
CASE SUMMARY
BACKGROUND: Coronary artery fistula (CAF) is a very rare clinical finding in itself. Here we report a case of a CAF complicated with myocarditis and discuss the diagnostic and therapeutic challenges.
INVESTIGATION: Clinical evaluation, laboratory tests, blood culture, coronary angiogram, cardiac computed tomography (CT), cardiac magnetic resonance (CMR), positron emission tomography (PET) CT scan (PET/CT).
DIAGNOSIS: Myocardial infarction in the setting of CAF caused by a possibly infected thrombus migrated downstream to the inferoseptal cavity followed by myocarditis.
MANAGEMENT: Medical pre-treatment (dual antiplatelet therapy, antibiotics), surgical treatment (resection of the CAF and coronary artery bypass grafting).
KEYWORDS: acute coronary syndrome, cardiac imaging, coronary artery fistula
PRESENTATION OF THE CASE
A 51-year-old male without cardiovascular risk factors was admitted for acute coronary syndrome (ACS) and high grade fever. The coronary angiogram showed a 50% stenosis of the left anterior descending artery (LAD) and an aneurysmal dolicho-artery of the diagonal branch (D) draining into a calcified structure and further into the coronary sinus (Figure 1, Moving image 1, Moving image 2). Cardiac computed tomography (CT) confirmed the diagnosis of a coronary artery fistula (CAF), localised within the inferior intraventricular septum (Figure 2A, Figure 2B). As the clinical status of the patient was collapsing with high grade fever, chills and hypotonia, in spite of normal blood cultures, the decision to carry out additional diagnostics was made. A positron emission tomography/CT (PET/CT) scan showed an active process inside and in the periphery of the cavity (Figure 2C). Cardiac magnetic resonance (CMR) showed a pediculated, floating thrombus within this cavity (Figure 3A, Moving image 3). In late gadolinium enhancement on CMR, myocardial ischaemia was detected in the anterior wall supplied by the LAD, coexisting with myocarditis in the area adjacent to the cavity (Figure 3B). A diagnosis of myocardial infarction in the setting of CAF caused by a possibly infected thrombus migrated downstream to the inferoseptal cavity followed by myocarditis was postulated in this patient.
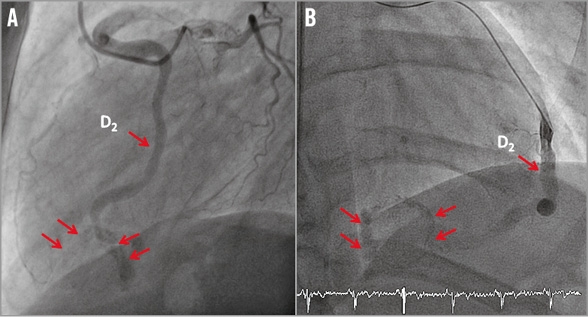
Figure 1. Coronary angiogram. 50% stenosis of the left anterior descending artery (LAD) (A) and an aneurysmal dolicho-artery of the second diagonal branch (D2) draining into a calcified inferoseptal cavity and further into the coronary sinus (A,B).
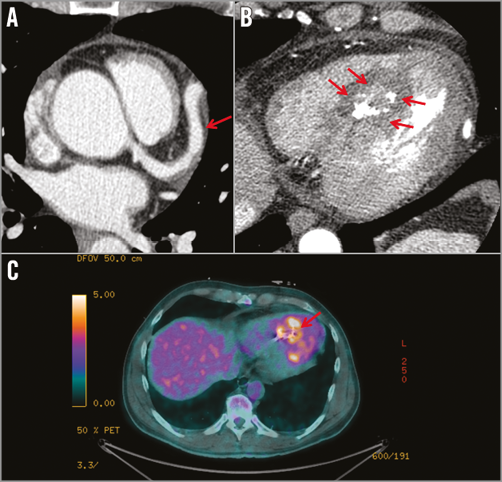
Figure 2. Non-invasive cardiac imaging. A) & B) Cardiac computed tomography (CT). Coronary artery fistula (CAF) within the inferior intraventricular septum. C) Positron emission tomography/CT scan. An active process in the cavity.
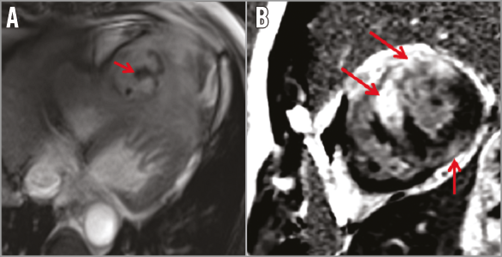
Figure 3. Cardiac magnetic resonance (CMR). A) A pediculated thrombus within the cavity. B) Late gadolinium enhancement. Myocardial ischaemia in the anterior wall supplied by the LAD, myocarditis in the area adjacent to the cavity.
The acute, complex clinical setting and uncommon anatomy of the CAF caused many concerns as to how to treat the patient optimally, in particular whether to choose percutaneous coronary intervention (PCI) with coil embolisation or cardiac surgery?
How would I treat?
THE INVITED EXPERT’S OPINION

The patient reported by Morawiec et al presents with an interesting but rare problem. Congenital coronary artery fistulas (CAF) are rare and usually isolated. They are the most common congenital coronary anomaly. The incidence is between 1/100,000 and 1/10,000. About 60% arise from the right coronary artery, 20% from the left anterior descending and 20% from the left main stem or the circumflex coronary arteries. The drainage is to the right heart in over 90% of cases1. They may cause a significant left-to-right shunt in early infancy or childhood, or coronary artery “steal” in older patients. The feeding artery may be large and aneurysmal or small with multiple bends. Towards the exit point, there may be aneurysmal dilation of the vessel.
This patient has a fistula between the diagonal branch of the left anterior descending coronary artery and probably the coronary sinus, with an aneurysm towards the distal part. Aneurysmal dilation with a smallish exit point may rarely cause sufficient stasis within the aneurysm to cause thrombosis. It is even rarer to have a thrombus floating around within the aneurysm or for myocarditis to occur.
Catheter closure of CAF is usually performed early in infancy because of a large left-to-right shunt causing heart failure or in adults causing myocardial ischaemia. The aim of closure is to close the fistula as distally as possible, near or at the drainage point into the right heart and beyond any normal myocardial branches arising from the dilated vessel2,3. The approach depends on the size and age of the patient and the type of fistula. In children, an arteriovenous guidewire circuit is established and the appropriate device is delivered from the femoral venous approach. In adults, the feeding artery tends to be more tortuous and a retrograde arterial approach is preferable. A coronary guiding catheter is positioned in the ostium of the coronary artery giving rise to the fistula. Through this, a 3 Fr infusion catheter, such as a Progreat® (Terumo Corp., Tokyo, Japan) or Cantata® catheter (Cook Medical, Bloomington, IN, USA) is passed over a 0.014” floppy coronary guidewire. This combination is successful in negotiating the bends to reach the desired location for occlusion, which in this patient is likely to be within the aneurysm. This type of fistula is amenable to the aneurysm being filled with numerous coils to form a tight nest. A variety of coils can be used, such as IDC (Boston Scientific, Marlborough, MA, USA) or Detach-18 Embolization Coil System (Cook Medical), which are made of platinum and can be passed through the 0.018” lumen of the infusion catheters. They have controlled-release mechanisms and, if in an unsatisfactory position, can be withdrawn prior to their release. After the first coil, the position of the infusion catheter is maintained and further coils are delivered. Repeat angiograms are performed through the guiding catheter to assess complete occlusion.
Occasionally, in larger diameter fistulas, a balloon angiographic catheter may be floated into the dilated vessel for temporary balloon occlusion for 10-15 minutes to assess for ischaemia. If none is induced, then the fistula can be occluded in that position. Alternatively, a balloon wedge catheter can be passed over the guidewire into a distal location, inflated, and angiography performed distally to visualise any small branches.
After complete occlusion of the fistula, the patient is maintained on dual antiplatelet therapy. For this patient, this may only be needed for six to 12 months, after which a single drug such as aspirin may be adequate. In this type of fistula, it is rare for thrombus to track back to the main vessel. There are no universal guidelines for the management of CAF, although Gowda et al have produced an algorithm for their management4,5. Nowadays, surgery is reserved for those patients in whom catheter closure has failed6,7.
In this patient, if no organisms are grown on blood cultures and there is haemodynamic deterioration, then catheter closure should be performed. Otherwise, apart from cultures, other immune investigations and viral studies are needed before proceeding.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

Morawiec et al present an interesting case of a patient with a coronary fistula arising from the first diagonal branch and communicating with the coronary sinus. The patient presented with signs of acute coronary syndrome, and also sepsis. While a 50% left anterior descending artery (LAD) stenosis was found, an infected thrombus and calcified cavity were identified in the coronary fistula. Blood cultures, which we believe were taken before antibiotic treatment was started, were negative.
From a diagnostic point of view this leaves us with two questions:
1. What is the origin/cause of the infected thrombus in the coronary fistula?
2. Are there signs of bacterial infection?
Regarding the first, embolisations of this kind have been reported for patients with endocarditis, particularly if the aortic valve is involved8-10. Therefore, we would ask for additional transthoracic, or, even better, transoesophageal echocardiography, to rule out this primary diagnosis. Echocardiography, best in 3D quality, would also provide more information on the anatomy of the fistula and the calcified cavity, which can be used to plan future treatment. Given that the patient presented with signs of acute coronary syndrome, we would also be interested in the patient’s left ventricular function.
For the differential diagnosis of a bacterial infection, blood results such as white blood-cell count, C-reactive protein and procalcitonin, would be interesting.
In addition, we would like to mention that we would be careful in making a diagnosis of classic myocarditis (as in the title) at all, as the changes seen on CMR may be more reactive to the infectious process and not affecting myocardial function as found in classic myocarditis.
Assuming that the additional diagnostic tests confirm an isolated, bacterially infected thrombus in front of the calcified cavity in the coronary sinus, one could, in principle, discuss medical, interventional or surgical treatment options.
If the patient remains haemodynamically stable, we would start with broad-spectrum intravenous antibiotic therapy for 48 hours, to improve his septic reaction and to optimise his condition. Although interventional closure of coronary fistulas has been reported11, we would not consider this option in this case as it would increase the risk of recurrent infections in the longer term. In fact, we would recommend open surgery to close the fistula at its origin and to remove the infected thrombotic material and calcified cavity12. Furthermore, coronary artery bypass grafting could be considered using the left internal thoracic artery to the LAD territory. If the patient were to become haemodynamically unstable and not respond to the initial antibiotic treatment, an operation may need to be considered earlier to remove the infectious focus.
In case endocarditis were to be confirmed by echocardiography, we would again recommend surgery, as the infected heart valve, the primary cause of the embolisation, needs to be treated at the same time. This surgery should be considered early after presentation as the patient already suffers from the consequences of an arterial embolisation and future complications such as cerebral embolisations need to be prevented.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
In the setting of the CAF with complex anatomy, complicated with myocarditis, with a coexisting thromboembolic process, the patient underwent medical pre-treatment with dual antiplatelet and antibiotic agents (imipenem, vancomycin for 10 days, then levofloxacin for six weeks). Resolution of the thrombus and myocarditis was observed on CMR after six weeks of this therapy (Figure 4). Three months after the introduction of pharmacological treatment, the patient was sent for surgery of the proximal D2 (resection of the CAF) and LIMA on the LAD (Figure 5). The surgery was carried out on acetylsalicylic acid alone. At one-year follow-up, the patient remains asymptomatic and free from adverse events.
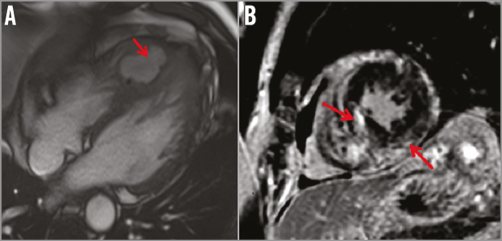
Figure 4. Cardiac magnetic resonance (CMR) after six weeks. A) Resolution of the thrombus within the cavity. B) Resolution of the inflammatory process.
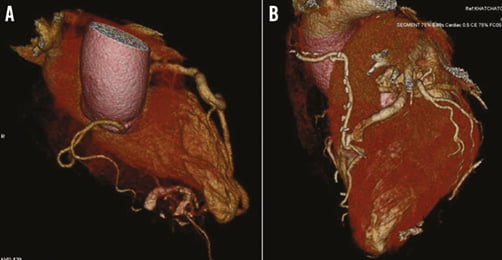
Figure 5. Cardiac computed tomography (CT) 3D reconstruction. A) Aneurysmal dolicho-artery of the second diagonal branch (D2) draining into a calcified inferoseptal cavity. B) Postoperative image. Recession of the D2 and LIMA-LAD.
As there are no guidelines or consensus to guide the decision-making process in the treatment of CAF, the individual management plan is based on the clinical presentation, anatomical conditions and the experience of the Heart Team. Surgery remains the main treatment of CAF, despite the lack of firm recommendations. Data on different treatment options are mainly available as case reports or small series. Branco et al described good long-term clinical outcome on medical treatment in a similar anatomical variant of acquired CAF draining into an interventricular septum cavity, in spite of atrial fibrillation and heart failure13. Recently, PCI has become an alternative to surgery in certain situations. Transcatheter and surgical closure are reported to have similar long-term outcome incidence of complications and mortality14,15. There are several parameters related to the fistula that are involved in the decision-making process, including size, flow, number of communications and terminations, pathway, side branches and procedure-related risk16. CAF that drain into the coronary sinus are at a higher risk of long-term complications after percutaneous closure15. According to the existing classifications of CAF17, our case represents a macro type I CAF (≥1.5 mm diameter, solitary coronary artery to cardiac chamber) and single channel type, i.e., a tortuous/single channel type with a dilatation of the fistula-related artery (considering the pathway).
Clinical presentation and additional information, obtained by multimodality imaging (CMR, CT and PET/CT scan), are critical factors in the decision making. In the described case, the treatment options included surgical exclusion of the fistula and LIMA bypass grafting or PCI coil embolisation of the dilated diagonal artery. Surgery was considered to be the better option as the fistula contained a large and probably infected thrombus. In addition, there was a significant step down in diameter between the LAD and the diagonal branch. Embolisation of the diagonal would have directed the flow from a huge proximal LAD into a small distal vessel with turbulence and potential complications. In addition, coil embolisation in an infected fistula is not recommended, even after antibiotic therapy. For similar reasons, in the context of unclear thromboembolic complications, the implantation of a stent graft was not considered as the first choice strategy but could be discussed in case of surgical treatment failure and recanalisation of the CAF18.
There are no ongoing trials which can help to answer the question of surgery versus PCI for CAF. Evidence-based medicine cannot be applied in this setting as CAF is a rare clinical condition. Randomisation would be at least difficult and probably impossible due to the great variety of anatomical and clinical situations. Treatment has to be individualised to each patient. Analyses of single case reports and case series may aid the decision-making process.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Coronary angiogram. 50% stenosis of the left anterior descending artery (LAD) and an aneurysmal dolicho-artery of the second diagonal branch (D2) draining into a calcified cavity and further into the coronary sinus.
Moving image 2. Coronary angiogram with the microcatheter. An aneurysmal dolicho-artery of the second diagonal branch.
Moving image 3. Cardiac magnetic resonance (CMR). A pediculated, floating thrombus within the inferoseptal cavity.
Supplementary data
To read the full content of this article, please download the PDF.
Coronary angiogram. 50% stenosis of the left anterior descending artery (LAD) and an aneurysmal dolicho-artery of the second diagonal branch (D2) draining into a calcified cavity and further into the coronary sinus.
Coronary angiogram with the microcatheter. An aneurysmal dolicho-artery of the second diagonal branch.
Cardiac magnetic resonance (CMR). A pediculated, floating thrombus within the inferoseptal cavity.

