Abstract
Aims: Patients undergoing transcatheter aortic valve replacement (TAVR) possess a higher risk of recurrent healthcare resource utilisation due to multiple comorbidities, frailty, and advanced age. We sought to devise a simple tool to identify TAVR patients at increased risk of 30-day readmission.
Methods and results: We used the Nationwide Readmissions Database from January 2013 to September 2015. Complex survey methods and hierarchical regression in R were implemented to create a prediction tool to determine probability of 30-day readmission. Boot-strapped internal validation and cross-validation were performed to assess model accuracy. External validation was performed using a single-centre data set. Of 39,305 patients who underwent endovascular TAVR, 6,380 (16.2%) were readmitted within 30 days. The final 30-day readmission risk prediction tool included the following variables: chronic kidney disease, end-stage renal disease on dialysis (ESRD), anaemia, chronic lung disease, chronic liver disease, atrial fibrillation, length of stay, acute kidney injury, and discharge disposition. ESRD (OR 2.11, 95% CI: 1.7-2.63), length of stay ≥5 days (OR 1.64, 95% CI: 1.50-1.79), and short-term hospital discharge disposition (OR 1.81, 95% CI: 1.2-2.7) were the strongest predictors. The c-statistic of the prediction model was 0.63. The c-statistic in the external validation cohort was 0.69. On internal calibration, the tool was extremely accurate in predicting readmissions up to 25%.
Conclusions: A simple and easy-to-use risk prediction tool utilising standard clinical parameters identifies TAVR patients at increased risk of 30-day readmission. The tool may consequently inform hospital discharge planning, optimise transitions of care, and reduce resource utilisation.
Introduction
Thirty-day readmissions are a closely scrutinised metric and are associated with increasing costs to the healthcare system, poorer quality of life, and greater resource utilisation. Interest in readmission rates remains high. Recent US government initiatives such as the Hospital Readmissions Reduction Program under the Affordable Care Act have led to a reduction in hospital readmission rates1. While readmission rates have improved, they remain elevated for several targeted conditions - acute myocardial infarction, heart failure, and pneumonia2.
Transcatheter aortic valve replacement (TAVR) is an evolving and rapidly growing treatment modality for selected symptomatic aortic stenosis patients who are at intermediate to prohibitive risk for surgical valve replacement3. Using the Nationwide Readmissions Database (NRD), we recently reported a 30-day all-cause readmission rate of 17.9% after TAVR4,5. These results mirror other studies reporting 30-day readmission rates of 14.6% to 20.9% after TAVR6,7,8,9. Non-cardiac causes of readmissions (respiratory, infection, and bleeding events) are more common than cardiac causes (heart failure and arrhythmias)4,9. Early hospital readmission after TAVR is associated not only with greater healthcare expenditure and resource utilisation, but also with poorer long-term outcomes9. A readmission risk prediction tool specifically designed for the TAVR population is much needed. Identification of those patients at greatest risk may facilitate the implementation of targeted interventions to reduce rehospitalisation rates, reduce healthcare expenditure, and improve clinical outcomes. We therefore sought to devise a 30-day hospital readmission risk prediction tool using routine administrative variables, which may facilitate discharge planning and post-discharge care coordination of patients undergoing TAVR.
Methods
DATA SOURCE
The NRD is a unique and powerful database designed to support analyses of national readmission rates for all payer types. The NRD contains discharge data from 27 geographically dispersed states, accounting for 57.8% of the total US resident population and 56.6% of all US hospitalisations, amounting to roughly 36 million discharges nationally. Developed through a Federal-State-Industry partnership sponsored by the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP), the data inform decision making at national, state, and community levels.
STUDY POPULATION
DERIVATION COHORT
We used the NRD database to identify admissions with endovascular TAVR (ICD-9-CM procedure code 35.05) between January 2013 and September 2015. Admissions that resulted in in-hospital mortality were excluded from the analysis. Figure 1 outlines our methods for isolating the study cohort. The methodology for extraction of index cases and 30-day readmissions was followed as recommended by the AHRQ10. When the same patient had more than one readmission within 30 days, only the first event was included. Comorbidities were obtained using relevant ICD-9-CM codes (Supplementary Appendix 1).
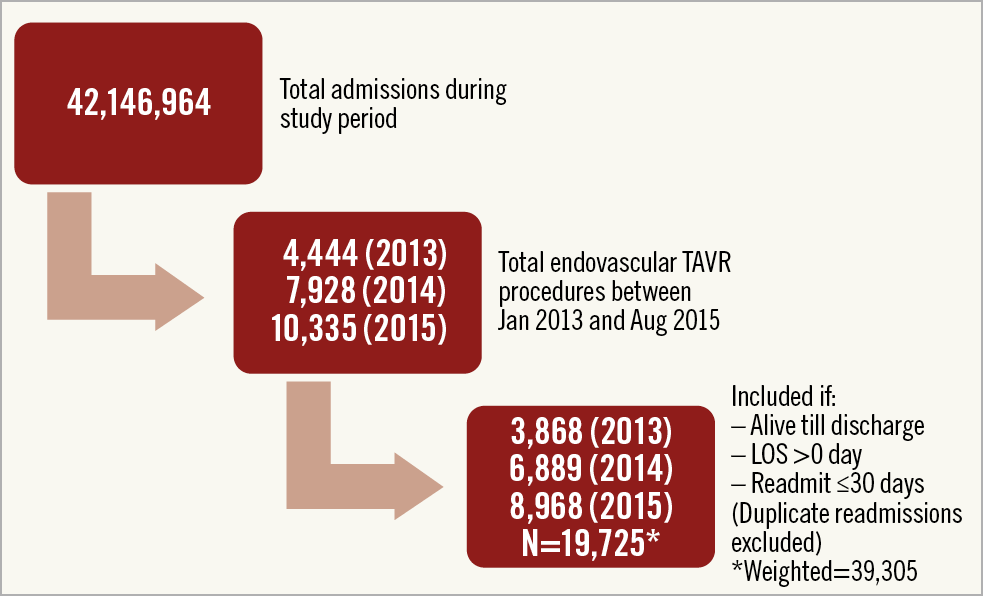
Figure 1. Flow chart depicting the selection of the study cohort. Endovascular includes all non-transapical TAVR approaches (predominantly transfemoral). LOS: length of stay
VALIDATION COHORT
TAVR procedures performed at University Hospitals, Cleveland from March 2011 to April 2018 comprised the validation cohort. The data were independently collected (J.R. Clevenger, E.J. Bansal, A. Kalra) after institutional review board approval (IRB #3-15-26). These data are regularly reviewed by the Society of Thoracic Surgeons and the American College of Cardiology for accuracy and reliability for the TVT Registry.
STATISTICAL ANALYSIS
Statistical analysis was performed using R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria)11. Categorical data are presented as frequency (percentage) while continuous data are presented as mean (SD). We stratified our study cohort into patients who did and did not experience a 30-day readmission. Categorical variables were compared using the chi-square test, and continuous variables using the Wilcoxon rank-sum test or two-tailed t-test as appropriate. National estimates were calculated using the “survey” package using appropriate weights provided in the NRD12. The NRD data sets contain three weighting variables (Hosp_nrd: hospital level clustering weight; Nrd_stratum: strata; Discwt: discharge weight). These three variables provide reliable national level estimates. The baseline table providing national estimates was a weighted analysis. However, all logistic regression was performed using unweighted data.
HCUP recommends using a hierarchical regression model to account for patient clustering within hospitals13,14. Thus, we initially implemented hierarchical regression with the hospital identifier as the random effect. However, given the non-significant contribution of the random effects model, we elected to develop a parsimonious model with fixed-effects logistic regression with the “rms” package (http://biostat.mc.vanderbilt.edu/rms) in R to identify preprocedural and post-procedural factors that impact on readmission. After starting with 39 preprocedural and post-procedural variables (Supplementary Table 1), we excluded those which did not contribute statistically (p>0.1). We also excluded comorbidities that did not appear clinically relevant to the model. For example, we excluded factors that were predominantly protective (dyslipidaemia, hypertension), most likely due to coding factors and without any clinical rationale for the effect. Centers for Medicare & Medicaid Services (CMS) has implemented this methodological strategy in the development of their readmission risk calculators15. A total of 23 variables based on p<0.1 on univariate analysis or clinical judgement (age) were finally used in the multivariable regression model for risk tool development (Supplementary Figure 1A, Supplementary Figure 1B). After stepwise backward selection in R using the default method of selection according to Akaike Index Criterion (AIC), the final model contained nine variables that influenced 30-day readmissions. A linear calculator was developed to determine the probability for 30-day readmission based on the individual score of each of the nine variables.
Internal calibration to assess model accuracy was conducted with bootstrapped calculations. K-fold cross-validation was used to determine the accuracy of the model (Supplementary Figure 1C). Data were stratified into ten derivation and validation cohorts using the jackknife method. The root mean square error and mean absolute error were calculated to determine model performance. All results are presented at the 95% confidence level.
Missing data were minimal in the NRD database. Payer status had 0.2% missing data; all other variables included in the logistic regression were complete. Hence, only complete cases were included in the regression models created. The study is reported according to the recommended guidelines16.
Data from University Hospitals (Case Western, Cleveland, OH, USA), comprising 885 TAVR procedures, were utilised for the external validation of the risk score. Our risk model was implemented to obtain prediction intervals for all patients included in the validation cohort. We further calculated a c-statistic for our externally validated model and present a comparative histogram of fitted probability values for our derivation and validation cohorts.
Results
Of 39,305 (19,725 unweighted) patients who underwent endovascular TAVR, 6,380 (16.2%) were readmitted within 30 days. On univariable analysis, there were no differences in the age (81.51±0.17 versus 81.3±0.11 years) or gender (47% versus 45% female) between readmitted and non-readmitted patients, respectively. There were no statistically significant differences in the 30-day readmission rates among the years of inclusion (17.1% in 2013, 16.5% in 2014 and 16.2% in 2015, p=0.26). Patients who were readmitted within 30 days were more likely to have anaemia, atrial fibrillation, chronic kidney disease, end-stage renal disease on dialysis (ESRD), chronic lung disease, chronic liver disease, and weight loss. Patients readmitted within 30 days had a longer index length of stay and were more likely to be discharged to a skilled nursing facility (Supplementary Table 1).
The final 30-day readmission risk prediction tool included nine variables (six preprocedural variables, one post-procedural complication, length of stay, and discharge disposition). The c-statistic of the prediction model was 0.63. Figure 2 illustrates the OR estimates of the predictors included in our model – chronic kidney disease (OR 1.22, 95% CI: 1.13-1.33), ESRD (OR 2.11, 95% CI: 1.7-2.63), anaemia (OR 1.15, 95% CI: 1.06-1.26), chronic lung disease (OR 1.24, 95% CI: 1.14-1.34), chronic liver disease (OR 1.24, 95% CI: 0.99-1.54), atrial fibrillation (OR 1.24, 95% CI: 1.14-1.35), length of stay ≥5 days (OR 1.64, 95% CI: 1.50-1.79), acute kidney injury (OR 1.29, 95% CI: 1.14-1.46), and discharge disposition (home health care, OR 1.21, 95% CI: 1.1-1.33; skilled nursing facility, OR 1.57, 95% CI: 1.41-1.75; short-term hospital, OR 1.81, 95% CI: 1.2-2.7).
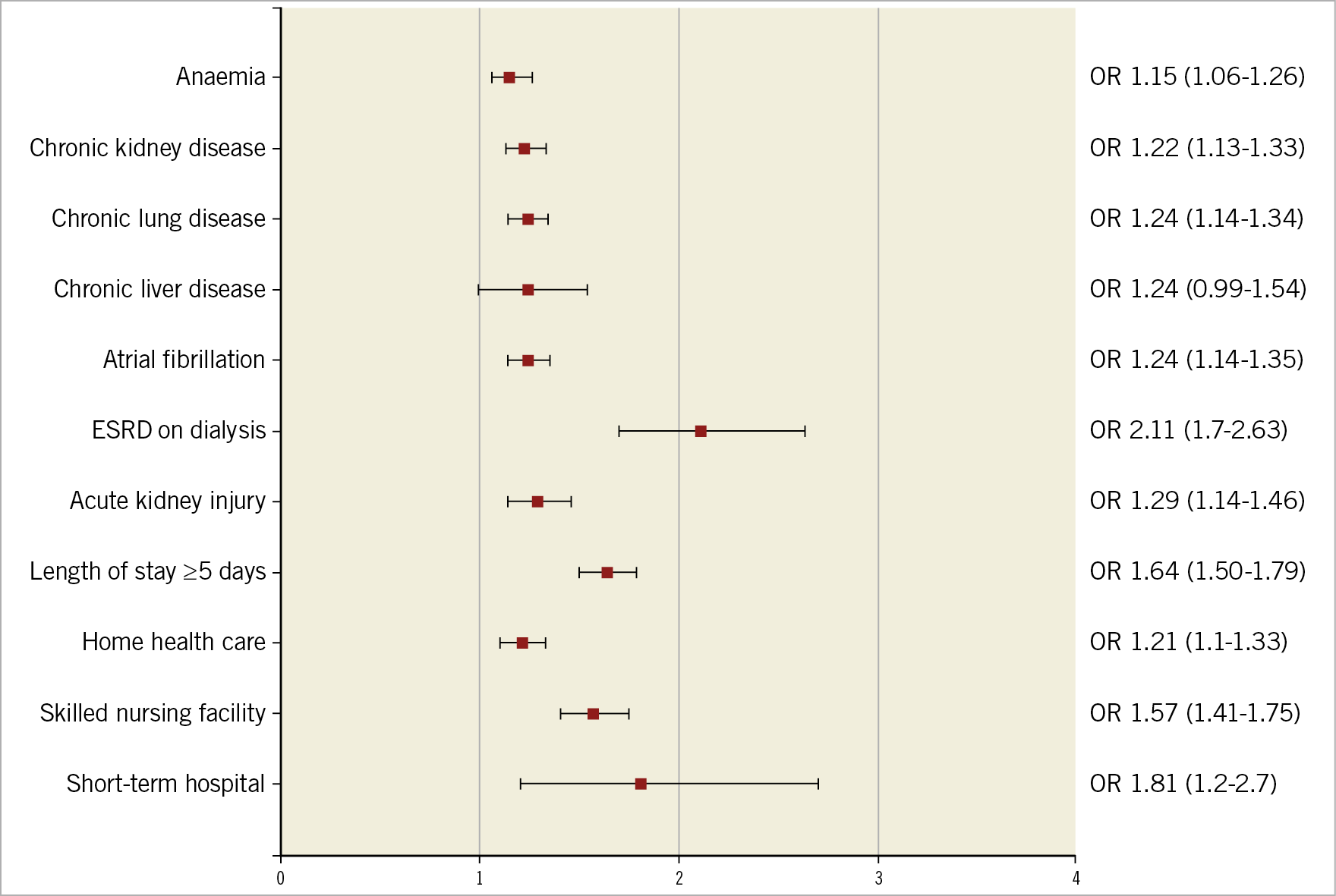
Figure 2. Forest plot depicting OR (95% CI) of the nine final variables included in the 30-day readmission risk tool.
The final risk prediction tool consisted of a ruler in which each predictive variable was assigned a score based on the raw estimates/standard error derived from the logistic regression model (Figure 3). The probability of 30-day readmission can be calculated from the scale by matching the total points for a given patient. For example, a hypothetical 82-year-old female with chronic lung disease, atrial fibrillation, and chronic kidney disease who develops acute kidney injury post TAVR requiring an extensive hospital stay (six days) and being discharged to home has a total score of >175 corresponding to a >25% predicted risk of 30-day readmission. We have provided the individual score for each predictor and the predicted 30-day readmission rate for a given cumulative score in Supplementary Appendix 1.
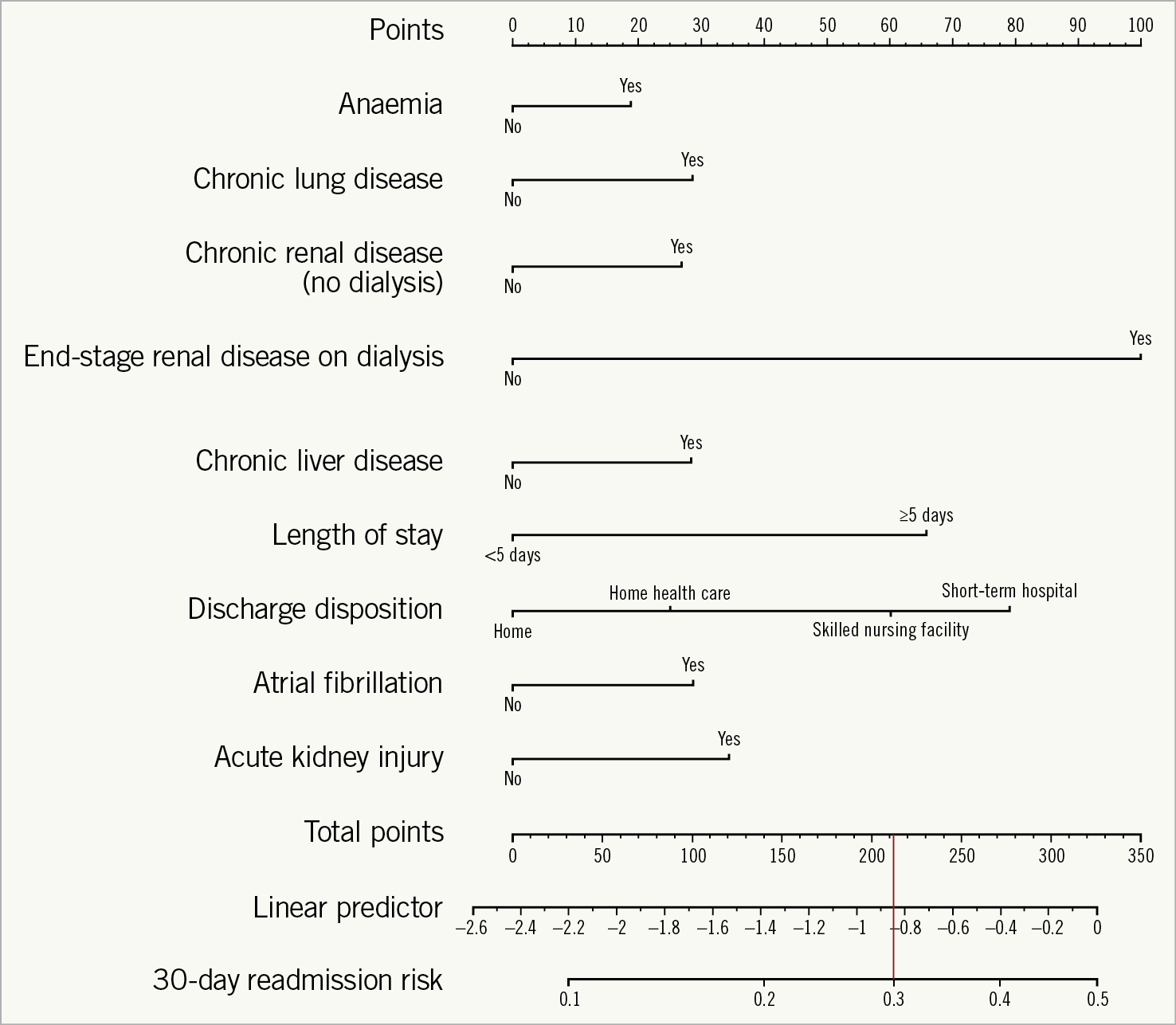
Figure 3. Nomogram for the TAVR 30-day readmission risk model. Each predictor has a ruler; adding the individual predictors provides the total score. The 30-day readmission risk is calculated by dropping a vertical line from the cumulative score to the risk ruler. – score of >210 corresponds to >30% 30-day readmission rate.
On internal calibration, our tool was extremely accurate in predicting readmissions up to 25%, and modestly accurate in predicting readmissions up to 40% (Figure 4). The results of tenfold cross-validation yielded a root mean square error (RMSE) of 0.9858 and mean absolute error (MAE) of 0.9783. The Hosmer-Lemeshow test p-value for the logistic regression model was 0.33. The Brier score for the derivation model was 0.130.
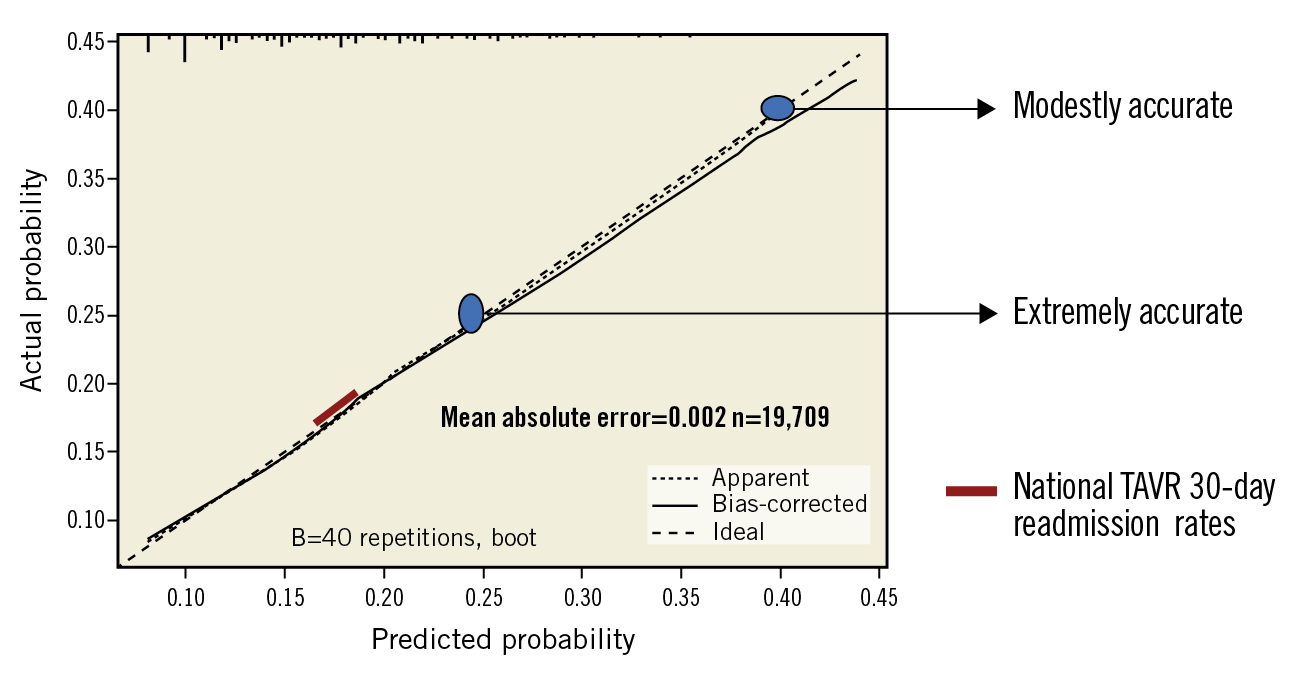
Figure 4. Internal calibration by boot-strapping. The 30-day readmission risk tool was extremely accurate in predicting readmissions up to 25%, and modestly accurate in predicting readmissions up to 40%.
External validation of our 30-day readmission risk tool was performed on the institutional data set from Case Western University Hospitals. Of the 885 patients (mean age 81.7 years) undergoing TAVR at this major academic centre, 167 (18.9%) patients were readmitted within 30 days. The validation c-statistic for our readmission risk tool was 0.69. Root mean square error (RMSE) of the external validation cohort was 0.9283. The Brier score for the validation model was 0.136. The fitted values of probability for our derivation data set closely mirror the fitted values of probability for our external validation cohort (Figure 5). We have provided the descriptive statistics of the external validation cohort in Supplementary Table 2.
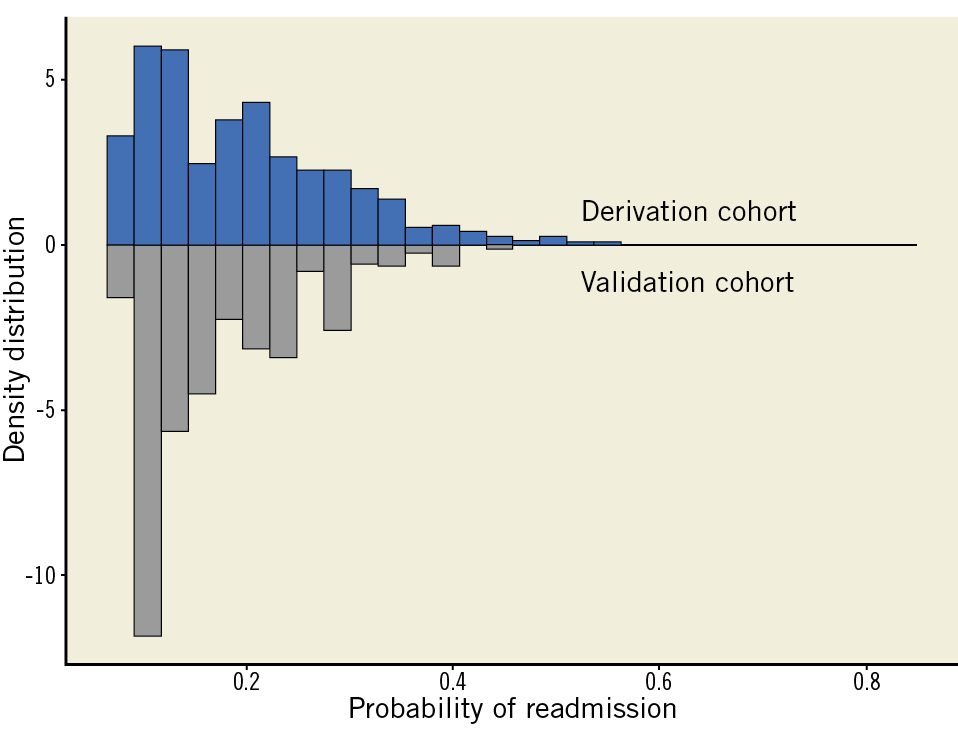
Figure 5. Bar graphs comparing fitted values of probability for the derivation and external validation cohorts.
Discussion
We report a simple, easy-to-use, 30-day readmission risk prediction tool for patients undergoing endovascular TAVR. The tool has a possibly helpful discriminant function. Strengths of the risk prediction tool include: i) the use of clinically relevant variables that are readily available from administrative data; ii) the incorporation of chronic liver disease as a variable; iii) variables that are difficult to generalise using administrative data sets such as vascular injury and bleeding complications are not included; iv) that it can be utilised in clinical practice for guiding discharge and follow-up planning, thereby improving transition of care for those with the highest predicted healthcare needs during the post-discharge period; and v) that it can be easily incorporated into the modern-day electronic health records.
In a recent analysis from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) registry utilising data from 2011-2015, and in our previous study from the 2013 NRD, the post-TAVR 30-day readmission rates were reported to be 17.9%4,17. Non-cardiac causes (61.8%/59%; respiratory, infection, bleeding events) were more frequently responsible for readmission after TAVR than cardiac causes (38.2%/41%; heart failure or arrhythmias)4,9. Independent predictors of 30-day readmissions from our prior study were length of stay >5 days, acute kidney injury, transapical TAVR, chronic kidney disease, chronic lung disease, and discharge to skilled nursing facilities4. In a smaller, multi-institutional study, Nombela-Franco and co-workers identified anaemia, bleeding complications, low ejection fraction (EF), and combination of antiplatelet and anticoagulant therapies at discharge as important predictors of early readmissions9. Data from the Bern TAVI Registry identified stage 3 kidney injury and male gender as predictors of all-cause readmission, and previous myocardial infarction and life-threatening bleeding as predictors of cardiovascular readmissions18. Irrespective of the data sources, and the construct and granularity of data sets, early readmissions are associated with higher mortality on medium and long-term follow-up, and poorer quality of life9,18.
Reduction of 30-day readmissions is a target for quality improvement and cost reduction for hospitals and the government alike. From the patient’s perspective, reducing readmissions can lead to improved quality of life. To our knowledge, this study is the first to develop a simple risk tool to predict 30-day readmission after TAVR. Our risk score has possibly helpful discrimination (c-statistic 0.63), which is similar to that for the CMS 30-day readmission models for pneumonia, heart failure, and acute myocardial infarction19. Limited discrimination for hospital readmission is probably due to its multifactorial nature and the impact of parameters not captured in administrative data sets, such as community and socioeconomic factors. Nonetheless, our risk score showed excellent calibration for predicting readmissions. Of the nine variables in the risk prediction tool, six preprocedural variables (baseline comorbidities) are non-modifiable. However, AKI and length of stay (LOS) (a surrogate for post-procedural complications) are modifiable, and measures to prevent post-procedural complications can help to reduce index LOS and risk of 30-day readmission in patients undergoing TAVR. Discharge disposition depends on several factors such as social support, frailty, etc., and may or may not be modifiable. Previous studies have shown that more than one fourth of 30-day readmissions after all-cause index hospitalisations are preventable, and some of the factors strongly associated with potentially preventable readmissions include failure to relay important information to outpatient healthcare providers, patients’ lack of awareness of whom to contact after discharge, inability to keep appointments after discharge, and emergency department decision making to admit a patient who may not have required an in-patient stay20. Some of these are also relevant to TAVR patients, and our readmission risk prediction tool can help to identify and target high-risk patients to facilitate better transition of care and post-discharge care aimed at modifying the factors associated with potentially preventable readmissions (Figure 6).
Figure 6. Application of the 30-day readmission risk tool during periprocedural discharge planning to inform hospital discharge planning, optimise transitions of care, and reduce resource utilisation.
Hospital discharge is an extremely complicated process involving the interaction and coordination of many professionals – nurses, house staff, physicians, social workers, and physical therapists. Ensuring safe discharge practices not only entails appropriate medication reconciliation and patient education, but also patient follow-up and transition of care to community caregivers, family members, and primary care providers. High-volume TAVR centres are reducing readmission rates and readmission costs by emphasising the appropriate discharge planning and adequate transition of care (communication with referring cardiologist, early patient follow-up, telephone calls, centralised follow-up care, and even telehealth systems to monitor daily vitals)21. In clinical practice, our risk prediction tool can be incorporated into electronic health records, or can be accessed via the free app (http://tavr30.com/) to calculate the patient’s risk of 30-day readmission prior to discharge (Figure 6, Supplementary Figure 2). The tool will help to identify TAVR patients who are at an increased risk of 30-day readmission, thereby enabling healthcare providers and hospitals to institute specific pre- and post-discharge measures aimed at preventing readmissions as outlined above in these “high-risk” patients, ultimately leading to improved quality of life. One of the reasons for increased readmissions after discharge to a facility may be the lack of attention to the subsequent transition of care from that facility to home. Identifying patients at increased risk of readmission can help facilities to institute specific measures during patient care and transition from facility to home.
Readmission reduction is an important modifiable target that can not only improve patient outcomes, but also impact on the cost-effectiveness of TAVR. TAVR has evolved into a widely accepted therapeutic modality for symptomatic patients with severe aortic stenosis who are at intermediate or higher operative risk for conventional surgical aortic valve replacement. Procedural planning, extensive engagement of multiple teams (surgeons, cardiologists, interventionalists, anaesthesiologists, and operating room staff), expensive valve platforms, and preprocedural optimisation of patient comorbidities drive the procedural cost. These are often fixed expenditures. TAVR valves (>$30,000/device) are six times more expensive than SAVR valves (≈$5,000/device). Mortality benefit, early ambulation, better quality of life, and prevention of complications thus become the primary drivers of cost-effectiveness of the procedure22. The PARTNER trial (cohort B) investigators estimated the cost-effectiveness of TAVR in inoperable patients to be $62,000 per quality-adjusted life-year gained23. Data from the CoreValve pivotal trial estimated the cost-effectiveness of TAVR in high-risk patients at $55,000 per quality-adjusted life-year gained24. In a recent analysis by Tripathi and co-workers, the cumulative cost of the index procedure and 30-day readmission was higher in the TAVR versus SAVR cohort ($51,025 versus $46,756, p=0.030)25. Our prior study showed that in TAVR patients the cost of 30-day readmission accounted for 16.4% of the total cost of the episode of care (index+readmission)4. By identifying patients at increased risk of readmission at the time of discharge, our tool can help to streamline the limited healthcare resources (e.g., visiting nurse services, early/frequent outpatient follow-up, etc.) towards such patients to prevent readmission. This, together with reduction in readmission-related costs, may improve the overall cost-effectiveness of TAVR.
Limitations
Due to the inherent nature of administrative databases, data on baseline echocardiographic variables, Society of Thoracic Surgeons (STS) risk score, frailty, metrics of exercise tolerance, and preprocedural/periprocedural pharmacotherapy (inotropes, antiplatelets, anticoagulation) were not available. ICD-9 coding can also introduce potential bias due to coding errors and reimbursement policies. Endovascular TAVR includes all percutaneous approaches; we were unable to differentiate between femoral or alternative endovascular access sites (direct aortic, subclavian, iliac, transcaval, or carotid access). Femoral access continues to be the predominant route for TAVR, and we believe that these data are accurately generalisable to femoral TAVRs. Information on valve type and size, preprocedural haemodynamics, procedural success, and post-procedural haemodynamics such as paravalvular leak was not available. Due to the observational nature of this study using administrative databases, residual measured and unmeasured confounding cannot be ruled out. A multitude of social factors that impact on readmissions (socioeconomic, community resources, family and social support) cannot be captured by administrative data sets. The NRD does not capture ethnicity and race, hence these data were not available. However, there are inherent strengths to the NRD data that make them appropriately suited for these analyses, for example, availability of data on all payer types, and availability of data on readmission irrespective of the index procedure site. The NRD is a stratified random sample of roughly 50% of all readmissions. Hence, although weighting is needed, national estimates are reliable and consistently accurate. Our model can be easily synced to the electronic medical records. Relevant clinical notes can be mined to identify keywords used to calculate an individual patient’s total score.
Conclusions
We report a simple and easy-to-use risk prediction tool to help identify TAVR patients at increased risk of 30-day readmission, and aid in appropriate transition of care upon discharge and post-discharge follow-up. The risk tool can be easily incorporated into the modern-day electronic health record systems and can be utilised by both physician and non-physician care givers.
|
Impact on daily practice We devised a simple and easy-to-use clinical risk tool using nine variables to help identify patients at risk of 30-day readmission after transcatheter aortic valve replacement. The risk tool can be easily incorporated into the modern-day electronic health record systems and is also available as an iOS app. The risk tool can aid in appropriate transition of care upon discharge and post-discharge follow-up, translating to lower readmissions and improved healthcare costs and patient outcomes. |
Appendix. Study collaborators
Igor Palacios, MD; Massachusetts General Hospital, Boston, MA, USA. Ignacio Inglessis, MD; Massachusetts General Hospital, Boston, MA, USA. Douglas E. Drachman, MD; Massachusetts General Hospital, Boston, MA, USA. Eric J. Bansal, MD; University Hospitals Cleveland Medical Center, Cleveland, OH, USA. Joshua R. Clevenger, MD; University Hospitals Cleveland Medical Center, Cleveland, OH, USA. Guilherme F. Attizzani, MD; University Hospitals Cleveland Medical Center, Cleveland, OH, USA. Michael Mack, MD; The Heart Hospital – Baylor Plano, Plano, TX, USA. Vinod Thourani, MD; MedStar Washington Hospital Center, Washington, DC, USA. Michael J. Reardon, MD; Houston Methodist, Houston, TX, USA.
Conflict of interest statement
N. Kleiman reports receiving educational and research support from Medtronic. A. Kalra is a consultant for Medtronic and Philips. E. Drachman is a consultant for Corindus Vascular Robotics and for Abbott Vascular, Inc., and also reports receiving uncompensated research support from Atrium Medical and from Bard/Lutonix. M. Mack serves as the Co-Principal Investigator for the PARTNER 3 trial (Edwards Lifesciences), study chair for the APOLLO trial (Medtronic), Co-Principal Investigator for the COAPT trial (Abbott Vascular). S. Elmariah serves as a consultant for Edwards and Medtronic and has also received research honoraria from Siemens and Boehringer Ingelheim. D.L. Bhatt discloses the following relationships - advisory board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; chair: American Heart Association Quality Oversight Committee; data monitoring committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; trustee: American College of Cardiology; unfunded research: FlowCo, Merck, PLx Pharma, Takeda. G.F. Attizzani is a consultant for Medtronic, Abbott and Edwards, and also serves on the Advisory Board for Medtronic. M.J. Reardon is on the Advisory Board for Medtronic. O. Khalique is a speaker for Edwards Lifesciences. S. Kodali is a consultant for Abbott Vascular and Claret Medical, and is on the scientific advisory board of Dura Biotech, Biotrace Medical, and Thubrikar Aortic Valve Inc. The other authors have no conflicts of interest to declare.

