Abstract
Aims: This new generation of DES has attempted to improve clinical safety by avoiding the presence of polymers. The present preclinical in vivo study was designed to investigate the safety profile of Cre8™ stent. This is a new coronary stent based on Amphilimus™, a sirolimus formulated with a polymer-free amphiphilic carrier released from reservoirs machined onto the abluminal stent surface.
Methods and results: Cre8™ stents were compared with two controls: R3 (the same platform only loaded with an amphiphilic carrier) and the Cypher Select Plus® stent (Cordis, Johnson & Johnson, Warren, NJ, USA). All devices (48 stents) were implanted in porcine coronary arteries with subsequent histological and morphometric evaluations at seven, 30 and 90 days. Early endothelisation at seven days was almost complete in all stents. Vessel wall histology at 30 days demonstrated a mild inflammation score in all groups (an inflammation score lower than 1 was observed in 100% of Cre8 stent, 71.5% for R3 and 66.7% for Cypher; p=n.s.) while morphometry showed a significantly smaller neointimal area in Cre8™ (Cre8 0.93±0.43mm2; R3 1.49±0.67 mm2; Cypher 1.81±0.94 mm2; Cre8 vs. Cypher p<0.05); this difference was maintained after 90 days (inflammation score lower than 1 in 100% of Cre8 stent, 100% for R3 and 66.7% for Cypher; p=n.s. Neointimal area was 1.27±0.56 mm2 for Cre8, 1.74±0.60 mm2 for R3 and 2.79±1.14 mm2 for Cypher; Cre8 and R3 vs. Cypher p<0.05 while neointimal thickness was 0.15±0.07mm for Cre8, 0.21±0.12mm for R3 and 0.31±0.15 mm for Cypher ; Cre8 vs. Cypher p<0.05).
Conclusions: The most significant experimental evidence appears to be the absence of chronic inflammatory response in Cre8™ stent. This is expressed by a reduced neointimal thickness and inflammatory score at all follow-ups. Such an outcome positively compares with the other DES where a trend to neointimal growth and increased cell infiltration was observed.
Introduction
After the initial excitement related to restenosis mitigation in first generation DES, safety warnings arose concerning the drawbacks of late stent thrombosis along with the need to maintain patients on long-term DAT1-5. These clinical concerns were linked to a delay of tissue healing in terms of stented segment re-endothelisation. The reasons for this biological condition can be found in a drug release not exclusively targeted to the vessel wall and to the chronic inflammatory stimulus related to the presence of permanent polymer coating used as the drug carrier6. The second generation of DES tried to deal with this issue using new polymers with lower impact on platelet activity, introducing bioerodible polymers, or by simply limiting the polymer coating to the abluminal surface of the struts7-13. This second approach is justified by the effort to transform the stent into a bare metal stent after the pharmacological phase of DES. A third attempt to improve the clinical safety of DES was to completely avoid the presence of polymeric substances. This solution requires an alternative method to load and keep appropriate quantities of drug on the metallic platform; different answers to such challenges have driven the development of ceramic coatings, microporous surfaces, holes, reservoirs or simply direct drug coating on the stent struts.
Most of these technologies showed difficulties in modulating the drug elution profile, or unavoidably required the presence of polymers to carry and target the release of active principles. On the other hand, creation of reservoirs abluminally machined on the strut surfaces has already been investigated, demonstrating that active principles can be effectively carried and eluted without the need of polymeric carriers obtaining the required drug bioavailability. Furthermore, abluminal reservoir technology allows the use of non-polymeric origin excipients to modulate the drug elution into the diseased vessel wall. In terms of antiproliferative drugs, macrolides immunosuppressant based on m-TOR inhibition have clinically demonstrated to be the safest and most effective drugs in controlling the neointimal proliferation that always follows coronary stenting procedures.
The present in vivo study has been designed to investigate the preclinical safety profile of the Cre8™ stent, a new generation coronary stent based on Amphilimus™ formulation (sirolimus formulated with a mixture of long-chain fatty acids to function as a polymer-free carrier) released from reservoirs created on the abluminal stent surface.
Methods
Implanted coronary stents
Cre8™ (manufactured by CID. S.p.A., Saluggia, Italy) is a polymer-free, sirolimus-eluting stent pre-mounted on a balloon catheter. The stent is a thin (80 µm) cobalt-chrome alloy L605 structure characterised by two main features: the presence of reservoirs on the stent’s outer surface, devoted to the containment of the drug, and a coating that promotes fast cellular growth constituted by a permanent ultra-thin and high-density turbostratic carbon film called i-Carbofilm™ (Figure 1). This coating is extremely thin (<0.3µm), with high adhesion force to the substrate (>700 Kg/mm2), and it is characterised by an improved haemocompatibility, explained by a selective albumin absorption that causes minimal platelets activation14-17. The Cre8’s uniqueness also consists in its formulation called Amphilimus™, characterised by a non-polymeric amphiphilic carrier and sirolimus loaded at 0.9 µg/mm2. Sirolimus is an m-TOR inhibitor widely applied in DES, while the amphiphilic carrier, typically a molecule with both hydrophobic and hydrophilic domains, is a mixture of long-chain fatty acids widely present in the body, and therefore able to enter normal metabolic pathways. This molecule is extensively used as an excipient in the pharmaceutical field as well as in cosmetic and food applications. Thanks to its amphiphilic properties it is able to modulate the drug elution from the abluminal reservoirs into the vessel wall. In the pharmacokinetic (PK) study (Harlan Laboratories Ltd., Itingen, Switzerland), the Cre8 stent presents a maximum drug-blood release concentration in rabbits virtually equal to zero (Cmax=1.74±0.37ng/mL). This was also confirmed by an extremely low drug systemic availability over 72 hours (AUC=35.8±19.4ng·h/mL). The tissue drug concentration profile shows an initial peak at one day followed by a sustained release for the subsequent 28 days. As evidence of this device-prolonged drug elution, the quantification of the drug remaining on the stent revealed that only 50% of the total drug amount was released in about 18 days. The Cre8 investigational stents were compared in vivo with the same stent platform loaded with just the amphiphilic carrier (R3) and with a Cypher Select Plus® stent (Cordis, Johnson & Johnson, Warren, NJ, USA).

Figure 1. Macro picture of the Cre8 stent. The stent structure with the abluminal reservoirs filled-in with Amphilimus formulation is visible.
PRECLINICAL IN VIVO IMPLANTS IN THE ORTHOTOPIC POSITION
EXPERIMENTAL ANIMAL MODEL AND IMPLANT PROCEDURE
All animals were implanted with two coronary stents (right coronary artery and left descending anterior coronary artery). The pigs were anesthetised and treated with antibiotics, then heparinised 150 (UI/Kg) by intra-arterial route (ACT>300 sec.). An arterial access was made in the common right carotid artery by means of a 7Fr introducer. A Judkins or Amplaz type guide catheter was introduced through the introducer by means of a J guidewire, size 0.035”. The coronary ostium was reached with injection of contrast media. The investigational devices were introduced through the 6Fr guide catheter over a 0.014” guidewire. The operation was monitored by angiography, and the device was advanced until the implantation site was reached. The devices were then expanded using appropriate pressures until they were well apposed to the vessel wall. All implanted stents were expanded to reach a vessel/device ratio of 1:1.1. The antiplatelet treatment was limited to aspirin® from two days before to the seventh day after the intervention and then suspended. No supplementary antiplatelet drugs (ticlopidine or clopidogrel) were administered. At the end of the scheduled follow-up, each group of animals underwent a further angiographic examination in order to assess the vessel patency.
The animals were anesthetised with intramuscular injections of 2mg/Kg di ketamine and 2mg/Kg of xylazine and by inhaling Isofluoran 1%. A7Fr introducer was positioned in the left carotid artery and a follow-up angiography was carried out on the implanted coronaries using a similar method to that described above. The animals received 14,000IU of heparin intravenously administered. The animals were then euthanised by an intravenous injection of barbiturate (60mg/kg) through the jugular access following the “AVMA guidelines on Euthanasia (June 2007)”. After the explant, the heart was submitted to x-ray analysis to confirm stent location, following which the coronary tree was perfused with a heparinised physiological solution. When the perfused liquid looked free from haematic traces, the coronaries were fixed with a solution of formaldehyde at 4% at 150mmHg pressure. Finally the coronary vessels were carefully dissected from epicardial surface preserving artery size and shape.
HISTOLOGY PROTOCOL
The samples were formalin post-fixed. Seven day follow-up stents were processed for scanning electron microscopy (SEM) to assess endothelisation. Stented segments devoted to SEM were longitudinally cut, critical points dried and, before gold coating, pictures were taken of each half-stent by a stereo microscope at high magnification. Thirty and 90 day follow-up coronary arteries were dissected, and stented coronary artery segments were processed for methyl-methacrylate embedding, while proximal and distal unstented vessel sections were paraffin embedded. A representative number of 4µm sections were cut perpendicularly to the long axis of the vessel by a precision microtome, at the proximal, mid and distal stent level and at proximal and distal unstented tracts. Histological sections were stained with Haematoxylin and Eosin (HE) and Movat’s pentachromic stains to evidence elastic fibres; photomicrographs were taken for each segment of interest. Injury scores as described by Schwartz18, inflammation scores after Kornowski19 and the percentage of struts with granulomas were also evaluated. Cellular type density of neointimal matrix (monocytes, polymorphonucleates and smooth muscle cells), in term of cells/mm2, were also evaluated.
Histomorphometry protocolEndothelisation was calculated from the stereoscopic images measuring the non-coated strut surfaces over the total extent of strut surfaces. Neointimal thickness and neointimal area were measured on histological images, and percentage stenosis was consequently calculated. Patency was evaluated as percent of in-stent area stenosis. Endothelisation percent and histomorphometric variables were analysed by Image-Pro Plus® software (Media Cybernetics Inc. Bethesda, MD, USA).
Statistical analysis
The data are presented as mean ±SD for parametric data and frequency percentage for scores. Comparison among continuous variables were performed using one way ANOVA as data was normally distributed, adopting the Bonferroni t test method for multiple comparison. Differences among inflammation scores were analysed with the Kruskal-Wallis test. Statistical analysis was performed using SAS 9.1.3 software (SAS Institute Inc., Cary, NC, USA). Ap-value of 0.05 or less was considered statistically significant.
Results
A total of 24 juvenile domestic pigs randomly received a double stent implant in orthotopic position (one stent in the left and one stent in the right coronary artery) using 48 investigational stents. The average implant pressure was 10.8 atm. Six pigs were sacrificed (12 stents) at seven days to conduct the SEM investigations on the explanted stents, while the remaining animals were sacrificed at longer term follow-up. For each follow-up, three pigs per group were implanted. Eighteen stents were explanted at 30 days and an additional 18 stents were explanted at 90 days follow-up for histological and histomorphometric examinations. The coronary angiographies carried out after implant and at each follow-up confirmed the patency of all treated vessels. Thrombus presence, incomplete stent strut apposition and myocardial infarctions were not detected in any of implanted groups either by morphological heart examination or by histology.
Vessel healing assessment at seven days
The specimens obtained from the stent longitudinal sections were observed by SEM in order to evaluate and measure the vessel healing in terms of quality and the percent extent of neo-endothelisation. In Figures2 and 3 the appearance at SEM examination of the three stent groups at seven days of implant is represented. Specimen surfaces for all three stent groups appear free from platelet aggregation and thrombotic clots. Morphometric assessment of the surface endothelisation was quantified in percent of strut coverage by a continuous carpet of endothelial cells. The results of these measurements indicate that in all groups the endothelial cells covered uniformly almost 100% of the struts surface. Only in the Cypher stents was the endothelial cell layer characterised by inhomogeneous cell growth.
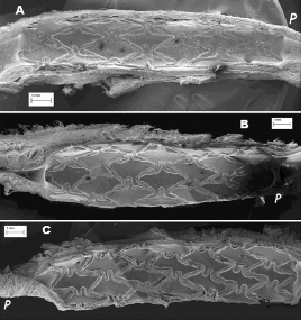
Figure 2. SEM (marker on picture) of the three stents at seven days (A: R3; B: Cre8; C: Cypher). The SEM pictures show at low magnification the status of vessel healing and strut coverage (the letter p indicates the proximal vessel segment in respect to blood flow).
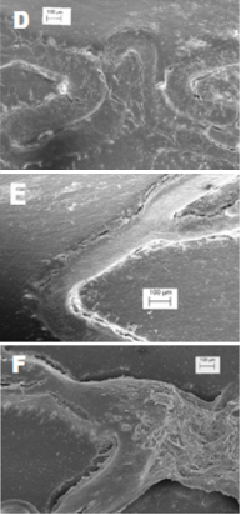
Figure 3. SEM (marker on picture) of the three stents at seven days (D: R3; E: Cre8; F: Cypher). At higher magnification, more details about the strut endothelisation are visible.
Histological evaluations
The 30-day tissue response was in general minimal, with negligible infiltration of inflammatory cells (Figures 4 and 5). The neointimal surface was lined by a regular carpet of endothelium. Neointimal growth was non-obstructive, characterised by an even distribution of a moderate number of cells in a proteoglycan matrix. Blood clots and minor fibrin deposits, mainly localised at strut proximity, were detectable for Cre8 and Cypher groups with the exclusion of R3 group.
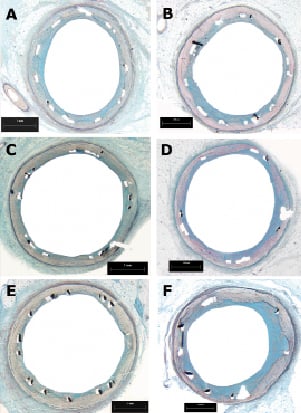
Figure 4. Histological section at low magnification shows the pattern of neointimal growth (marker on picture) at 30 days (A,C,E) and 90days (B, D, F) for all three stent groups (A and B: R3; C and D: Cre8; E and F: Cypher).
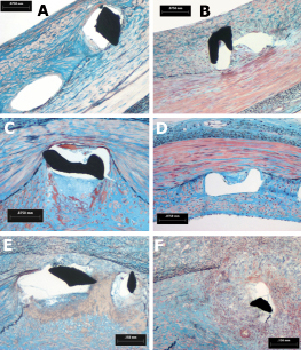
Figure 5. Histological section seen at high magnification showing tissue response to single struts; an example of granuloma is reported for the Cypher stent, (marker on picture) at 30 days (A,C,E) and 90days (B, D, F) for all three stent groups (A and B: R3; C and D: Cre8; E and F: Cypher).
Cell populations (monocytes, polimorphonucleates [PMN] with smooth muscle cells the most numerous) and their density were assessed in mid-stent sections and showed no statistical differences among the groups (Table1). Cre8 stents less stimulated the infiltration of monocytes, with a very low density, while, on the contrary, their density was higher in the Cypher group (Cre8 10±25 cells/mm2; R3 401±691 cells/mm2; Cypher 679±1133 cells/mm2; p=n.s.). PMN were totally absent in the Cre8 group and mildly present in the other groups (Cre8 0 cells/mm2, R3 77±133 cells/mm2, Cypher 81±126 cells/mm2, p=n.s.).
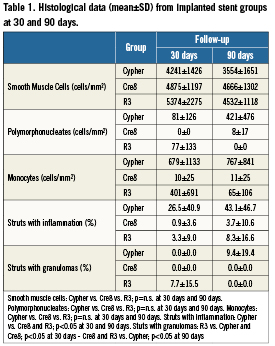
The inflammation score was in general mild in all groups, without statistical differences. An inflammatory score lower than 1 was observed in 100% of the Cre8 stents; while it was 71.5% for the R3 and 66.7% for the Cypher groups, respectively. Struts with granulomas were 0% for Cypher and Cre8, 7.7±15.5% for R3 stents (R3 vs. Cypher and Cre8 p<0.05).
The histological appearance at 90 days was characterised by a qualitative tissue response not significantly different from 30days (Figures 4 and 5). Neointimal growth was different among the implanted stent groups, nevertheless it appeared to be evenly distributed around the lumen circumference. The small blood clots around the struts, residual of the initial focal haemorrhages, were no longer present in the Cre8 stents, but still visible, together with fibrin traces, in histological sections from the Cypher stents. The cellular density at 90 days was still higher in the Cypher group concerning monocytes, and the same trend in PMN was observed. Monocyte density was 11±25 cells/mm2 for Cre8, 65±106 cells/mm2 for R3 and 767±841 cells/mm2 for Cypher (p= n.s.) while for PMN it was 8±17 cells/mm2 for Cre8, absent for R3 and 421±476 cells/mm2 for Cypher (p=n.s.). Inflammatory situations remained non-significantly different among groups at 90 days. Furthermore, the minimal inflammation score was reported for Cre8 stent (100% of stents had a score lower than 1) while the R3 group reduced its score to amilder level than at 30 days (100% lower than score 1). Cypher stents did not improve their inflammation score at 90 days, with only 50% scoring lower than 1 and the remaining 50% at a score of3. This observation was confirmed by the number of granulomas detected: in fact, even if the overall inflammatory picture is substantially mild, strut numbers concerned by the inflammatory reaction were higher in the Cypher stent (Cypher 9.4±19.4 %; Cre8 and R3 0%; p<0.05).
Histomorphometric evaluations
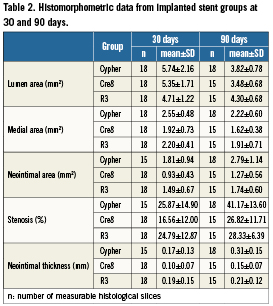
All histomorphometric parameters evaluated at 30 and 90 days are summarised in Figure 6. After 30 days, from implants, all three experimental groups were evaluated for their neointimal growth, cellular composition and vessel lumen patency (Table2). The measured neointimal thickness was lower for the Cre8 (0.10±0.07mm) as compared to the two other groups (R3 is 0.19±0.15mm and Cypher is 0.17±0.13mm), even if no statistical differences were detected among the groups. The same trend was reported for the neointimal area of the Cre8 group, with a significant difference towards the Cypher group (Cre8 0.93±0.43mm2; R3 1.49±0.67mm2; Cypher 1.81±0.94mm2; Cre8 vs. Cypher p<0.05). The percent area stenosis did not show statistical differences among the groups (Cre8 16.56±12.00 %; R3 24.79±12.87 %; Cypher 25.87±14.9%; p= n.s.). The Cypher group showed the largest medial area in comparison with the two other groups, with statistical difference versus the Cre8 group (Cre8 1.92±0.73mm2; R3 2.20±0.41mm2; Cypher 2.55±0.48mm2; Cypher vs. Cre8 p<0.05). The 90 days implant results are also summarised in Table2. The measured neointimal thickness was milder for non-polymeric stents and for Cre8 in particular. Cypher stent showed a statistically higher neointimal growth than Cre8 stent (Cre8 0.15±0.07mm; R3 0.21±0.12mm; Cypher 0.31±0.15mm; Cre8 vs. Cypher p<0.05). The neointimal area and percent area stenosis had the same trend of neointimal thickness, with significant differences between non-polymeric stents and Cypher. Neointimal area was 1.27±0.56mm2 for Cre8, 1.74±0.60mm2 for R3 and 2.79±1.14mm2 for Cypher respectively (Cre8 and R3 vs. Cypher p<0.05) while the percent area stenosis was 26.82±11.71% for Cre8, 28.33±6.39% for R3 and 41.17±13.60 for Cypher (Cre8 and R3 vs. Cypher p<0.05). The observed medial area statistical difference at 30 days between Cypher and Cre8 groups was maintained at 90 days (Cre8 1.62±0.38mm2; R3 1.91±0.71mm2; Cypher 2.22±0.60mm2; Cre8 vs. Cypher p<0.05).
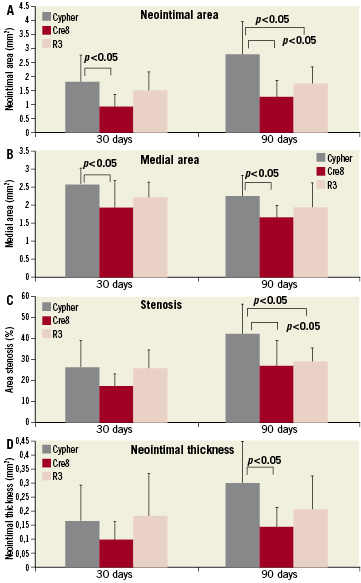
Figure 6. Histomorphometric data plotted for 30 and 90 days comparing the three stent groups. A) neointimal area; B) medial area; C) percent area stenosis; D) neointimal thickness
Discussion
The Cre8 stent is a third generation DES characterised by a permanent biocompatible i-Carbofilm strut coating and abluminal reservoirs loaded with a polymer-free sirolimus formulation (Amphilimus) to obtain a more efficient targeted elution towards the vessel wall. It is further characterised by a pharmacokinetic profile in which the active principle is modulated by a polymer-free carrier. The drug release profile distinguishes Cre8 stent from other DES for its minimal blood drug washout (1.74±0.4ng/mL), for example the Biofreedom™ polymer-free stent reports a4µg/mL drug loss with a peak after 2 hours20, while the Cypher stent is 12.8±2.3ng/mL after 1 hour from implant (data on file CID). These differences in drug-blood dispersion can be explained with the protection offered by the deep abluminal reservoirs and the Amphilimus formulation. As a consequence, the Cre8 system differentiates itself from other non-polymeric DES20 for its ability to control and sustain the drug elution along time. In fact, a significant tissue concentration for more than 30 days is maintained with 50% of the drug released in the first 18 days, the formulation is expected to be completely released after 90 days from implant.
This technology represents an effort towards the reduction in the incidence of late stent thrombosis that has been reported for polymeric DES1-5. without giving up the efficacy of DES in controlling excessive neointimal formation. Absent or late endothelisation and vessel healing are strong predictors of late stent thrombosis21.The early endothelialisation demonstrated by the Cre8 stent in this study is an important preclinical indicator of its clinical safety. This gentle approach to the endothelium may be ascribed to the targeted release of Cre8 that almost eliminates the drug leakage from nearby struts after implant, avoiding its cytotoxic effects; this is typical of polymeric DES. In addition, the turbostratic carbon coating on stent struts already promotes a complete endothelialisation at seven days22. This result confirms the safety window of the Cre8 formulation, even when a double drug dosage is administered. Further confirmation has been seen through other porcine coronary implants where a triple sirolimus dose has been loaded onto a Cre8 platform, and where the tissue response was not different from the single dosage (data on file L&D). The other post-stenting biological indicators of tissue response show differences between the implant times, but also among the different investigated groups. Neointimal thickness, neointimal area and percent area stenosis grow from 30 to 90days, but not in the same amount among the investigated groups. The Cre8 stent neointima is the mildest at 30 day follow-up, and remains mild at 90 days as well (from 0.10µm to 0.15µm) without any significant statistical difference (p=n.s.), while the Cypher neointima is the thickest at 30 days and significantly increases from 0.17 µm to 0.31 µm at 90 days (p<0.05). After 30 days, Cre8 demonstrates intact internal elastic laminae, some fibrin deposits and limited haemorrhagic blood clots around struts, without inflammation and granulomas; while the Cypher stents have larger clots and some inflammatory responses. Fibrin deposition and clots must be interpreted as an effect of drug activity on healthy porcine vessels. At 90 days, inside the neointima, the healing process appears completed in the Cre8 stent where all fibrin and haemorrhagic traces around the struts disappear without residual inflammation, reservoirs are very frequently fully colonised by cells (Figure5). The same appearance at 90 days is observed for R3 stents, where the few granulomas present at 30 days are healed. On the contrary, the tissue reaction to the Cypher stent is still marked by fibrin persistence, not fully reduced blood clots, inflammation signs and several granulomas (9.4±19.4% of struts). The same trend in inflammatory response is in line with previous data published on the Cypher stent by Wilson23. Such differences lead us to speculate on the hypothesis that the polymer coating may be the cause6 of a chronic inflammatory response after complete drug elution. In fact, the cell density of monocytes and PMN in the Cypher stent group, already high during the acute inflammatory response at 30 days, remains remarkable as well at 90 days; in particular, PMN cells increase. The mild reaction observed with the R3 group, corroborates the innovative choice of a polymer-free carrier to formulate a potent antiproliferative substance such as sirolimus. The absence of hypersensitivity to the Amphilimus formulation is linked to a limited inflammatory infiltrate at all follow-up times. The negligible presence of monocytes and PMN at 30 days in the R3 group with a carrier alone is, on the contrary, mitigated in Cre8 group by the drug activity, while at threemonths the healing is completed for both devices. The Cypher group trends towards an increasing chronic inflammatory reaction and, as previously reported in animal model20,24, it is estimated to peak for neointimal growth at longer follow-up time.
After stent implant, the integral i-Carbofilm coating offers a polymer-free, non-thrombotic surface on luminal stent side in contact with blood. This luminal stent side is quickly endothelised in absence of both cytotoxic drug effects and polymer that could induce inflammatory responses. On the abluminal side, the strut/vessel wall interface is characterised by i-Carbofilm coating and reservoirs filled with the drug formulation. Likewise, after the complete elution of the formulation, the presence of carbon coating, also in the reservoirs, will not trigger any residual chronic inflammatory stimuli, allowing for complete cellular colonisation. The Cre8 stent was developed in order to release drugs in a targeted and efficient manner, reaching significant drug concentration in the vessel wall. Nonetheless, negligible cytotoxic effect is visible, allowing fast and complete bio-induced endothelial growth. At 30 days, the biological effects of the drug are clearly evident and, in spite of that, the vessel expresses a very thin neointimal growth. However, at longer follow-up, the vessel healing appears complete, with a still limited neointimal deposition and absence of chronic inflammatory signs (the Cre8 neointimal thickness at 30 vs. 90 days; p=n.s.). The Cypher’s typical polymer-related inflammatory pattern was not detectable in the Cre8 at any of the follow-up times. The in vivo porcine orthotopic implant model, as discussed by Virmani25, has shown many limitations as a predictor for clinical efficacy in human subjects, however, it is effective in assessing the preliminary safety of DES. It appears that safety is mainly associated with the drug’s pharmacological effects, with chronic inflammatory response to polymers and with acute and late pro-thrombotic activity.
Conclusion
This preclinical, in vivo investigation confirms that the biological safety of the Cre8 stent with its polymer-free sirolimus elution, is superior to the Cypher Select Plus stent, even if the same pharmacological effect of m-TOR inhibitors are observed. Although definite speculations cannot be made about future clinical outcomes, good efficacy and improved safety is expected from the Cre8 clinical trials in correlation with current DES.
Acknowledgement
We acknowledge C.I.D. S.p.A., the manufacturer of the Cre8 and R3 stents in conducting this preclinical in vivo investigation and providing pharmacokinetic data as well as any additional technical materials and informative supports, from their Research & Development files, that were useful in writing this paper.
Conflict of interest statement
The authors have no conflict of interest to declare.

