Abstract
Aims: To evaluate the characteristics and clinical outcome of patients with new formation of left ventricular (LV) thrombus after percutaneous edge-to-edge mitral valve repair.
Methods and results: Between 2009 and 2012 we intended to treat 150 patients with severe mitral regurgitation (MR) with percutaneous edge-to-edge mitral valve repair in our centre. Post-procedural transthoracic echocardiographic examinations scheduled during the hospital stay revealed the new formation of LV thrombi in three out of 150 patients. All three patients suffered from end-stage systolic heart failure with a LV ejection fraction (LVEF) below 20% and were successfully treated in terms of MR reduction (reduction of at least two MR grades). No thrombus formation was observed in patients with a LVEF >20% treated in our centre (a total of 136 patients). The frequency of new LV thrombus formation in the cohort of patients with a LVEF ≤20% treated in our centre was 21% (three out of 14 patients).
Conclusions: New formation of LV thrombus was detected in patients with severely depressed LVEF (≤20%) after successful reduction of MR following percutaneous edge-to-edge mitral valve repair. This phenomenon could be a play of chance, but percutaneous edge-to-edge mitral valve repair using the MitraClip® system is a new procedure. Special care is needed when performing new procedures, and the unexpected post-procedural finding of LV thrombus formation in approximately 20% in this cohort is worth reporting.
Abbreviations
CRT: cardiac resynchronisation therapy
LV: left ventricle, left ventricular
LVEF: left ventricular ejection fraction
MR: mitral regurgitation
PCI: percutaneous coronary intervention
Introduction
Percutaneous edge-to-edge mitral valve repair is a non-surgical treatment option for patients with symptomatic mitral regurgitation (MR)1-4. Several studies have indicated that patients at high operative risk with or without severely depressed left ventricular (LV) function who are not eligible for conventional surgery may profit particularly from this non-surgical approach5,6. Left ventricular thrombus formation has not been detected systematically so far as a major adverse event in patients after successful percutaneous edge-to-edge mitral valve repair. Evidence is limited to a single case report from our group of a patient with a left ventricular ejection fraction (LVEF) of 15% and MR grade 4+ who was successfully treated with percutaneous edge-to-edge mitral valve repair and developed a new LV thrombus after the successful procedure7. Since LV thrombus formation is a major adverse event per se, we reviewed all our patients who were treated in our centre with this percutaneous edge-to-edge mitral valve repair retrospectively for new LV thrombus formation. After we detected a total of three patients with new formation of LV thrombus we sought to identify patient characteristics and describe patient outcome.
Methods
PATIENTS
We conducted a retrospective analysis in terms of LV thrombus formation of all patients treated in our centre with percutaneous edge-to-edge mitral valve repair. Between June 2009 and July 2012 we intended to treat 150 patients with severe MR by this approach. Eligible patients with symptomatic functional or degenerative MR were primarily at high risk for conventional mitral valve repair, suffered from cardiogenic shock or declined surgery. Degenerative MR was defined as disruption in any part of the mitral apparatus, i.e., mitral valve prolapse and/or rupture of the chordae tendineae or papillary muscles. Functional MR was defined as compromised coaptation of the mitral leaflets due to LV and annular dilatation and/or restricted leaflet motion. Key exclusion criteria were active endocarditis or history of endocarditis and mitral valve orifice area <3.0 cm². In contrast to the EVEREST trials, patients with an LVEF ≤25%, left ventricular end-systolic diameter (LVESD) >55 mm, lateral and medial leaflet pathologies (MR origin outside the A2/P2 segment) and coaptation length <2 mm were not excluded. Before percutaneous edge-to-edge mitral valve repair, standard heart failure therapy including optimal medical therapy as well as cardiac resynchronisation therapy was applied for at least three months.
PROCEDURAL TECHNIQUE
Percutaneous edge-to-edge mitral valve repair was performed using the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA) by a standard protocol1,4,8,9. MR classification was performed according to the American Society of Echocardiography guidelines and graded from 1+ to 4+10,11. Transthoracic and transoesophageal echocardiographic examinations were scheduled pre-procedurally at admission and transthoracic echocardiographic examinations pre-discharge. No pre-procedural testing was performed for contractile reserve in patients with LVEF ≤20%. Intraprocedural transoesophageal echocardiography was performed as previously described12. There was no routine use of contrast medium for the detection of LV thrombus formation in transthoracic echocardiographic examinations at admission and pre-discharge.
PERIPROCEDURAL ANTICOAGULATION AND ANTIPLATELET THERAPY
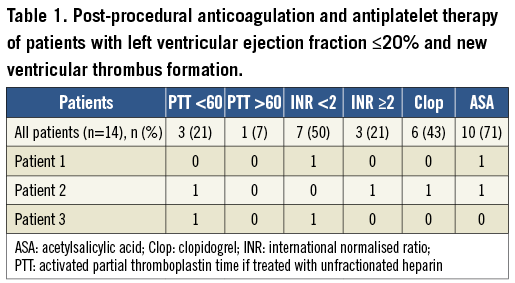
Patients without an indication for oral anticoagulation were treated with aspirin (100 mg BID) and with clopidogrel (loading dose of 600 mg before the procedure and a maintenance dose of 75 mg daily) and with intravenous heparin during the procedure. In patients with an indication for oral anticoagulation (atrial fibrillation, previous cryptogenic stroke), the following periprocedural anticoagulation and antiplatelet regimen was adapted with respect to the individual patient: in patients without the need for additional ASS and clopidogrel, either they were bridged with unfractionated heparin (UFH) post-procedurally until the international normalised ratio (INR) was close to the respective range or phenprocoumon was started again post-procedurally without bridging. If the percutaneous edge-to-edge mitral valve repair was scheduled within six months after percutaneous coronary intervention (PCI) using a drug-eluting stent, the patient received triple therapy containing ASS, clopidogrel and phenprocoumon with a recommended INR of 2.0. Post-procedural anticoagulation and antiplatelet therapy of patients with an LVEF ≤20% are shown in Table 1. During the procedure heparin dosage was adjusted to an activated clotting time of above 250 seconds.
FOLLOW-UP
Follow-up echocardiographic examinations were scheduled one, six and 12 months after the procedure. If patients were prevented from attending clinical follow-up, we conducted telephone interviews.
DATA ANALYSIS
We used Fisher’s exact test to compare categorical variables. The Mann-Whitney U test was used for the comparison of continuous variables. The log-rank test was used to compare overall survival. A two-tailed p-value <0.05 was regarded as statistically significant.
Results
Transthoracic echocardiographic examinations scheduled during the hospital stay after the procedure revealed the new formation of LV thrombi in three out of all 150 patients treated in our centre. All three patients suffered from end-stage systolic heart failure with a LVEF below 20% and were successfully treated in terms of MR reduction (reduction of at least two MR grades). No thrombus formation was observed in patients with a LVEF >20% treated in our centre after the procedure (a total of 136 patients) either during the initial hospital stay or during follow-up echocardiographic examinations. The frequency of new LV thrombus formation during the hospital stay in the cohort of patients with a LVEF ≤20% treated in our centre was 21% (three out of 14 patients). Patients with a LVEF ≤20% without LV thrombus formation during the hospital stay also had not developed LV thrombi by the time of follow-up examinations.
COHORT OF PATIENTS WITH A LVEF ≤20%
The mean LVEF of patients with an LVEF ≤20% was 17% ± 3.2%. All but one patient suffered from MR grade ≥3+. The aetiology of MR in all but one patient was functional due to either ischaemic (six patients, 46%) or non-ischaemic (seven patients, 54%) cardiomyopathy. Severe LV dilation (LV end-systolic diameter ≥55 mm) was present in 10 patients (77%). The mean logistic EuroSCORE was 29.7% ± 25.3%. All patients suffered from NYHA functional Class ≥III. Clip implantation was performed in 13 of the 14 patients. In those 13 patients MR was reduced successfully by at least 1 grade (MR reduction displayed in Figure 1A and Figure 1B). The patient characteristics are listed in Table 2.
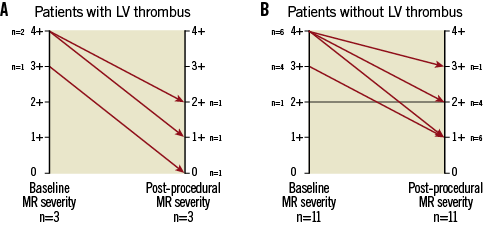
Figure 1. Changes in MR grade baseline vs. post-procedural. A) Changes of MR grade pre- compared to post- clipping in patients with LV thrombus. B) Changes of MR grade pre- compared to post- clipping in patients without LV thrombus. MR: mitral regurgitation
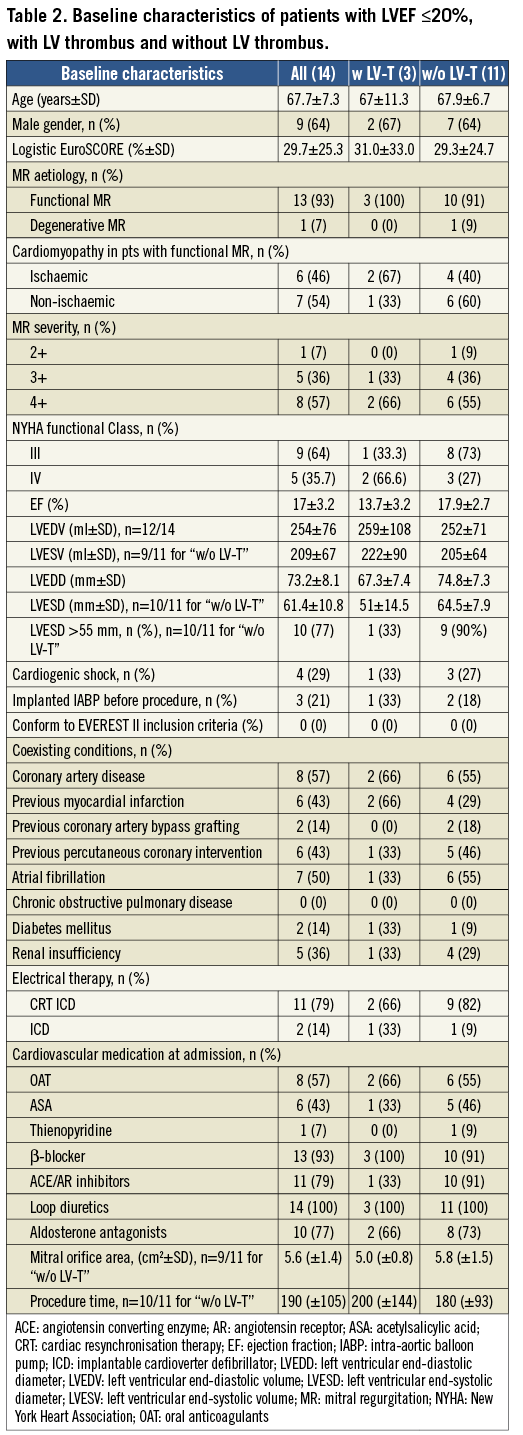
Patients who developed a LV thrombus after mitral valve repair had a worse LVEF (13.7±3.2) compared to those without thrombus formation (17.9±2.7). Mortality in this cohort of patients was high. In patients with new LV thrombus formation after mitral valve repair 12-month mortality was 100% (mean follow-up three months), whereas in patients without LV thrombus formation mortality was 36% (mean follow-up 6.4 months, Kaplan-Meier curve) (Figure 2). There were no other relevant differences in the variables between patients with new LV thrombus formation compared to patients without LV thrombus.
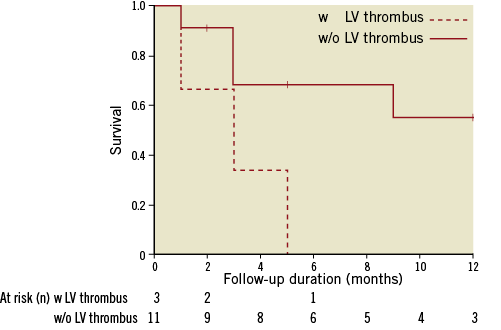
Figure 2. Overall survival of patients with LV thrombus vs. without LV thrombus. Vertical markings within patients without LV thrombus indicate last live contact.
On-clopidogrel platelet reactivity was assessed with the Multiplate Analyzer® (Verum Diagnostica, Munich, Germany) in five out of six patients in the cohort of patients with an LVEF ≤20% who were on clopidogrel treatment after the procedure. All five patients showed an adequate response (<468 AU x min) to the drug according to consensus definitions13. One out of the five patients developed a new LV thrombus after the procedure. This patient´s platelet reactivity was highest among all five patients (243 AU x min compared to 70, 91, 142 and 164 AU x min).
CHARACTERISTICS AND OUTCOME OF ALL THREE PATIENTS WITH NEW POST-PROCEDURAL LEFT VENTRICULAR THROMBUS FORMATION
A 73-year-old male patient (patient 1) suffered from severe functional MR due to LV dilation (LV end-diastolic volume [LVEDV]: 371 ml) and large dyskinetic areas caused by ischaemic cardiomyopathy and a LVEF of approximately 16% despite cardiac resynchronisation therapy (CRT). Due to a small, partly calcified structure within the inferoapical wall of the LV detected in prior echocardiographic examinations, the patient had received therapeutic anticoagulation for three months. The patient was successfully treated, reducing the MR from grade 4+ to grade 1+ (Figure 3A pre- and Figure 3B post-procedurally). The patient recovered well from the procedure. On the evening of the procedure we started with oral anticoagulation with phenprocoumon without bridging with UFH. A follow-up echocardiogram performed two days after the procedure revealed the new formation of left ventricular thrombus within the apex of the LV which was clearly absent before the procedure (Figure 3C pre- and Figure 3D post-procedurally). Therapeutic heparinisation was started. Unfortunately, the patient suffered from a lateral wall myocardial infarction the following day. Coronary angiography revealed a thromboembolic occlusion of the left circumflex coronary artery (Figure 3E) which was successfully treated with balloon angioplasty (Figure 3F). The embolus within the coronary artery most likely originated from the new LV thrombus. He recovered during his hospital stay and was referred to a secondary hospital for further rehabilitation. Unfortunately the patient died due to LV failure two and a half months after the procedure.
A 74-year-old male patient (patient 2) with severe functional MR due to LV dilation (LVEDV: 252 ml) and large dyskinetic areas caused by ischaemic cardiomyopathy and an LVEF of approximately 10% despite CRT suffered from cardiac decompensation resulting in cardiogenic shock. The patient was treated with catecholamines, intra-aortic balloon counterpulsation and mechanical ventilation. Due to atrial fibrillation, he was treated with oral anticoagulants. After excluding new relevant coronary artery stenosis, a mitral clip was considered. The patient was treated successfully during an uneventful procedure which reduced the MR from grade 3+ to grade ≤1+ (Figure 4A pre- and Figure 4B post-procedurally). After the procedure the patient recovered quickly from cardiogenic shock. We performed follow-up routine echocardiography before discharge which showed the new formation of a large thrombus within the apex of the LV, which was absent before the procedure (Figure 4C during procedure and Figure 4D post-procedurally). The INR was already within the therapeutic range and phenprocoumon was continued. The patient did not show any major adverse events during his hospital stay but died due to LV failure three months after the procedure.
A 55-year-old female patient (patient 3) suffered from dilative cardiomyopathy (LVEDV: 155 ml) with severely depressed LVEF of approximately 15% and severe functional MR. Due to a history of cryptogenic stroke, the patient received phenprocoumon. She was successfully treated, with a reduction of MR from grade 4+ to grade 2+ (Figure 5A pre- and Figure 5B post-procedurally). Unfortunately, during bridging with UFH, the patient suffered from a new stroke which was confirmed in cranial computed tomography. Echocardiography demonstrated new formation of a large left ventricular thrombus adherent to the inferoapical wall, which was absent before the procedure (Figure 5C pre- and Figure 5D post-procedurally). The patient died one week later due to pneumonia complicating the thromboembolic stroke (detailed information of the clinical course of patient 3 has been reported previously7).
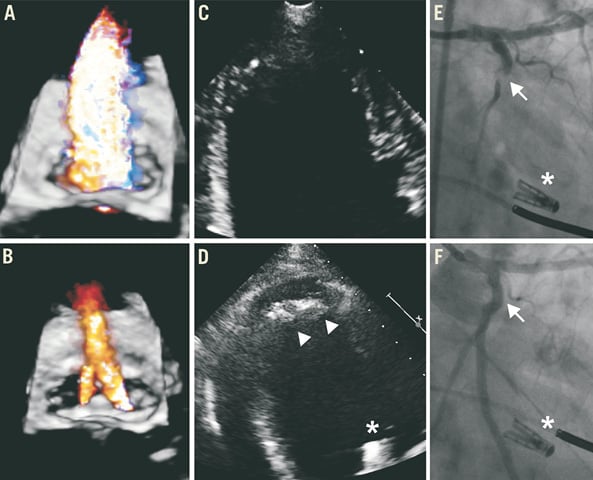
Figure 3. Patient 1.
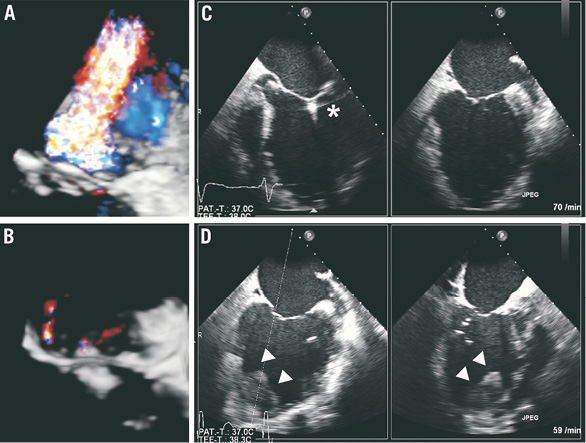
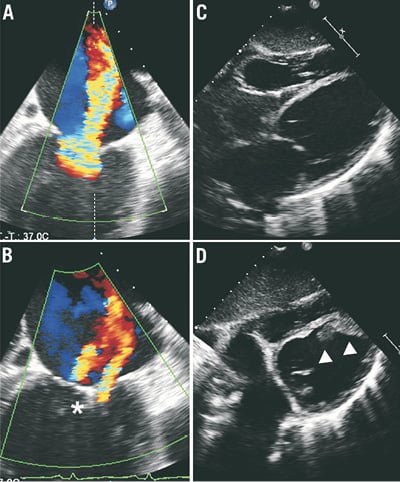
Figure 4. Patient 2.Figure 5. Patient 3.
Figure 3-Figure 5. Intraventricular thrombus formation after successful percutaneous edge-to-edge mitral valve repair.
In all three patients panel A shows colour flow transoesophageal echocardiography of severe mitral regurgitation pre-procedurally (60°, three-dimensional depicted from the left atrium in patients 1 and 2, two-dimensional in patient 3, grade 3+ in patient 1, grade 4+ in patients 2 and 3) and after successful reduction in panel B (grade 1+ in patients 1 and 2, grade 2+ in patient 3). In all patients left ventricular thrombus was absent before and during the procedure in panel C (transthoracic echocardiography in patients 1 [apical 4-chamber view] and 3 [subxiphoidal view], transoesophageal echocardiography in patient 2, x-plane 0°/90°) whereas panel D shows new formation of apical left ventricular thrombus post-procedurally (echocardiography corresponding to panel C in patients 1 and 3, transoesophageal echocardiography in patient 2, x-plane 75°/12°). In patient 1, panel E shows thromboembolic occlusion of the left circumflex artery (arrow) which was treated with balloon angioplasty (panel F, arrow). Asterisks indicate clip, arrowheads indicate new thrombus.
Review of all three percutaneous procedures did not reveal any abnormal device manoeuvres or potential injuries of the left ventricle.
Discussion
REVIEW OF LITERATURE
The randomised Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) and the registry of high-risk patients within the EVEREST patient population which compared percutaneous edge-to-edge mitral valve repair to conventional surgery excluded patients with LVEF ≤25%4,6. Several other patient populations treated with MitraClip® have been reported1,2,5,14-17. No LV thrombus formation has been described so far in recently published studies. Some of these studies also investigated patients with severely depressed LV function and no study reported the described phenomenon in this cohort of patients2,5,14-17.
Successful percutaneous edge-to-edge mitral valve repair may lead to a substantial reduction of intraventricular flow especially in patients with severely depressed LV function, which may increase the risk for intracavity thrombus formation. The incidence of LV thrombus formation in patients with severely depressed LV function with and without severe MR has been described in several studies. Blondheim et al described an overall frequency of 40% of LV thrombi in 91 patients with dilated cardiomyopathy and severely depressed LVEF but without significant MR, whereas the frequency of LV thrombi in the same cohort in patients with severe MR was only 8%18. Another work from Kalaria et al described LV thrombus formation in 12 out of 103 patients with dilated cardiomyopathy and severely depressed LVEF. No patient with LV thrombus suffered from severe MR, while in contrast no patient out of 11 patients with severe MR developed a LV thrombus19. Özdemir et al studied 1,311 patients with ischaemic cardiomyopathy. The overall rate of LV thrombus formation in patients with severe MR was significantly lower than in patients without severe MR (4% versus 15.6%, p<0.001)20. In summary, a “protective” effect of a coexisting MR against LV thrombus formation due to higher intraventricular flow was postulated in these manuscripts.
PATHOPHYSIOLOGIC ASPECTS
The manuscript raises a note of caution towards the possibility that mitral valve repair may lead to reduced intraventricular flow through a sudden reduction of a relevant regurgitation volume in patients with severely depressed LV function, increasing the risk of thrombus formation as a potential complication of this otherwise successful procedure in this cohort. Another aspect promoting the formation of a LV thrombus might be the sudden increase in afterload after a successful reduction of the MR which may cause a further decrease in LV function. Although not investigated in this study, a pre-procedural testing for contractile reserve in patients with LVEF ≤20% (e.g., dobutamine stress echocardiography) might be useful to identify left ventricles which are more likely to cope with the increase in afterload. Of note - as a third aspect - large dyskinetic areas in patients with ischaemic cardiomyopathy causing dilation of the LV might be more prone to post-procedural thrombus formation.
PERIPROCEDURAL AND POST-PROCEDURAL ANTICOAGULATION
In our patient cohort the delicate balance between blood flow and thrombosis within a dilated, almost failing left ventricle might have deteriorated towards thrombosis because one of Virchow´s triad components, blood stasis17, suddenly increases after successful percutaneous edge-to-edge mitral valve repair. Since thrombus formation occurred post-procedurally during the hospital stay of the three patients and no thrombus formation was seen in any other patients during the follow-up period, it might have been necessary to prolong or intensify the intra- and early post-procedurally performed anticoagulation beyond the procedure in this cohort.
REDUCTION IN MR GRADE
Data on the optimal grade of reduction of MR in patients with severely depressed LVEF and severe MR to prevent a sudden and relevant drop of left ventricular flow are limited. It is unclear if a two-step successive reduction from severe to medium and from medium to mild MR might be safer due to smaller changes in afterload that might allow a better LV remodelling and adjustment of left ventricular flow conditions.
LIMITATIONS
The small sample size, its retrospective character and the single-centre experience are the limitations of this study.
Conclusion
New formation of LV thrombus was detected in patients with severely depressed LVEF below 20% after successful reduction of MR following percutaneous edge-to-edge mitral valve repair. In the setting of our study, LV thrombus formation after the MitraClip® procedure is associated with high morbidity and mortality. This phenomenon could be a play of chance, but percutaneous edge-to-edge mitral valve repair using the MitraClip® system is a new procedure. Special care is needed when performing new procedures and the unexpected post-procedural finding of LV thrombus formation in approximately 20% in this cohort is worth reporting. Since an increasing number of patients with severely depressed LVEF and severe MR are treated with this percutaneous approach, the phenomenon of new LV thrombus formation might be as yet under-recognised. As a consequence, special care should be taken at pre-discharge and follow-up echocardiography to identify new LV thrombus formation after successful percutaneous edge-to-edge mitral valve repair. Further investigations are warranted to clarify if patients with an LVEF ≤20% would profit from a prolonged post-procedural therapeutic administration of heparin to prevent LV thrombus formation after successful edge-to-edge mitral valve repair.
Conflict of interest statement
J. Mehilli and J. Hausleiter received speaker honoraria from Abbott Vascular. The other authors have no conflicts of interest to declare.

