Abstract
Coronary-cameral fistulas (CCF) are anomalous connections between one or more coronary arteries and a cardiac chamber, most commonly the right ventricle or right atrium. The major indications for closure are: significant left to right shunt, myocardial ischaemia, prevention of endoarteritis or rupture. Nowadays, the first option for treatment is transcatheter closure. According to the morphology of the fistulas the most appropriate occluder device should be selected: coils (e.g., Gianturco coils, controlled-release coils, PFM coils), vascular plugs or a patent ductus arteriosus (PDA) device or muscular ventricular septal defect (VSD) device. The way to deploy the occluders could be direct arterial or venous through an arteriovenous loop, according to the anatomy of the fistulas assessed by multiple angiograms in different projections. A test occlusion of the fistula with balloon catheter and simultaneous coronary angiogram is recommended for choosing the proper device type and size and the best position for deployment to achieve complete occlusion of the fistula without compromising the flow in coronary side branches.
Pathophysiology
Coronary-cameral fistulas (CCF) are anomalous connections between one or both coronaries, most commonly the right coronary, then the left or circumflex, and a cardiac chamber, most frequently the right ventricle or right atrium. The fistula may drain into the pulmonary artery, in which case it is referred to as a coronary arteriovenous fistula. The origin of the fistula may be from a proximal main coronary or from its branches; the fistula frequently has a tortuous course and can be very dilated or aneurysmatic. Multiple feeding vessels may also be present.
Small fistulas found incidentally in asymptomatic patients need no treatment. The indications for closure are left to right shunt with significant left ventricular volume overload, congestive heart failure, myocardial ischaemia due to blood steal from the normal coronary branches, or prevention of endoarteritis or rupture in cases of aneurysmal dilatation of fistulous vessels.
Treatment
Surgical repair is recommended in young symptomatic patients with body weight less than 5 kg or in cases of complex anatomy where transcatheter closure is judged to be unfeasible; however, the first choice of treatment is transcatheter intervention in the majority of patients. Pre-procedural assessment is usually obtained by CT scan; however, aortography and selective left and right coronary angiography in the cathlab are essential to confirm the diagnosis.
Transcatheter procedure
Standard right and left catheterisation is performed through femoral access. One hundred U/kg heparin is given. An aortogram and selective coronary angiograms are performed: it is particularly important to delineate the anatomy of the fistula, in terms of origin, course, size, site of drainage, and relationships with normal coronary branches1.
A variety of catheters and wires should be available, including non-tapered catheters, guiding catheters, microcatheters, floppy 0.014 inch or superfloppy wires, to be able selectively to enter tortuous vessels, as frequently observed. Temporary occlusion of the fistula with a balloon catheter and simultaneous selective coronary injection is recommended, in order to select the most appropriate device type and size, and the exact point for deployment to achieve complete occlusion of the fistula without interfering with flow in normal coronaries.
Occluder devices
The most commonly used occluder devices are the following: coils (e.g., Gianturco coils and controlled-release coils [both Cook Medical, Bloomington, IN, USA], PFM coils [pfm medical ag, Cologne, Germany]), a patent ductus arteriosus (PDA) device or a muscular ventricular septal defect (VSD) device or vascular plugs by a manufacturer such as Occlutech (Helsingborg, Sweden) or St. Jude Medical (St. Paul, MN, USA). When using coils, a single coil can be sufficient for closing relatively small fistulas; however, multiple coils in sequence are frequently used to achieve complete occlusion of larger fistulas or, in fistulas with aneurysmal dilation, filling them like a nest. In very high-flow fistulas an AMPLATZER™ (St. Jude Medical) or an equivalent occluder device is preferable1-4. The size of the occluder device should be 20 to 40% larger than the size of the fistulous vessel.
The occlusion of the fistula should occur distally to the origin of normal side branches. The route for device deployment is chosen according to the anatomy. It could be direct arterial, most commonly by using microcatheters, particularly helpful when navigating in long and tortuous vessels. The occluder device has to be placed at the distal end of the fistula; while treating a large tortuous vessel the venous approach is preferable, obtained by entering the fistula from the arterial side, passing an exchange wire to the right heart, snaring it and establishing an arteriovenous circuit, allowing a safe and easy advancement of the delivery sheath over the wire from the venous route into the fistulous vessel to be closed. The use of braided “kink-resistant” long sheaths is recommended, particularly when navigating in tortuous vessels. Examples of such sheaths are from Cook Medical and from St. Jude Medical (TorqVue™ delivery system), and there are others available.
Results and complications
The decision to treat should be based on balancing the symptoms and the risk of the fistula and the risk of complications. Effective occlusion of CCF can be obtained in more than 90% of cases5,6. An example of closure of a fistula between the circumflex artery and the right ventricle is shown in Figure 1 and Figure 2.
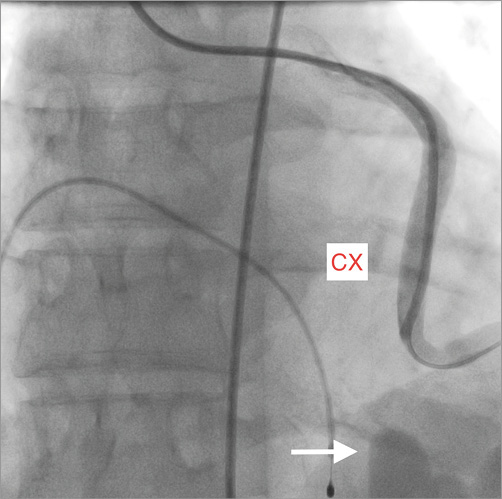
Figure 1. Selective contrast injection in a case of a fistula between the circumflex (CX) and the apex of the right ventricle. Aneurysmal dilatation of the tortuous vessel at its end is indicated by the arrow.
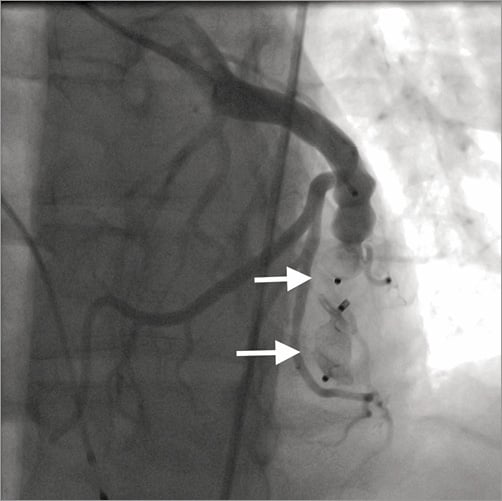
Figure 2. Angiogram taken after implantation of two AMPLATZER Vascular Plug II (arrows). The fistula is completely closed; normal flow in coronary branches originating proximally to the fistula can clearly be seen.
Appropriate sizing is mandatory to avoid device embolisation that may occur, particularly in high-flow fistulas. Retrieval devices should therefore be available when starting the procedure. Careful planning of the procedure can usually prevent occlusion of normal coronary branches, leading to myocardial ischaemia/infarction. Should this occur, selective thrombolytic treatment has proven to be effective.
Follow-up
Regular rest and exercise ECGs to rule out possible myocardial ischaemia and echocardiography to check possible regional wall motion abnormalities are mandatory to rule out late sequelae. Sometimes recanalisation of the fistula has been described, requiring additional interventions in selected cases7,8. In a few cases, late myocardial ischaemia due to retrograde thrombus propagation may also occur5-8. The best way to guarantee normal flow in the coronary is to close the CCF as close to the coronary origin as possible and simultaneously to close the distal end, if possible, to exclude as far as possible a cul de sac with flow stasis possibly promoting thrombosis. The appropriate anticoagulation regimen has not yet been definitively established: antiplatelet therapy for six months or lifelong, or anticoagulation at least in selected cases with very dilated and “low flow” fistulous vessels could be considered. Repeat coronary angiography after one year is recommended in these cases5-8.
Conflict of interest statement
M. Carminati is a consultant for St. Jude Medical. The other authors have no conflicts of interest to declare.

