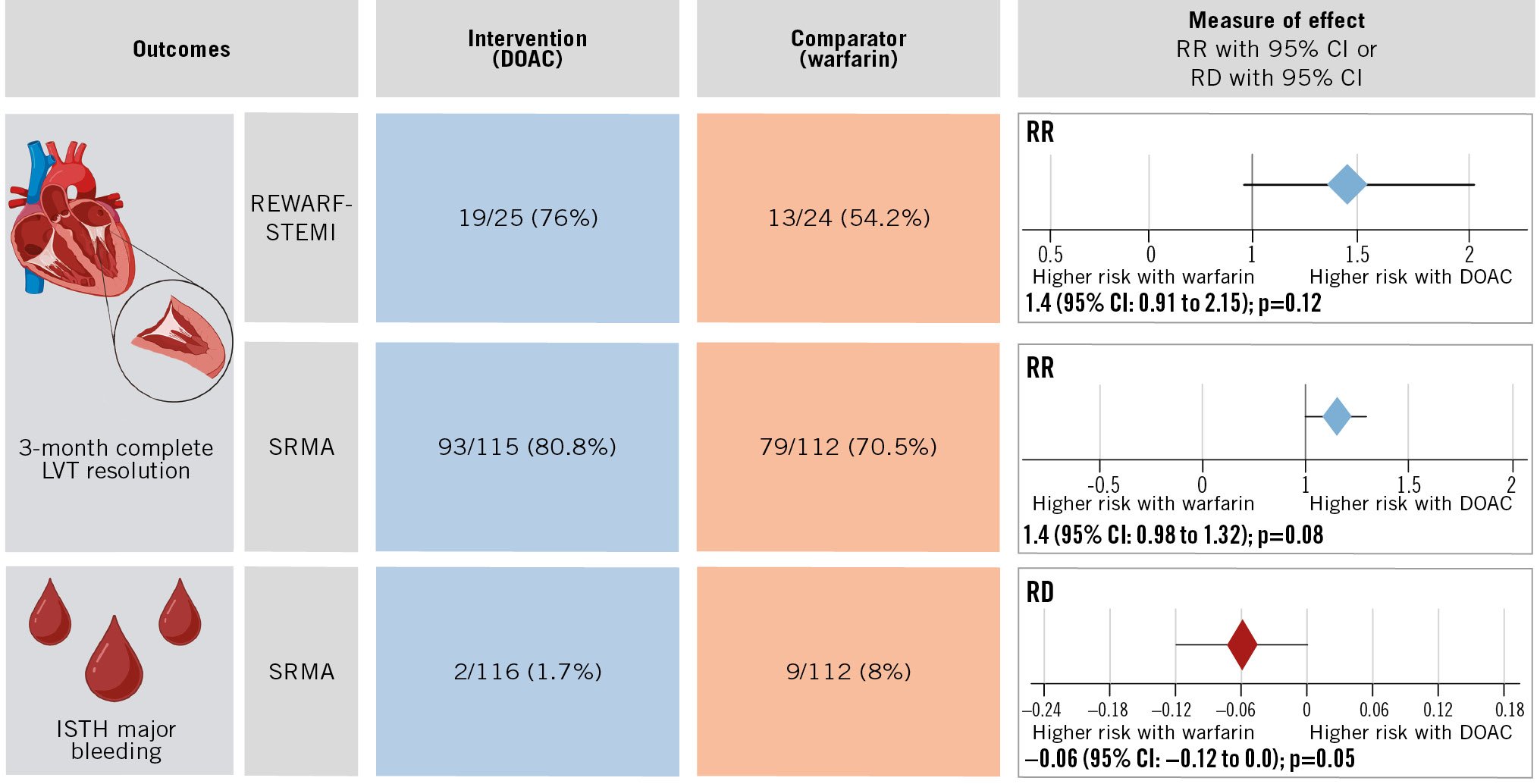
Central illustration. Summary of REWARF-STEMI trial results and published RCTs on the role of DOAC versus warfarin in patients with echocardiographically diagnosed LVT after STEMI. No ISTH major bleeding event was registered in REWARF-STEMI. CI: confidence interval; DOAC: direct oral anticoagulant; ISTH: International Society on Thrombosis and Haemostasis; LVT: left ventricular thrombus; RCT: randomised clinical trial; RD: risk difference; REWARF-STEMI: Rivaroxaban vErsus Warfarin for Antithrombotic TheRapy in Patients with LeFt Ventricular Thrombus After Acute ST-Elevation Myocardial Infarction; RR: relative risk; SRMA: systematic review and meta-analysis; STEMI: ST-elevation myocardial infarction
Discussion
In this RCT of 50 patients with STEMI complicated by LVT, three-quarters and nearly a half of the patients treated with rivaroxaban- and warfarin-based antithrombotic regimens, respectively, had complete LVT resolution. No major thromboembolic, ischaemic, or bleeding events were observed in either group. More importantly, in the pooled analysis of available RCTs, including the present study, there were no significant differences between DOAC- and warfarin-based regimens in terms of complete LVT resolution or major bleeding events, with the 95% CI estimates suggesting that it would be very unlikely that DOACs fared worse than warfarin for either effectiveness or safety (Central illustration). Although none of the individual trials nor the pooled analysis were formally planned for non-inferiority testing18, the lower bound of the 95% CI for reduced efficacy (for LVT resolution) of DOACs, compared with warfarin, is far smaller than the margin of reduced efficacy for RCTs of stroke prevention in atrial fibrillation (AF; a margin of relative excess risk of 1.38-1.46 in prior trials)19 or acute treatment of venous thromboembolism (a margin of relative excess risk of 1.50-2.75)20. Similarly, for bleeding, pooled results were favourable for DOACs, with 95% CIs indicating that bleeding events with DOACs were at least not higher than with warfarin (upper bound of the 95% CI for risk difference: 0.00). In summary, the current best evidence, albeit still limited by the relatively small sample size, is suggestive that DOACs are at least as effective and as safe as warfarin for the treatment of LVT.
For STEMI patients with LVT, the ideal primary efficacy outcome is SSE events1. To date, as shown by our systematic search, no RCTs have been powered to compare different antithrombotic strategies for hard endpoints. In fact, embolic events, particularly systemic embolism other than stroke, were not even consistently reported in the available trials14151617. However, the 3-month complete LVT resolution is often regarded as a measure to stop anticoagulation due to the negligible risk of future embolic events after LVT resolution6. It is conceivable that if participants had major embolic events, the trialists would have reported such outcome data. Our pooled analysis showed complete LVT resolution in the majority of DOAC-treated patients (80.8%), which is statistically not different from patients treated with warfarin (70.5%).
Some professional societies have already considered DOACs as a potential alternative to warfarin for the treatment of LVT621. However, prior experience related to the reduced efficacy of DOACs in conditions such as thrombotic APS8 or AF in patients with rheumatic heart disease22 raised uncertainty about those recommendations4. Findings from the current RCT and the pooled analysis of RCTs presented in this manuscript are in agreement with statements by professional societies such as the American Heart Association6, suggesting that DOACs can be a viable option for the treatment of LVT. In a recently published meta-analysis on 22 studies comparing DOACs versus warfarin in patients with LV thrombosis, the use of DOACs was not associated with a significant increase in stroke or systemic embolism (odds ratio [OR] 0.81, 95% CI: 0.57-1.15) compared with warfarin23. The odds of thrombus resolution were not significantly different between the groups (OR 1.12, 95% CI: 0.86-1.46). The authors reported lower odds of all-cause mortality (OR 0.65, 95% CI: 0.46-0.92) and a composite bleeding endpoint (OR 0.67, 95% CI: 0.47-0.97) with the use of DOACs compared to warfarin. Of note, 18 of the 22 included studies were retrospective, patients with different aetiologies (ischaemic vs non-ischaemic) of LV thrombosis were all eligible for the final analysis, major bleeding was not separately reported, and REWARF-STEMI was not included in that meta-analysis23.
It should, however, be considered that the sample sizes for the existing individual RCTs are small, ranging from 27-79 patients, making it unfeasible to assess hard clinical endpoints with sufficient power (Figure 2). In the existing RCTs, apixaban (5 mg twice daily) and rivaroxaban (15 to 20 mg once daily) were the DOACs administered. Different dual antiplatelet therapy regimens and durations are assigned for different studies, and thus, the safety of dual versus triple therapy is still inconclusive in the LVT population. Findings from some additional ongoing trials24 (ClinicalTrials.gov: NCT03764241, NCT04970576, NCT05892042, NCT05973188, NCT05794399, NCT03415386, as summarised in the Supplementary Appendix and Supplementary Table 3) have the potential to improve the confidence in alternative strategies to warfarin for LVT.
Limitations
This study has several limitations. First, the sample size and the pilot nature of the original trial rendered the trial underpowered for its results. The pooled analysis of findings from REWARF-STEMI and previous pilot RCTs was prespecified and had consistent results. Second, the trial included few female individuals. However, this is largely reflective of the disease epidemiology, which is consistent with the disproportionately higher relative frequency of LVT post-STEMI in male individuals compared to females25262728. Third, contrast echocardiography was not performed in the trial, in large part due to resource limitations. However, prior RCTs on this subject14151617 also used non-contrast 2D TTE which had high specificity (98%)29 and reasonable positive predictive value (72%) (Supplementary Appendix, Supplementary Figure 5), and this has been endorsed by the American Heart Association statement6 as one of the acceptable modalities for the diagnosis of LVT. Given the randomised design, any limited sensitivity would have affected the results similarly in both groups. Lack of clinical (stroke) events further reduces the possibility of missing major clinically relevant residual LVT. Of note, the majority of included patients had anterior STEMI, in whom non-contrast 2D TTE has higher sensitivity2. Finally, only two centres participated in patient recruitment for REWARF-STEMI, which limits the generalisability of the findings. However, the results are consistent with other independent trials reported in the pooled analysis.
Conclusions
Findings from the REWARF-STEMI pilot trial of patients with STEMI complicated by LVT, paired with the preplanned meta-analysis of RCTs presented herein, suggest that DOACs are at least as effective and safe as warfarin with respect to LVT resolution and the risk of major bleeding. Therefore, despite the limitations of the existing evidence, DOACs appear to be a reasonable option for the management of patients with LVT after STEMI.
Impact on daily practice
The results of the current pilot trial and preplanned meta-analysis of available randomised clinical trials (RCTs) showed direct oral anticoagulants are at least as effective and safe compared to warfarin in patients with ST-elevation myocardial infarction complicated with left ventricular thrombus. This message should be interpreted cautiously as the sample size of currently published RCTs is limited. Large RCTs are required for a definitive recommendation.
Acknowledgements
The authors would like to thank Dr W. Yus Haniff W. Isa, MMed, for his kind cooperation in responding to our inquiries regarding the data from his RCT, as well as Hamidreza Rafieetari, PhD, Nasim Jafari, Maryam Zarinsadaf, and Sara Tayyebi, MS, (Rajaie Cardiovascular Institute) for their support and contribution to the study.
Funding
This study was funded by the Tehran Heart Center and Rajaie Cardiovascular Institute.
Conflict of interest statement
G. Piazza has received research grants (paid to his institution) from BMS/Pfizer, Janssen, Alexion, Bayer, Amgen, Boston Scientific, Esperion, and the NIH (1R01HL164717-01); and has received consulting fees for advisory roles from Boston Scientific, Amgen, PERC, NAMSA, BMS, Janssen, Penumbra, and Thrombolex. H.M. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Reality Labs, Tesseract/4Catalyst, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, and F-Prime; he is a co-founder of Refactor Health and Hugo Health; and has contracts through Yale New Haven Hospital from the Centers for Medicare & Medicaid Services and through Yale University from Johnson & Johnson. G.Y.H. Lip is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Anthos, although no fees are received personally; and he is a National Institute for Health and Care Research (NIHR) senior investigator and co-principal investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 899871, outside the submitted work. B. Bikdeli is supported by a Career Development Award from the American Heart Association and VIVA Physicians (#938814); was supported by the Scott Schoen and Nancy Adams IGNITE Award; is supported by the Mary Ann Tynan Research Scientist award from the Mary Horrigan Connors Center for Women’s Health and Gender Biology at Brigham and Women’s Hospital, and the Heart and Vascular Center Junior Faculty Award from Brigham and Women’s Hospital; reports that he was a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters but was not involved in the litigation in 2022-2024 nor did he receive any compensation in 2022-2024; he reports that he is a member of the Medical Advisory Board for the North American Thrombosis Forum; and serves on the Data Safety and Monitory Board of the NAIL-IT trial funded by the National Heart, Lung, and Blood Institute, and Translational Sciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

