Abstract
Aims: The aim of this study was to provide contemporary outcome data for patients with de novo coronary disease and Medina 1,1,1 lesions who were treated with a culotte two-stent technique, and to compare the performance of two modern-generation drug-eluting stent (DES) platforms, the 3-connector XIENCE and the 2-connector SYNERGY.
Methods and results: Patients with Medina 1,1,1 bifurcation lesions who had disease that was amenable to culotte stenting were randomised 1:1 to treatment with XIENCE or SYNERGY DES. A total of 170 patients were included. Technical success and final kissing balloon inflation occurred in >96% of cases. Major adverse cardiovascular or cerebrovascular events (MACCE: a composite of death, myocardial infarction [MI], cerebrovascular accident [CVA] and target vessel revascularisation [TVR]) occurred in 5.9% of patients by nine months. The primary endpoint was a composite of death, MI, CVA, target vessel failure (TVF), stent thrombosis and binary angiographic restenosis. At nine months, the primary endpoint occurred in 19% of XIENCE patients and 16% of SYNERGY patients (p=0.003 for non-inferiority for platform performance).
Conclusions: MACCE rates for culotte stenting using contemporary everolimus-eluting DES are low at nine months. The XIENCE and SYNERGY stents demonstrated comparable performance for the primary endpoint.
Abbreviations
BBK: Bifurcations Bad Krozingen
CERC: European Cardiovascular Research Center
CVA: cerebrovascular accident
DES: drug-eluting stent
DK: double kissing
EBC: European Bifurcation Club
IVUS: intravascular ultrasound
LMS: left main stem
MACE: major adverse cardiovascular events
MACCE: major adverse cardiovascular or cerebrovascular events
MI: myocardial infarction
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
POT: proximal optimisation technique
TAP: T-and-protrusion
TVF: target vessel failure
TVI: target vessel inadequacy
TVR: target vessel revascularisation
Introduction
Percutaneous coronary intervention (PCI) for bifurcation lesions is still viewed as a complex procedure. Bifurcations show substantial variation in their anatomical presentation, either by Medina classification1, by angulation from the main vessel to the daughter branches or by discrepancy in the diameter of the parent and daughter branches. Historically, randomised controlled trials have tended to favour a less complex strategy to treat these lesions2-5. Lower rates of major adverse cardiovascular events (MACE) were reported with a provisional (preferred single-stent) strategy at the bifurcation. Whilst this concept may be attractive for a number of bifurcation lesions, many Medina 1,1,1 lesions create a significant burden of ischaemia in both branches and an initial provisional approach is not always appropriate for the patient. Under these circumstances, up-front two-stent strategies may be necessary for complete and durable resolution of ischaemia. Numerous different two-stent strategies are described6, with the strategic approach best dictated by the specific anatomy that is encountered during the case, whether this is a bail-out manoeuvre after a provisional approach, or as an up-front strategy where clinically indicated.
Culotte stenting has been evaluated against other techniques in randomised trials7-11. Results have been mixed, with some favourable outcomes compared to crush or T-and-protrusion (TAP)7,9,10 and some unfavourable compared with double kissing (DK) crush in the left main8. The European Bifurcation Club (EBC) 2 study found improved outcomes with a provisional approach versus culotte, although in a variety of Medina classified lesions with ≥50% stenosis11. Furthermore, none of these trials exclusively evaluated contemporary everolimus-eluting stents that have the broadest evidence base for both use and outcomes. One aim of the current study was to assess outcomes for patients who were exclusively treated with these devices.
There are also a number of mechanical features of modern DES platforms that have the potential to influence mechanical and ultimately patient outcomes in bifurcation lesions. These include the overexpansion capability that dictates the ability to appose the device in a larger proximal main vessel. The number of connectors through the body of the stent affects the stiffness of the device, bending moments and fracture risk. These are important considerations at areas of significant vasomotion or hinge potential, including at a bifurcation. Finally, the number of connectors and their construction also influence guidewire access as well as the maximum area that can be achieved at the ostium of the side branch. Therefore, a second aim of the study was to explore whether the type of stent used during culotte stenting would potentially influence the procedural or clinical outcome.
Methods
This was an investigator-led, prospective, randomised, multicentre trial that was undertaken in nine centres in Ireland and the United Kingdom. The trial was administered and overseen by a clinical research organisation (European Cardiovascular Research Center [CERC]) and the data were overseen, assessed, and adjudicated by a clinical events committee, angiographic core laboratory (CERC core laboratory using CASS QCA V7.3 software by Pie Medical Imaging BV, Maastricht, the Netherlands) and data and safety monitoring board. The study protocol was approved by the relevant authorities in each country/centre and there was compliance with the Declaration of Helsinki. All patients provided written, informed consent prior to trial participation. The research was funded by an unrestricted educational grant from Boston Scientific. The trial was registered on clinicaltrials.gov (NCT02232815). Procedural technique as well as inclusion and exclusion criteria are shown in Supplementary Appendix 1.
STUDY ENDPOINTS
The primary endpoint of the study was a composite of death, myocardial infarction (MI), cerebrovascular accident (CVA), target vessel failure (TVF), stent thrombosis (definite or probable) and binary angiographic restenosis assessed at nine months. Secondary endpoints included predefined procedural parameters. These included technical success (deployment of stents in both branches with <20% residual stenosis and kissing balloon inflation at end of procedure), assessment of the equipment used and evidence of longitudinal stent deformation (index implant) or stent fracture at angiographic follow-up. Patients will be followed for 24 months for adverse events.
Major adverse cardiovascular or cerebrovascular events (MACCE) were defined as a composite of death, MI, CVA and target vessel revascularisation (TVR).
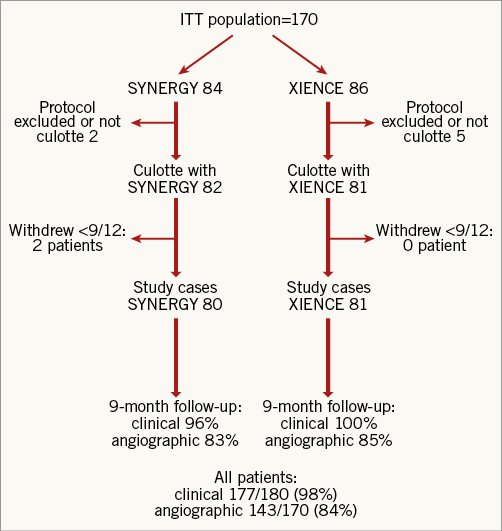
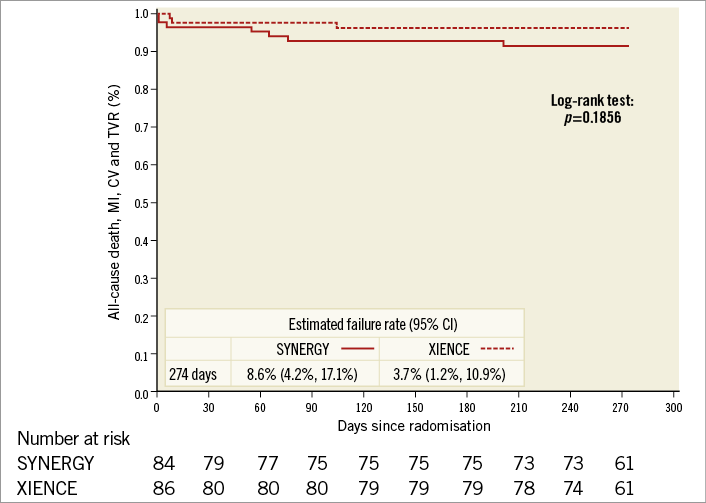
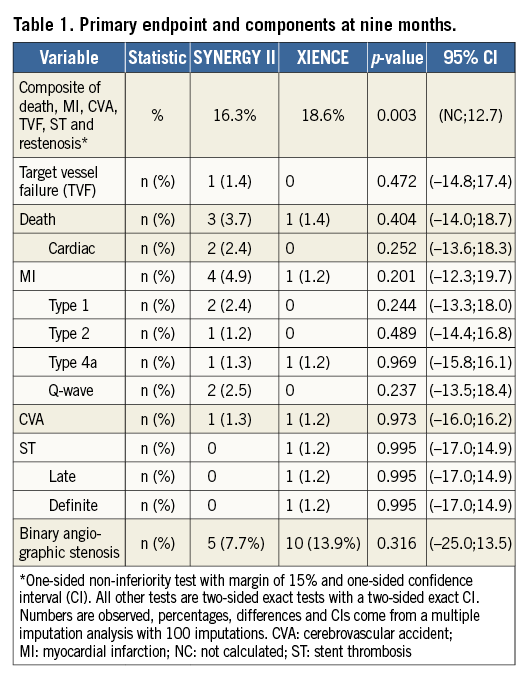
Death was considered cardiac unless there was another clear cause. MI was defined according to the third universal definition of MI12. TVR was defined by any balloon being inflated within the target vessel, or if any new stent was implanted, or if the vessel was treated with a bypass graft. Target vessel inadequacy (TVI) was defined as complete or sub-total occlusion ( STATISTICAL ANALYSIS A primary endpoint rate of 10% was anticipated on the basis of historical trial results2,4,13. This investigation was a pilot study for the comparison of the two study stents, with the potential for a larger subsequent trial based on the results. As such, a perfunctory power calculation was performed. It was calculated that 85 patients per group were required (with α of 5% and 1-β of 90%) to assess a one-sided non-inferiority limit of 15% for SYNERGY. A more detailed description of statistical methods is available (Supplementary Appendix 2). Results One hundred and seventy patients were consented for and randomised in the study. The patient flow is outlined in Figure 1. A small number did not proceed to culotte stenting for a variety of reasons (presence of previously undiagnosed significant left main disease, presence of stents in both target vessels not apparent on diagnostic angiography, occlusion of the target vessel subsequent to diagnostic angiography, a side branch diameter <2.5 mm at PCI and physiology-based deferment of a two-stent strategy). Patient characteristics are provided in Supplementary Table 1 and procedural characteristics are described in Supplementary Table 2. Patients were allowed to have a non-study vessel treated at the time of the index PCI. In total, 11 patients (six XIENCE [Abbott Vascular, Santa Clara, CA, USA], five SYNERGY™ [Boston Scientific, Marlborough, MA, USA]) had 12 non-study lesions treated (six XIENCE, six SYNERGY). Figure 1. Patient flow through the study. ITT: intention-to-treat Both groups were well matched at inclusion. There was good adherence to the protocol with POT and final kissing inflation rates exceeding 90%. As one might expect with patients who had coronary disease that was amenable to culotte stenting, the diameter of the side branch stent matched the main vessel stent. Procedure-related parameters are presented in Supplementary Table 3. These demonstrated very similar results in both groups, inferring that there was little difference in procedural performance between a thin-strut 2-connector and 3-connector DES. Follow-up rates were high at 98% for clinical follow-up and 84% for angiographic follow-up for the entire study cohort. A Kaplan-Meier curve for MACCE (death, MI, CVA or TVR) is shown for the intention-to-treat population in Figure 2. The overall MACCE rate was low for all patients, occurring in 5.9% by nine months. Numerically, more MACCE events occurred in the SYNERGY group (Figure 2, Table 1) although this was not statistically significantly different. Detailed analysis of the MACCE events revealed that a number of these were specific neither to the culotte technique, nor to stent type. These included an atheromatous embolic CVA periprocedurally that led to in-patient (non-cardiac) mortality, a traumatic subdural haematoma that also led to a later (non-cardiac) mortality, a CVA one week post procedure and a perforation during post-dilation with a non-compliant balloon that led to in-patient coronary artery bypass grafting. Figure 2. Kaplan-Meier curve for MACCE for the intention-to-treat population. The primary endpoint and its components are presented in Table 1. The non-inferiority test for device performance was met (16.3% vs. 18.6% for SYNERGY vs. XIENCE, p=0.003). The components were similar for both groups, although the XIENCE had an in-stent restenosis (ISR) rate almost double that of the SYNERGY stent. This did not achieve statistical significance. For both devices, this occurred almost exclusively in the distal segments, was moderate, was not associated with geographical miss and did not require repeat PCI. Discussion There has been considerable evolution in the practice of PCI over the last decade. Many of these changes in practice are reflected in the CELTIC Bifurcation Study. In contrast to the reports of Ferenc9, Zheng10 and Hildick-Smith11, our patients had exclusively Medina 1,1,1 lesions. This higher disease burden led to longer stent lengths in the side branches relative to previous reports. However, irrespective of the higher lesion complexity and disease burden, our cases were performed almost exclusively with transradial access (96%) compared to <2/3 of patients in other recent contemporary series10,11. A transradial approach is viewed by some clinicians as a step that offers less guide catheter support and potentially hampers complex PCI procedures. Our experience demonstrated very high rates of technical success and final kissing balloon inflation. Furthermore, the recorded procedural parameters including procedure time, fluoroscopy time, radiation and contrast doses are comparable to other recent publications that examine culotte stenting9-11 and more closely mirror those of the provisional stenting group in the EBC-2 study11. These findings may in part reflect more modern X-ray equipment14, the specific stents that were used in addition to operator experience. The MACCE rate of 5.9% at nine months provides reassurance with regard to outcomes with the two-stent culotte technique for more complex disease subsets. These results are difficult to compare to older studies due to different endpoint definitions, especially of MI. For example, MACE was defined as cardiac death, non-procedure-related MI, stent thrombosis, or TVR (PCI or coronary artery bypass surgery) after six months in the Nordic Stent Technique Study7. Despite being almost a decade old, low MACE rates were noted in this study for sirolimus-eluting culotte stents at six months (3.7%). However, using the same definition of MI as in the CELTIC Bifurcation Study, this rose to 12.5% if type 4a MI was added to the composite endpoint7. The finding of a 16.3% MACE rate at 12 months in the DKCRUSH-III study in left main stem (LMS) bifurcations is surprising in the context of the CELTIC Bifurcation outcomes8. The DKCRUSH-III definition of MACE was MI, cardiac death, and/or TVR. Whilst the majority of implanted stents were XIENCE in this trial (almost 2/3), a significant proportion were not everolimus-eluting stents. It is likely that the high adverse event rate was driven by the small final diameters achieved in the LMS and its branches (only 3 mm in the main vessel, with no description of systematic POT). A mean LMS diameter of ~5 mm would be anticipated on the basis of population-derived IVUS data assessing the LMS bifurcation15. Indeed, the “side branch” stent diameter (i.e., LAD or LCx) in DKCRUSH-III is comparable to those described for diagonal, marginal and distal RCA branches in our study. Whilst speculative, it is possible that a double layer (culotte) of relatively undersized stent in the LMS would lead to worse outcomes than a comparable single layer (the DK crush group) in suboptimal mechanical circumstances. The CELTIC Bifurcation outcomes also compare favourably to more contemporary studies. The results of the Bifurcations Bad Krozingen (BBK) 2 study, which used a variety of contemporary DES, demonstrated a “target lesion failure” rate (composite of cardiac death, target vessel MI, and target lesion revascularisation) of 6.7% for culotte stenting at 12 months9. Presumably, the same definition of MACCE would have led to a higher adverse event rate. The study of Zheng and colleagues used cardiac death, MI, stent thrombosis, and/or TVR as the composite for MACE10. Culotte stenting showed a MACE rate of 5.3% at 12 months, although it is not clear which stents were used in the study or whether the universal definition of MI was applied. The EBC-2 primary endpoint (composite of all-cause death, MI, and TVR) more closely reflects our definition of MACCE11. The 12-month event rate of 10.3% and adverse procedural parameters compared to our study are more likely to reflect the specific biolimus-eluting stent that was used in this study, given the similarity of the technical approach to the culotte procedure. The comparison of the different stents in the CELTIC Bifurcation Study was hypothesis-generating. Whilst the power calculation anticipated a primary endpoint event rate of 10%, this was noted to be 18% overall. Therefore, the trial retained 80% power to test the initial hypothesis despite a lower number of patients having angiographic follow-up. Overall, the finding of comparable performance for the primary endpoint was unsurprising (SYNERGY 16% vs. XIENCE 19%, p=0.003 for non-inferiority). In a relatively small population, it is important to understand the MACCE events in context, where a low number of adverse events occurred. Whilst the MACCE rate was numerically different between the two groups, a number of these events were generic complications of PCI procedures and their relevance to the stent platforms should be interpreted cautiously. The binary angiographic restenosis rate was 11% overall. This is higher for culotte stenting than the 6.6% in-segment restenosis at six months reported in the Nordic Stent Technique Study7, 6% in BBK 29 and 6% reported by Zheng and colleagues10 at 12 months. The latter two investigations did not use an angiographic core laboratory that may in part have contributed to the outcome differences. The finding of differing rates of in-stent restenosis observed between the two platforms in our study is at most hypothesis-generating. The adverse event rate observed in a large all-comer PCI population was similar when 2-connector and 3-connector durable fluoropolymer everolimus-eluting stents were compared in the PLATINUM PLUS study16. The 12-month results of the EVOLVE II clinical trial would also suggest that there is unlikely to be a major early influence of a durable versus biodegradable polymer with modern everolimus-eluting stent platforms17, although the rates of two-stent bifurcation treatment in these all-comer studies were likely to be low and are not specifically reported. It is possible that these features may be of some relevance to longer-term outcomes in more complex lesions. Nevertheless, the importance of a moderate angiographic restenosis that does not lead to TVI or repeat intervention is of questionable clinical relevance. There was a very low stent thrombosis rate at nine months (0.6% overall) in our patients, with one longitudinal stent deformation noted that did not hamper the technical result after intraprocedural correction. The stent fracture rate of 2.1% overall is similar to that described for a larger population of >1,000 patients with less complex lesions who were treated with 3-connector everolimus-eluting stents and had systematic follow-up angiography at six to nine months (2.9% per lesion fracture rate)18. A similar study of 2-connector everolimus-eluting stents in >800 patients found a per lesion fracture rate of 1.7%19. Both registries reported low rates of two-stent bifurcation treatments. This finding would suggest that two-stent culotte bifurcation stenting is not an added risk factor for stent fracture. Limitations This study did not directly compare culotte stenting to a provisional approach for the patients who were enrolled in the trial. Therefore, implications regarding the results when compared to a provisional approach should be interpreted with caution. The procedures were carried out by high-volume PCI operators with extensive experience of bifurcation stenting; similar results may not be achieved by less experienced operators who are not familiar with this technique. Conclusions The CELTIC Bifurcation Study confirms that a transradial approach for a two-stent culotte bifurcation strategy is associated with high rates of technical success and acceptable procedural parameters. The overall MACCE rate is low at nine months and provides reassurance that patients with true bifurcation disease can be safely treated with this two-stent technique. The XIENCE and SYNERGY stents were comparable in performance for the primary endpoint. Contrary to prior conclusions and current dogma that a “provisional approach” is preferable for bifurcation lesions, this randomised multicentre study demonstrates that culotte stenting with either device is safe and effective and may also be considered as the primary strategy in appropriately selected, symptomatic Medina 1,1,1 lesions with large side branches. Impact on daily practice This study demonstrates that culotte stenting can be performed safely and efficiently from a transradial approach. Cardiologists can be reassured that, if two-stent techniques are indicated for either bail-out after a provisional approach or as an up-front technique, outcomes are favourable when modern everolimus-eluting stents are used with culotte stenting. The SYNERGY and XIENCE stents both perform well under these circumstances. Funding Investigator-sponsored research funded by an unrestricted grant from Boston Scientific. Conflict of interest statement S. Walsh is a consultant to and has received research funding from Abbott Vascular and Boston Scientific. C. Hanratty is a consultant to Abbott Vascular and Boston Scientific. J. Spratt is a consultant to Abbott Vascular and Boston Scientific. M-C. Morice is the CEO of the clinical research organisation that conducted the trial. The other authors have no conflicts of interest to declare. Supplementary data Supplementary Appendix 1. Inclusion, exclusion criteria and procedural technique. Supplementary Appendix 2. Detailed statistical methods. Supplementary Table 1. Patient characteristics of intention-to-treat population. Supplementary Table 2. Procedural characteristics of intention-to-treat population. Supplementary Table 3. Procedural parameters for both treatment groups. To read the full content of this article, please download the PDF.